Abstract
The prevalence (7.5%, 19/255) and genotypes of Enterocytozoon bieneusi in children of various age categories and clinical presentations were determined herein. The co-occurrence of the known genotypes (CS-4, EbpC, and Henan-IV) in children and pigs in the same study area, the phylogenetic characterization of novel genotypes (NEC1 to NEC5), and the assessment of potential risk factors associated with zoonotic transmission robustly suggested that pigs could be a significant source of human E. bieneusi infections in northeast China.
TEXT
Enterocytozoon bieneusi, a ubiquitous unicellular microsporidian fungal pathogen with a broad host range, infects many species of humans, livestock, wildlife, and birds and causes significant infectious diarrhea in immunocompromised hosts, notably AIDS patients and children (1–3). Approximately 90% of reported cases of human microsporidiosis are caused by E. bieneusi; the risk factors for infections include an immunosuppressive condition (AIDS patients), young age (children), and contact with contaminated food and water and other infected humans and animals (1).
Investigations of both human and animal samples for E. bieneusi genotypes that rely on hypermutation of the ribosomal internal transcribed spacer (ITS) have been helpful in elucidating the transmission routes of microsporidiosis (3). Genotypic typing and phylogenetic analysis facilitated classification of the almost 200 genotypes identified thus far into genetic group 1 with zoonotic potential and several other host-adapted genetic clusters (4–7). Infections with E. bieneusi in humans have been repeatedly reported worldwide, although epidemiologic data to indicate the prevalence and risk of human microsporidiosis in China are limited (8–10). The frequent and close contact of humans with livestock in China is of potential zoonotic concern (6, 7). This study investigated 255 fecal specimens from children in the cities of Harbin and Daqing in northeast China for E. bieneusi genotypes and analyzed the risk for potentially zoonotic transmission of microsporidiosis.
Study population.
Fecal specimens (n = 255) from children (one specimen per child) were collected in a community hospital (IH) (n = 40), two nursery schools (NS) (NS1, n = 20; NS2, n = 17), and a primary school (PS) (PS1, n = 57) in suburban Harbin in June 2013 and in four primary schools (PS2, n = 41; PS3, n = 11; PS4, n = 29; PS5, n = 40) in suburban Daqing in July 2014 (Table 1). Only four children in the community hospital had diarrhea, while the others had nongastrointestinal illnesses. The nursery and primary school children were all healthy at the time of sampling. The specimens were divided into four age groups: group I included 31 preweaned in-hospital children <1 year of age; group II included nine weaned in-hospital children aged 1 to 3 years; group III included 37 nursery school children aged 3 to 6 years; and group IV included 178 primary school children aged 6 to 12 years (Table 1). Background information on age, gender, and clinical presentations (diarrhea or nondiarrhea) is shown in Table 1. Body weights were fully available only for preweaned children in age group I.
TABLE 1.
Infection rates of Enterocytozoon bieneusi in children in the cities Harbin and Daqing of Heilongjiang Province, China
| Group | Age (yr) | City | Sourcea | Infection rate (% [positive no./total no.]) |
||||
|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Diarrhea | Nondiarrhea | ||||
| I | <1 | Harbin | IH | 25.8 (8/31) | 30.8 (4/13) | 22.2 (4/18) | 25 (1/4) | 25.9 (7/27) |
| II | 1–3 | Harbin | IH | 11.1 (1/9) | 20 (1/5) | 0.0 (0/4) | 0.0 (0/0) | 11.1 (1/9) |
| III | 3–6 | Harbin | NS1 | 0.0 (0/20) | 0.0 (0/12) | 0.0 (0/8) | 0.0 (0/0) | 0.0 (0/20) |
| Harbin | NS2 | 0.0 (0/17) | 0.0 (0/9) | 0.0 (0/8) | 0.0 (0/0) | 0.0 (0/17) | ||
| IV | 6–12 | Harbin | PS1 | 12.3 (7/57) | 10.7 (3/28) | 13.8 (4/29) | 0.0 (0/0) | 12.3 (7/57) |
| Daqing | PS2 | 0.0 (0/41) | 0.0 (0/17) | 0.0 (0/24) | 0.0 (0/0) | 0.0 (0/41) | ||
| Daqing | PS3 | 0.0 (0/11) | 0.0 (0/2) | 0.0 (0/9) | 0.0 (0/0) | 0.0 (0/11) | ||
| Daqing | PS4 | 0.0 (0/29) | 0.0 (0/14) | 0.0 (0/15) | 0.0 (0/0) | 0.0 (0/29) | ||
| Daqing | PS5 | 7.5 (3/40) | 0.0 (0/24) | 18.8 (3/16) | 0.0 (0/0) | 7.5 (3/40) | ||
IH, in hospital; NS, nursery school; PS, primary school.
