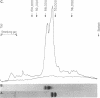
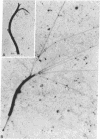
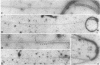
Abstract
Tubulin purified by phosphocellulose chromatography and free of accessory proteins will not form microtubules in the absence or presence of microtubule initiating sites (flagellar microtubules). The capacity for growth onto pre-existing "seeds" can be restored by the addition of small quantities of partially purified tau protein. Larger quantities restore the capacity for spontaneous assembly. These results suggest that tubulin requires tau for both initiation and growth of microtubules and that tau is incorporated into the microtubule throughout its length.
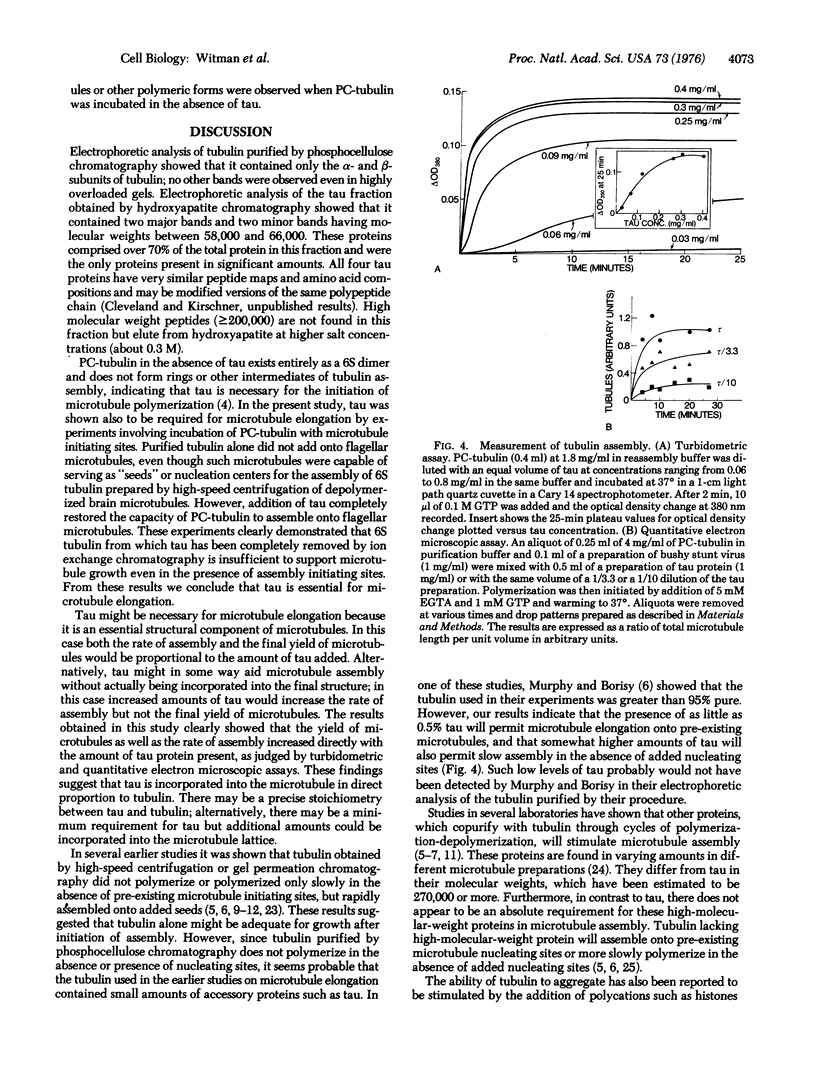
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C., Borisy G. G. Structural polarity and directional growth of microtubules of Chlamydomonas flagella. J Mol Biol. 1974 Dec 5;90(2):381–402. doi: 10.1016/0022-2836(74)90381-7. [DOI] [PubMed] [Google Scholar]
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Dentler W. L., Rosenbaum J. L. Assembly of chick brain tubulin onto flagellar microtubules from Chlamydomonas and sea urchin sperm. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1122–1126. doi: 10.1073/pnas.72.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B. Nucleated assembly of microtubules in porcine brain extracts. Science. 1972 Sep 29;177(4055):1196–1197. doi: 10.1126/science.177.4055.1196. [DOI] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Rosenbaum J. L. Ultrastructural localization of the high molecular weight proteins associated with in vitro-assembled brain microtubules. J Cell Biol. 1975 Apr;65(1):237–241. doi: 10.1083/jcb.65.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. L., Granett S., Witman G. B., Rosenbaum J. L. Directionality of brain microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1974 May;71(5):1710–1714. doi: 10.1073/pnas.71.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P. Assembly of microtubules from preformed, ring-shaped protofilaments and 6-S tubulin. J Supramol Struct. 1974;2(2-4):393–411. doi: 10.1002/jss.400020228. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Haga T., Kurokawa M. Microtubule formation from two components separated by gel filtration of a tubulin preparation. Biochim Biophys Acta. 1975 Jun 12;392(2):335–345. doi: 10.1016/0304-4165(75)90015-x. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Bennett P. M., Dickens M. J. Duplex microtubule is a new form of tubulin assembly induced by polycations. Nature. 1975 Oct 23;257(5528):707–709. doi: 10.1038/257707a0. [DOI] [PubMed] [Google Scholar]
- Keates R. A., Hall R. H. Tubulin requires an accessory protein for self assembly in microtubules. Nature. 1975 Oct 2;257(5525):418–421. doi: 10.1038/257418a0. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W., Honig L. S., Williams R. C. Quantitative electron microscopy of microtubule assembly in vitro. J Mol Biol. 1975 Dec 5;99(2):263–276. doi: 10.1016/s0022-2836(75)80144-6. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C. The mechanism of microtubule assembly in vitro. J Supramol Struct. 1974;2(2-4):412–428. doi: 10.1002/jss.400020229. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Miki-Noumura T. Light-microscopic observations of individual microtubules reconstituted from brain tubulin. J Cell Sci. 1975 Dec;19(3):607–620. doi: 10.1242/jcs.19.3.607. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975 Nov 18;14(23):5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele R. B., Borisy G. G. Comparison of the sedimentation properties of microtubule protein oligomers prepared by two different procedures. Biochem Biophys Res Commun. 1976 May 3;70(1):1–7. doi: 10.1016/0006-291x(76)91100-1. [DOI] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Suter M. M., Littman D. R., Kirschner M. W. Properties of the depolymerization products of microtubules from mammalian brain. Biochemistry. 1974 Dec 31;13(27):5529–5537. doi: 10.1021/bi00724a012. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B. The site of in vivo assembly of flagellar microtubules. Ann N Y Acad Sci. 1975 Jun 30;253:178–191. doi: 10.1111/j.1749-6632.1975.tb19199.x. [DOI] [PubMed] [Google Scholar]