Abstract
A case of fungal necrotizing fasciitis that appeared in an immunocompetent Mexican woman after a car accident is described. The patient did not respond to antifungal treatment and died 4 days later. The fungus was molecularly identified as a new species of Apophysomyces, namely, Apophysomyces mexicanus.
CASE REPORT
A 46-year-old woman was admitted to an emergency department 6 days after she suffered a rollover car accident, which occurred on a highway in a semiarid region with 75% relative humidity; the patient was ejected 10 meters from the car during the rollover, with consequent T12 vertebral fracture, Frankel grade B spinal cord injury, and superficial abrasions and lacerations over the neck. The patient was immobilized with a Philadelphia collar immediately after the accident, and her medical history showed type 2 diabetes mellitus, controlled with metformin-glibenclamide, and an allergy to nonsteroidal anti-inflammatory drugs. She received initial treatment in a regional hospital and was transferred to the emergency department of the Lic. Adolfo López Mateos Hospital 6 days later. After the collar was removed, a 3-cm-diameter fixed, irregular, red, swollen area was observed with a central necrosis of about 1 cm in length over the right posterior cervical triangle (Fig. 1A), along with multiple abrasions of the neck. Initial laboratory test results showed leukocytosis of 17 × 109/liter, glucose at 10.77 mmol/liter, hemoglobin at 121 g/liter, hematocrit at 0.352 liter/liter, urea nitrogen at 3.92 mmol/liter, urea at 3.92 mmol/liter, and creatinine at 53.04 mmol/liter. A computed tomography (CT) scan of the neck after administration of contrast material revealed isodense homogeneous soft tissue bulking posterior to the sternocleidomastoid muscle, with no evidence of foreign bodies. An escharectomy was carried out, and ceftriaxone (2 g/day) and clindamycin (1.2 g/day) were administered. On the second day, the cellulitis and necrosis areas expanded to 10 cm in length (Fig. 1B), affecting the posterior, muscular, and submandibular cervical triangles. The patient underwent surgical exploration of the neck and debridement, which showed necrotic tissue that extended as far as the muscle. Material was collected and sent to the laboratory for histopathological examination and microbial culture. Direct mountings on KOH (10% [wt/vol]) showed dichotomously branched, broad coenocytic hyphae (Fig. 1C), similar to those observed on hematoxylin-eosin histopathology preparations (Fig. 1D). Culturing on Sabouraud dextrose agar (Difco/Becton Dickinson, Mexico) produced white, cottony fungal colonies made of pauciseptated hyphae evocative of a fungus belonging to Mucorales. Consequently, primary cutaneous mucormycosis (PCM) was diagnosed; therefore, amphotericin B deoxycholate (0.5 mg/kg of body weight/day) and fluconazole (400 mg/day) were administered. A second debridement removed all necrotic tissue, reaching the free edges of the lesion and leaving a surgical mark (10 by 15 cm) (Fig. 1E). Despite antifungal treatment, the patient showed significant overall deterioration and hemodynamic instability that required management with amines and antiarrhythmics. Two days later, the ulcer enlarged, with edges that were more necrotic; a further surgical debridement was carried out, and the dose of amphotericin B was increased to 0.75 mg/kg/day. After the third debridement, a 20-cm-diameter surgical ulcer with clean edges was left. The borders reached the jaw angle, the temporal and occipital scalp regions, the posterior cervical line, and the supraclavicular edge. The renal and hepatic function remained within normal ranges, but the patient developed metabolic acidosis, insulin therapy being necessary to control glucose levels. The cumulative dose of amphotericin B was 380 mg. Although the patient's wound was clean and did not grow after the last debridement and glucose levels were controlled, she was hemodynamically unstable, developed ventricular fibrillation, and died 4 days after admission.
FIG 1.
(A) The initial phase of the lesion. (B) Dry-ulcer expansion. (C) Broad, coenocytic, dichotomously branched hyphae seen on a 10% KOH direct mounting (bar, 10 μm). (D) Hematoxylin-eosin staining of biopsy material, showing many wide, coenocytic hyphae (bar, 10 μm). (E) An ulcer after the second surgical debridement. (F) Apophysomyces mexicanus (CBS 136361) sporangiophore (scanning electron microscope) (bar, 15 μm). (G) Apophysomyces mexicanus (CBS 136361) sporangiospores (Nomarski) (bar, 10 μm).