Specimen processing and data analysis.
Specimens were stored in 2.5% potassium dichromate solution, washed twice in distilled water, and subjected to DNA extraction using a TIANamp stool DNA kit (Tiangen, China) following the manufacturer's recommended procedures. PCR amplification of the hypervariable region (ITS) of the rRNA gene was conducted as described previously (6). PCR amplicons of the expected size (392 bp) were sequenced in both directions. The procedures or methods to determine E. bieneusi genotypes and construct a neighbor-joining tree were the same as those described previously (6). The differences in infection rates between any two of the four age groups (chi-square test) and in mean body weights between pathogen-infected and uninfected children in age group I (independent-samples t test) were considered statistically significant at a P value of <0.05. Analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, IL).
Infection rates of E. bieneusi.
Enterocytozoon bieneusi was detected in children in the community hospital (22.5%, 9/40; 95% confidence interval [CI], 0.078 to 0.372) and in primary school PS1 (12.3%, 7/57; 95% CI, 0.032 to 0.214) in Harbin and in primary school PS5 (7.5%, 3/40; 95% CI, −0.010 to 0.160) in Daqing (Table 1). Infection rates for the pathogen in males and females and in diarrheic and nondiarrheic individuals from different locations are shown in Table 1. In total, 19 of 255 children were infected with the pathogen (7.5%; 95% CI, 0.041 to 0.108), giving an infection rate of 11.9% (16/134; 95% CI, 0.061 to 0.178) in Harbin and 2.5% (3/121; 95% CI, −0.003 to 0.053) in Daqing. Males and females had total infection rates of 6.5% (8/124; 95% CI, 0.020 to 0.109) and 8.4% (11/131; 95% CI, 0.034 to 0.134), respectively. One of four (25%; 95% CI, −0.240 to 0.740) diarrheic children and 18 of 251 nondiarrheic children (7.2%; 95% CI, 0.039 to 0.105) were infected with the pathogen (Table 1).
PCRs of the ITS locus revealed the presence of E. bieneusi in 8 of 31 (25.8%; 95% CI, 0.079 to 0.437) in-hospital children (group I), 1 of 9 (11.1%; 95% CI, −0.107 to 0.329) in-hospital children (group II), 0 of 37 nursery school children (group III), and 10 of 178 (5.6%; 95% CI, 0.021 to 0.091) primary school children (group IV). The difference in infection rates between age groups I and IV was significant (P < 0.01, χ2 = 11.2), whereas there were no significant differences between any other age groups. The difference in average body weights between E. bieneusi-positive and -negative children in age group I was not significant.
ITS genotyping and phylogeny.
Nucleotide sequences were obtained from all E. bieneusi-positive specimens (Table 2). Multiple-sequence alignment and analysis identified three known genotypes, CS-4 (n = 2), EbpC (n = 11), and Henan-IV (n = 3), and five novel genotypes named NEC1 to NEC5 (Table 2). The dominant genotype in children was EbpC (11/19), followed by Henan-IV (3/19), CS-4 (2/19) and one case each for NEC1 to NEC5. Genotypes Henan-IV and NEC1 were found in a 12-month-old male hospitalized child and EbpC and NEC4 in a 6-year-old female school child (Table 2). The distribution of genotypes in various age groups is shown in Table 2.
TABLE 2.