Mycology.
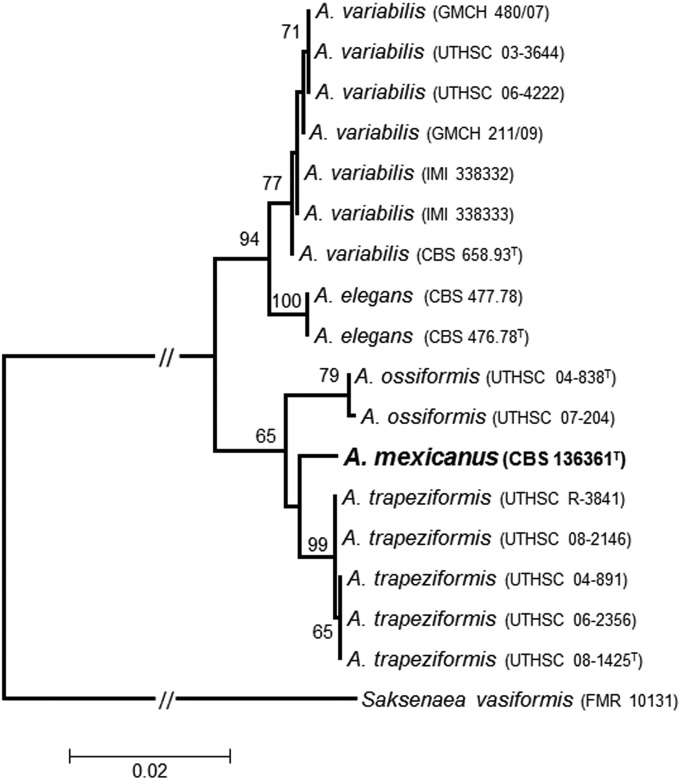
The fungus was identified as Apophysomyces sp., characterized by culturing on potato dextrose agar (PDA; Pronadisa, Spain), Czapek-Dox agar (CZA; Difco/Becton Dickinson, France), and malt extract agar (MEA; 10 g of malt extract, 20 g of agar-agar, and 1,000 ml of distilled water) for 4 days at 15, 25, 35, 37, 42, and 45°C incubation temperatures. The sporangiophores were mounted on water and on lactic acid from CZA plates and incubated for 6 days at 37°C. They showed characteristics similar to those of the other species of Apophysomyces, with the exception of A. elegans (which produces both vase-shaped and funnel-shaped apophyses). The sporangiophore wall of our isolate (including the apophysis) was verrucose at maturity (Fig. 1F), remaining smooth walled in the other species of the genus. The shape and the size of the sporangiospores of our isolate (Fig. 1G) were comparable to those of A. ossiformis and A. trapeziformis. A carbon source assimilation profile was determined using an API 50 CH commercial kit (bioMérieux, Marcy l'Etoile, France). The fungus assimilated glycerol, d-ribose, d-xylose, d-adonitol, d-glucose, d-fructose, d-mannose, d-mannitol, d-sorbitol, N-acetylglucosamine, d-maltose, d-trehalose, starch, glycogen, xylitol, d-lyxose, d-arabitol, l-arabitol, and potassium gluconate. Nitrogen source assimilation and growth in the presence of 2%, 5%, 7%, and 10% NaCl, 2% MgCl2, and 0.1% cycloheximide, carried out as described by Alvarez et al. (1), showed the ability of the fungus to assimilate arginine, cadaverine, creatine, creatinine, l-cysteine, l-leucine, l-lysine, l-ornithine, l-proline, l-tryptophan, and nitrate as well as to grow on 2% NaCl and on 2% MgCl2, like all the species of the genus. Results of esculin splitting, tested after inoculation on bile esculin agar (BEA; Panreac Quimica SA, Castellar del Vallés, Spain) in small petri dishes (5 cm in diameter) and incubation at 37°C for up to 1 week, were negative despite the fungus being able to grow on this medium. We followed the previously described protocols in the DNA extraction, amplification, and sequencing of the internal transcribed spacer (ITS) region, D1 and D2 (D1/D2) domains of the 28S nuclear ribosomal RNA (nrRNA) gene, and a fragment of the histone H3 gene (H3) (1). The phylogenetic analysis of the combined data set (ITS, D1/D2 and H3; 1,429 bp) encompassed the sequences of our isolate and others corresponding to type or reference strains of the Apophysomyces spp. that we had used in a previous study (1). Saksenaea vasiformis (FMR 10131) was used as the outgroup. The alignments and phylogenetic analyses were carried out using MEGA v. 5.05 with Clustal W and the maximum-likelihood (ML) algorithm, with the Kimura two-parameter model as the substitution model. The robustness of the branches was assessed by bootstrap (bs) analysis of 1,000 replicates. In the ML tree (Fig. 2), we observed two main clades, one with high bootstrap support (94% bs) and another with lower bootstrap support (65% bs). In the first one, we were able to distinguish two subclades: the A. variabilis subclade (77% bs) and another corresponding to the type species of the genus, A. elegans (100% bs). The second main clade split in two well-supported subclades corresponding to A. ossiformis (79% bs) and A. trapeziformis (99% bs) and an intermediate branch between them with our isolate (CBS 136361).