Genotype distribution of Enterocytozoon bieneusi in children by sequence analysis of the ITS region of the rRNA gene
| City | Group | Sourcea | Gender | Age | Wt (kg) | Genotypeb | Host (locationc) | References |
|---|---|---|---|---|---|---|---|---|
| Harbin | I | IH | Male | 7 mo | 8.35 | EbpC | Pig (China, Thailand, Japan, Germany, and Switzerland), dog (China), human (China, CZE, Vietnam, and Thailand), and wild mammals (China, Austria, CZE, Poland, and United States) | 6, 7, 13, 15 |
| Harbin | I | IH | Female | 9 mo | 8.2 | EbpC | See above | 6, 7, 13, 15 |
| Harbin | I | IH | Male | 3 mo | 7.5 | Henan-IV | Pig (China) and human (China) | 6, 8 |
| Harbin | I | IH | Female | 5 mo | 11.2 | CS-4 | Pig (China) | 6, 7 |
| Harbin | I | IH | Male | 12 mo | 10.1 | Henan-IV/NEC1d | See above/this study | 6, 8 |
| Harbin | I | IH | Female | 1 mo | 5.7 | NEC2 | This study | |
| Harbin | I | IH | Male | 6 mo | 10.4 | CS-4 | See above | 6, 7 |
| Harbin | I | IH | Female | 2 mo | 6 | NEC3 | This study | |
| Harbin | II | IH | Male | 27 mo | 14.6 | EbpC | See above | 6, 7, 13, 15 |
| Harbin | IV | PS1 | Female | 6 yr | EbpC | See above | 6, 7, 13, 15 | |
| Harbin | IV | PS1 | Male | 7 yr | Henan-IV | See above | 6, 8 | |
| Harbin | IV | PS1 | Female | 5 yr | EbpC | See above | 6, 7, 13, 15 | |
| Harbin | IV | PS1 | Male | 6 yr | EbpC | See above | 6, 7, 13, 15 | |
| Harbin | IV | PS1 | Male | 6 yr | EbpC | See above | 6, 7, 13, 15 | |
| Harbin | IV | PS1 | Female | 6 yr | EbpC/NEC4d | See above/this study | 6, 7, 13, 15 | |
| Harbin | IV | PS1 | Female | 6 yr | EbpC | See above | 6, 7, 13, 15 | |
| Daqing | IV | PS5 | Female | 6 yr | 36 | EbpC | See above | 6, 7, 13, 15 |
| Daqing | IV | PS5 | Female | 8 yr | 35 | NEC5 | This study | |
| Daqing | IV | PS5 | Female | 6 yr | 35 | EbpC | See above | 6, 7, 13, 15 |
IH, in hospital; PS, primary school.
The Genotype terminology is based on that of Santin and Fayer (16). NEC1 to NEC5 are new genotypes found in this study.
Location where the genotypes were reported before this work. CZE, Czech Republic.
Mixed infection.
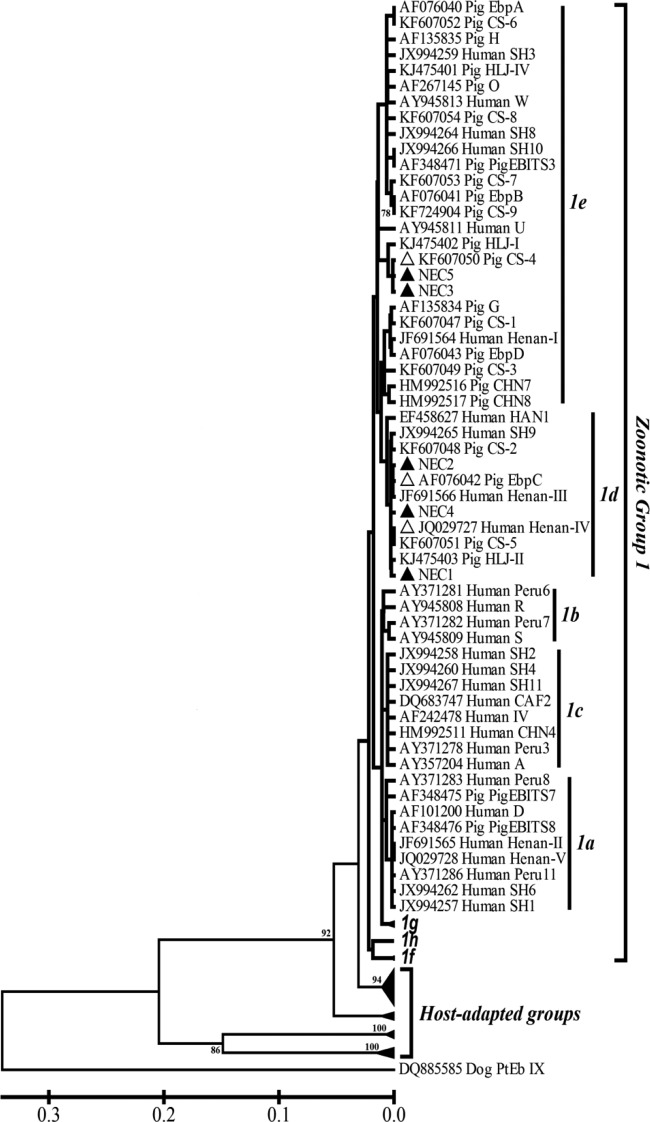
The extent of genetic relationships among E. bieneusi genotypes identified in this study was evaluated by molecular phylogenetic analysis using the neighbor-joining method, which showed clustering of the known genotypes CS-4 (subgroup 1e), EbpC (1d), Henan-IV (1d), and the new genotypes NEC1 (1d), NEC2 (1d), NEC3 (1e), NEC4 (1d), and NEC5 (1e) in genetic group 1 with the zoonotic potential described by Thellier and Breton (4) (Fig. 1). Group 1 also included genotypes CHN7, D, EbpA, EbpB, EbpC, EbpD, EBITS3, G, H, Henan-I, Henan-III, Henan-IV, O, and CS-1 to CS-9 identified in diarrheic and nondiarrheic pigs in multiple cities (including Harbin and Daqing) in northeast China (6, 7), genotypes CS-1, D, EbpA, H, LW1, O, and HLJ-I to HLJ-IV in asymptomatic pigs in the cities of Harbin and Suihua in northeast China (11), genotypes CHN7 and CHN8 in pigs in the city of Changchun in northeast China, genotypes D, EbpC, EbpD, IV, Peru8, Peru11, PigEBITS7, and Henan-I to Henan-V in HIV-positive and -negative individuals in Henan province in central China (8), genotypes D, EbpA, EbpC, Henan-I (reported as SH7), IV (reported as SH12), Peru11, SH1 to SH4, SH6, and SH8 to SH11 in children in the city of Shanghai in central China (9), and some other genotypes previously reported in humans and pigs in other geographic areas of the world (3).
FIG 1.
Phylogenetic relationship of ITS nucleotide sequences of Enterocytozoon bieneusi in this study and known E. bieneusi genotypes, as inferred by a neighbor-joining analysis (Mega 4 software, http://www.megasoftware.net/) based on genetic distances calculated using the Kimura two-parameter model. The ITS tree was rooted with GenBank sequence DQ885585. Bootstrap values >75% from 1,000 pseudoreplicates are shown. NEC1 to NEC5, marked with black triangles, are new genotypes found in this study; the known genotypes detected are indicated by white triangles.
Zoonotic potential and public health significance.
Infections with E. bieneusi in humans have occurred worldwide, with infection rates ranging from 1.4% to 78% (1). The variances mainly resulted from the detection methods used, the types of clinical specimens analyzed, the geographic areas investigated, and the gender, age, socioeconomic, clinical, and immune features of the patients (1). Two recent studies in central China reported the occurrence of E. bieneusi in 5.7% (39/683) of adult AIDS patients, 4.2% (29/683) of HIV-negative adult participants, 10.8% (8/74) of hospitalized case children with an average age of 20.7 months, and 3.2% (16/499) of control children with an average age of 42.6 months (8, 9). The pathogen was also detected in 22.5% (9/40) of in-hospital children with diarrhea (age unknown) in Changchun (10). The present study ascertained infection with the organism in diarrheic (25%, 1/4) and nondiarrheic (22.2%, 8/36) in-hospital children mostly aged <12 months and 5.6% (10/178) of healthy primary school children aged 6 to 12 years. Nevertheless, there were no cases of infection found in nursery school children. Interestingly, we observed a significantly higher infection rate of E. bieneusi in preweaned children (group I) than in primary school children (group IV), corresponding to the disease susceptibility of the former. Although E. bieneusi can lead to symptomatic or asymptomatic infections in immunocompromised and immunocompetent individuals (1), it is uncertain whether the appearance of diarrhea in the hospitalized child was associated with the fungal pathogenicity of E. bieneusi in this study. One previous study conducted in the city of Benin, Nigeria, indicated the significant association between E. bieneusi infections and weight loss in HIV-positive persons (12), whereas this was not the case for the immunocompetent children in this study.