FIG 2.
Maximum-likelihood (ML) tree based on Kimura two-parameter model constructed using the combined data set (ITS, D1/D2, and H3) sequences of our isolate and type and reference strains of other Apophysomyces spp. used in a previous study (1). Numbers on the branches are bootstrap ML values above 65. Branch lengths are proportional to distance. Type strains of the different species are indicated with a superscript capital T. The new species proposed in this study is indicated in boldface.
The combination of morphological, physiological, and molecular results demonstrates that our isolate has enough differential characters from the other Apophysomyces species for us to propose the new species A. mexicanus to accommodate it.
Taxonomy.
Apophysomyces mexicanus Bonifaz, Cano, Stchigel et Guarro sp. nov. MycoBank MB 11223344 (Fig. 1F and G). Colonies on CZA attaining a diameter of 90 mm after 4 days of incubation at 37°C, whitish, with scarce aerial mycelium, hyphae branched, hyaline, smooth walled, 3 to 5.5 μm in diameter; reverse concolorous. Sporangiophores erect, arising singly, at first hyaline but soon becoming light greyish-brown, straight to slightly sinuous, tapered toward the apex, unbranched, 100 to 700 μm in length, 3.5 μm wide below the apophyses and 5 to 7 μm wide at the base, at first smooth walled but finally strongly verrucose, thin walled to slightly thick walled, bearing hyaline rhizoids at the base and 1 to 2 lateral stolons. Sporangia apophysate, terminal, obpyriform, multispored, white at first, becoming light greyish-brown when mature, and 25 to 30 μm diameter. Columella lens shaped to subspherical, 8 to 15 μm by 12 to 20 μm. Apophyses short, cup shaped to funnel shaped. Sporangiospores slightly trapezoidal in side view, cylindrical in front view, with flattened to slightly concave lateral walls, hyaline to light brown in mass, smooth walled and thin walled, 5 to 6.5 (to 10) μm by 3 to 4 μm. Colonies on PDA and MEA floccose, whitish, and with less sporulation than on CZA. Minimum and maximum temperatures of growth, 15 and 45°C, respectively.
Holotype.
CBS H-21410, isolated from biopsy material from a necrotic lesion in the neck of a patient (female, diabetic), Mexico City D.F., Mexico City, Mexico, 05-XI-2009, collected by L. Pintos and E. Guevara, isolated by A. Bonifaz. Living cultures: CBS 136361, FMR 12552.
In vitro antifungal susceptibility results as measured according to the Clinical and Laboratory Standards Institute M38-A2 guidelines for filamentous fungi (2) were as follows: amphotericin B MIC, 4 μg/ml; posaconazole MIC, 0.5 μg/ml.