Despite repeated reports of E. bieneusi in humans and domestic and wild animals in China, the transmission routes of human microsporidiosis remain unclear (1, 8–10, 13–15). Contact with pigs was considered a risk factor for acquisition of E. bieneusi genotype EbpC in HIV+ farmers in Henan (8). Genotype EbpC has also been sporadically reported in dogs (city of Xian), monkeys (Henan), hospitalized children (Shanghai), and wastewater (Wuhan and Qingdao) in China (5, 9, 13). Our two previous studies demonstrated that EbpC was the most dominant genotype in diarrheic (23.5%, 12/51) and nondiarrheic pigs (34.1%, 30/88) in multiple cities (including Harbin and Daqing where this study was conducted) in northeast China (6, 7). Genotype EbpC was also confirmed to be the most prevalent genotype (57.9%, 11/19) affecting in-hospital and primary school children in Harbin and primary school children in Daqing in this study. As summarized in Table 2, infections with EbpC also existed in humans, pigs, and wild mammals in several other geographical areas of the world. The known genotype Henan-IV originally documented in human infections in Henan also appeared in pigs in Harbin and Suihua (a close neighbor to the east of Harbin) (6). It is of interest to observe its widespread presence in hospitalized and primary school children in Harbin in this study. Likewise, the known genotype CS-4 previously recorded in pig infections in Harbin (our unpublished data) and Hegang (a neighbor to the south of Harbin) (6) also was found in infected hospitalized children in Harbin in the present study. All together, the known E. bieneusi genotypes EbpC, Henan-IV, and CS-4 found herein colonized both humans and pigs residing in the same study area, strongly supporting the likelihood of zoonotic transmission of microsporidiosis between humans and pigs.
Almost all E. bieneusi genotypes reported in pigs thus far belonged to the zoonotic phylogenetic group 1 (3, 4, 6, 7). Here, the clustering pattern of the evolutionary tree (Fig. 1) explicitly exhibited a close genetic relationship of the new genotypes NEC1 to NEC5 to the existing genotypes with zoonotic potential in group 1; thus, they were considered of public health significance.
Heilongjiang is a poor agricultural province in China, with an extensive livestock breeding industry and low socioeconomic status. Harbin and Daqing are located at the southern end of Heilongjiang, and persons are in close contact with the livestock kept free range or housed individually there, notably in the suburban regions. It is possible that human microsporidiosis could be transmitted through a direct fecal-oral route from free-range pigs by frequent daily contacts. Furthermore, the environmental shedding of E. bieneusi spores by pigs from huge pig factory farms could contaminate the groundwater and surface water during the rainy season (June and July in Heilongjiang) when our specimen collection was processed. Considering the extensive existence of E. bieneusi in the wastewater in multiple cities in central China (5), waterborne transmission of microsporidiosis may occur. In addition, farmers in Heilongjiang normally use pig manure in vegetable gardens or compost piles, and E. bieneusi spores that may be in this type of manure are more likely to survive and infect people than those in other types of manure. Judging by the above risk factors, children in Harbin and Daqing might acquire E. bieneusi infections from pig fecal sources.
In conclusion, this study confirmed the infections of E. bieneusi in children from Harbin and Daqing of various age categories and with various clinical presentations. The co-occurrence of the known genotypes CS-4, EbpC, and Henan-IV in children and pigs in the same study area, identification of novel genotypes being the members of zoonotic group 1, and assessment of potential risk factors associated with zoonotic transmission robustly suggested that pigs could be a significant source of human E. bieneusi infections in northeast China.
Nucleotide sequence accession numbers.
Nucleotide sequences for NEC1 to NEC5 were deposited in GenBank under the accession numbers KM272392 to KM272396.
ACKNOWLEDGMENTS
We thank Wenhe Li and Xingchao Li for their help in specimen collection.
This study was supported by the 7th Special Financial Grant from the China Postdoctoral Science Foundation (grant 2014T70307), the Heilongjiang Postdoctoral Research Fund (grant LBH-Z13024), the Scientific Research Funds of the Heilongjiang Provincial Education Department (grant 1253HQ015), the State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute (grant SKLVBF201307), and funds from Northeast Agricultural University (grant 2012RCA01).
Footnotes
Published ahead of print 1 October 2014
REFERENCES
- 1. Matos O, Lobo ML, Xiao L. 2012. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 2012:981424. 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathis A, Weber R, Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18:423–445. 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santín M, Fayer R. 2011. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 90:363–371. 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 4. Thellier M, Breton J. 2008. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite 15:349–358. 10.1051/parasite/2008153349. [DOI] [PubMed] [Google Scholar]
- 5. Li N, Xiao L, Wang L, Zhao S, Zhao X, Duan L, Guo M, Liu L, Feng Y. 2012. Molecular surveillance of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Negl. Trop. Dis. 6:e1809. 10.1371/journal.pntd.0001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W, Diao R, Yang J, Xiao L, Lu Y, Li Y, Song M. 2014. High diversity of human-pathogenic Enterocytozoon bieneusi genotypes in swine in northeast China. Parasitol. Res. 113:1147–1153. 10.1007/s00436-014-3752-9. [DOI] [PubMed] [Google Scholar]
- 7. Li W, Li Y, Li W, Yang J, Song M, Diao R, Jia H, Lu Y, Zheng J, Zhang X, Xiao L. 2014. Genotypes of Enterocytozoon bieneusi in livestock in China: high prevalence and zoonotic potential. PLoS One 9:e97623. 10.1371/journal.pone.0097623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Zhang H, Zhao X, Zhang L, Zhang G, Guo M, Liu L, Feng Y, Xiao L. 2013. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J. Clin. Microbiol. 51:557–563. 10.1128/JCM.02758-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Xiao L, Duan L, Ye J, Guo Y, Guo M, Liu L, Feng Y. 2013. Concurrent infections of Giardia duodenalis, Enterocytozoon bieneusi, and Clostridium difficile in children during a cryptosporidiosis outbreak in a pediatric hospital in China. PLoS Negl. Trop. Dis. 7:e2437. 10.1371/journal.pntd.0002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Wang Z, Su Y, Liang X, Sun X, Peng S, Lu H, Jiang N, Yin J, Xiang M, Chen Q. 2011. Identification and genotyping of Enterocytozoon bieneusi in China. J. Clin. Microbiol. 49:2006–2008. 10.1128/JCM.00372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao W, Zhang W, Yang F, Cao J, Liu H, Yang D, Shen Y, Liu A. 2014. High prevalence of Enterocytozoon bieneusi in asymptomatic pigs and assessment of zoonotic risk at a genotype level. Appl. Environ. Microbiol. 80:3699–3707. 10.1128/AEM.00807-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akinbo FO, Okaka CE, Omoregie R, Dearen T, Leon ET, Xiao L. 2012. Molecular epidemiologic characterization of Enterocytozoon bieneusi in HIV-infected persons in Benin City, Nigeria. Am. J. Trop. Med. Hyg. 86:441–445. 10.4269/ajtmh.2012.11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karim MR, Dong H, Yu F, Jian F, Zhang L, Wang R, Zhang S, Rume FI, Ning C, Xiao L. 2014. Genetic diversity in Enterocytozoon bieneusi from dogs and cats in China: host specificity and public health implications. J. Clin. Microbiol. 52:3297–3302. 10.1128/JCM.01352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye J, Xiao L, Ma J, Guo M, Liu L, Feng Y. 2012. Anthroponotic enteric parasites in monkeys in public park, China. Emerg. Infect. Dis. 18:1640–1643. 10.3201/eid1810.120653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karim MR, Wang R, Dong H, Zhang L, Li J, Zhang S, Rume FI, Qi M, Jian F, Sun M, Yang G, Zou F, Ning C, Xiao L. 2014. Genetic polymorphism and zoonotic potential of Enterocytozoon bieneusi from nonhuman primates in China. Appl. Environ. Microbiol. 80:1893–1898. 10.1128/AEM.03845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santín M, Fayer R. 2009. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J. Eukaryot. Microbiol. 56:34–38. 10.1111/j.1550-7408.2008.00380.x. [DOI] [PubMed] [Google Scholar]