Cases of cutaneous mucormycosis can be divided into two types: primary and secondary. The latter is the most frequent clinical presentation and in most cases derives from the rhinocerebral form. In our experience, approximately 70% of cases have presented a cutaneous expansion related to uncontrolled diabetes mellitus and immunosuppression (neutropenia) (3). PCM is usually acquired by inoculation of material contaminated with spores, as in cases of sites of venipuncture and application of adhesive tapes in severely immunocompromised hosts (3, 4). However, PCM can also occur in immunocompetent individuals, where the only predisposing factors identified are serious injuries (burns, car crashes) (3, 4). There have been recent reports and small outbreaks caused by lacerations and injuries due to tornadoes (5, 6) and other natural disasters, such as tsunamis and volcanic eruptions (4, 7).
The present case, from a clinical perspective, is similar to others reported (3, 8, 9). We believe that the infection was acquired through a skin laceration and inoculation of a foreign body of small diameter during the accident and encouraged by the location of the Philadelphia collar, which probably created an optimal microenvironment for the fungus. A microscopic examination of KOH mountings of biopsy material from surgical debridement allowed the diagnosis of mucormycosis to be confirmed. We need to draw attention to the rapid progress of the infection, which spread and deepened easily in a manner typically characteristic of necrotizing fasciitis (4, 10, 11). Although the patient would have been considered immunocompetent, the history of controlled type 2 diabetes mellitus, which is a high-risk factor for infection by Mucorales (3, 12), should be emphasized. Roden et al. (13) stated that there is a 19% incidence of PCM, with 34% of cases due to traumatic inoculation and only 3% associated with car crashes. However, Chakrabarti et al. (14) and Chakrabarti (15) reported the prevalence of PCM associated with car accidents to be higher.
In the present case, despite treatment that included administering amphotericin B and fluconazole and carrying out extensive surgical debridement, the infection spread extremely rapidly. The reported therapeutic response of Apophysomyces spp. to antifungals is highly variable. Most cases had previously been managed in a manner similar to that employed with our patient, with amphotericin B and surgical debridement treatment. Recent reports have shown that posaconazole has a good in vitro response (MIC, 0.1 g/liter) (16, 17, 18) as well as in vivo response (3, 16, 18), although in the reported cases of mucormycosis due to Apophysomyces trapeziformis, as with the Joplin tornado, only 6 of 13 patients responded to treatment with that compound (6, 19).
The phenotypic characterization of isolate CBS 136361 allowed us to find certain differences from the other species of the genus, such as the production of verrucose sporangiophores (smooth walled in the other species of the genus), a spore shape intermediate between those A. ossiformis and A. trapeziformis, and the assimilation of potassium gluconate, while l-arabinose, d-cellobiose, and d-melezitose were not assimilated. However, the pattern of assimilation of nitrogen sources, and also the resistance to NaCl, MgCl2 and cycloheximide, was similar to those seen with the other species of the genus (1). The ITS, D1/D2, and H3 nucleotide sequences, as well as the phylogenetic tree inferred from them, clearly show that our isolate is taxonomically separate from the other species of Apophysomyces. Consequently, we consider that this isolate represents a new species of the genus. Although only the present isolate of A. mexicanus so far exists, it is important to be aware of the high MIC of amphotericin B against this strain, since this drug is recommended for the treatment of mucormycosis. An experimental study tested different isolates of Apophysomyces variabilis, the most common species of the genus, showing better susceptibility to posaconazole than to amphotericin B (18).
Accession numbers.
The ITS, D1/D2, and H3 sequence data of the case isolate have been deposited into GenBank (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers HG974255, HG974256, and HG974254, respectively. A. mexicanus data have been deposited into the MycoBank database under accession number MB 11223344.
Footnotes
Published ahead of print 8 October 2014
REFERENCES
- 1.Alvarez E, Stchigel AM, Cano J, Sutton DA, Fothergill AW, Chander J, Salas V, Rinaldi MG, Guarro J. 2010. Molecular phylogenetic diversity of the emerging mucoralean fungus Apophysomyces: proposal of three new species. Rev. Iberoam. Micol. 27:80–89. 10.1016/j.riam.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard—2nd ed. Document M38-A2. CLSI, Wayne, PA. [Google Scholar]
- 3.Bonifaz A, Vázquez-González D, Tirado-Sánchez A, Ponce-Olivera RM. 2012. Cutaneous zygomycosis. Clin. Dermatol. 30:413–419. 10.1016/j.clindermatol.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Skiada A, Petrikkos G. 2013. Cutaneous mucormycosis. Skinmed 11:155–159. [PubMed] [Google Scholar]
- 5.Etienne KA, Gillece J, Hilsabeck R, Schupp JM, Colman R, Lockhart SR, Gade L, Thompson EH, Sutton DA, Neblett-Fanfair R, Park BJ, Turabelidze G, Keim P, Brandt ME, Deak E, Engelthaler DM. 2012. Whole genome sequence typing to investigate the Apophysomyces outbreak following a tornado in Joplin, Missouri, 2011. PLoS One 7:e49989. 10.1371/journal.pone.0049989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 367:2214–2225. 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 7.Gomes MZ, Lewis RE, Kontoyiannis DP. 2011. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 24:411–445. 10.1128/CMR.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayala-Gaytán JJ, Petersen-Morfín S, Guajardo-Lara CE, Barbosa-Quintana A, Morfín-Otero R, Rodriguez-Noriega E. 2010. Cutaneous zygomycosis in immunocompetent patients in Mexico. Mycoses 53:538–540. 10.1111/j.1439-0507.2009.01735.x. [DOI] [PubMed] [Google Scholar]
- 9.Guarro J, Chander J, Alvarez E, Stchigel AM, Robin K, Dalal U, Rani H, Punia RS, Cano JF. 2011. Apophysomyces variabilis infections in humans. Emerg. Infect. Dis. 17:134–135. 10.3201/eid1701.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. 2006. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Mod. Pathol. 19:1221–1226. 10.1038/modpathol.3800639. [DOI] [PubMed] [Google Scholar]
- 11.Kindo AJ, Shams NR, Kumar K, Kannan S, Vidya S, Kumar AR, Kalyani J. 2007. Fatal cellulitis caused by Apophysomyces elegans. Indian J. Med. Microbiol. 25:285–287. 10.4103/0255-0857.34778. [DOI] [PubMed] [Google Scholar]
- 12.Meis JF, Chakrabarti A. 2009. Changing epidemiology of an emerging infection: zygomycosis. Clin. Microbiol. Infect. 15(Suppl 5):10–14. 10.1111/j.1469-0691.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- 13.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634–653. 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarti A, Ghosh A, Prasad GS, David JK, Gupta S, Das A, Sakhuja V, Panda NK, Singh SK, Das S, Chakrabarti T. 2003. Apophysomyces elegans: an emerging zygomycete in India. J. Clin. Microbiol. 41:783–788. 10.1128/JCM.41.2.783-788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti A. 2010. Cutaneous zygomycosis: major concerns. Indian J. Med. Res. 131:739–741. [PubMed] [Google Scholar]
- 16.Alastruey-Izquierdo A, Castelli MV, Cuesta I, Monzon A, Cuenca-Estrella M, Rodriguez-Tudela JL. 2009. Activity of posaconazole and other antifungal agents against Mucorales strains identified by sequencing of internal transcribed spacers. Antimicrob. Agents Chemother. 53:1686–1689. 10.1128/AAC.01467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarti A, Shivaprakash MR, Curfs-Breuker I, Baghela A, Klaassen CH, Meis JF. 2010. Apophysomyces elegans: epidemiology, amplified fragment length polymorphism typing, and in vitro antifungal susceptibility pattern. J. Clin. Microbiol. 48:4580–4585. 10.1128/JCM.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salas V, Pastor FJ, Calvo E, Sutton DA, Chander J, Mayayo E, Alvarez E, Guarro J. 2012. Efficacy of posaconazole in a murine model of disseminated infection caused by Apophysomyces variabilis. J. Antimicrob. Chemother. 67:1712–1715. 10.1093/jac/dks090. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson TD, Schniederjan SD, Dionne-Odom J, Brandt ME, Rinaldi MG, Nolte FS, Langston A, Zimmer SM. 2007. Posaconazole treatment for Apophysomyces elegans rhino-orbital zygomycosis following trauma for a male with well-controlled diabetes. J. Clin. Microbiol. 45:1648–1651. 10.1128/JCM.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]