Abstract
Background
The outcome of treatment for pediatric Hodgkin lymphoma (HL) is excellent using chemotherapy and radiation. However, a minority of patients will relapse after treatment, but additional therapy achieves durable second remission in many cases. The optimal surveillance strategy after modern therapy for HL has not been well-defined.
Procedures
We reviewed the outcomes of pediatric patients with HL treated between 1990 and 2006 to determine the primary event that led to the detection of relapse. We determined the probability of relapse detection by routine follow-up procedures, including history, physical examination, laboratory tests, and imaging, and determined the impact of each of these screening methods on the likelihood of survival after relapse.
Results
Relapse occurred in 64 of 402 evaluable patients (15.9%) at a median of 1.7 years from the time of diagnosis. The majority of relapses (60%) were diagnosed at a routine visit, and patient complaint was the most common initial finding that led to a diagnosis of relapse (47% of relapses). An abnormal finding on physical examination was the primary event in another 17% of relapses, and imaging abnormalities led to the diagnosis in the remaining 36%. Laboratory abnormalities were never the primary finding. The method of detection of relapse and timing (whether detected at a routine visit or an extra visit) did not impact survival.
Conclusions
In pediatric Hodgkin lymphoma, most relapses are identified through history and physical examination. Frequent imaging of asymptomatic patients does not appear to impact survival and is probably not warranted.
Keywords: pediatric, childhood, Hodgkin lymphoma, relapse, surveillance, outcome
INTRODUCTION
The treatment outcome for pediatric patients with Hodgkin lymphoma (HL) is excellent with combined modality therapy that includes multi-agent chemotherapy and low dose involved-field radiotherapy. Relapses after treatment are uncommon and there is a reasonable survival rate for those patients who do experience a relapse. The optimal surveillance for recurrent disease after completion of therapy has not been well defined, though an increasing number of imaging modalities are available to clinicians. In particular, fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scanning has evolved as the standard of care in the United States (U.S.) to supplement anatomic imaging for initial staging evaluation of HL and assessment of early response to treatment. However, its role for surveillance after treatment has been completed is less clear since it has a high sensitivity but a low specificity for detection of relapse.1-4 These scans are burdensome to patients and families, expensive, and expose the patient to ionizing radiation. Furthermore, false positive results provoke anxiety and result in additional workup with imaging and sometimes invasive procedures. The challenge for clinicians is to determine a rational, cost-effective monitoring strategy that has a positive influence on disease outcome.
Few studies have evaluated the optimal plan for off-treatment surveillance. In the U.S., most children and adolescents with common childhood cancers are treated on or according to clinical trials which provide a specific follow-up schedule. These vary considerably and justification for a particular follow-up strategy is usually not provided. In order to be effective, a follow-up strategy should improve outcome by detecting disease in an earlier, more treatable stage.
In 1990, investigators from several institutions in the U.S. formed a multi-institutional consortium to conduct clinical trials in pediatric HL. Sequential therapeutic trials for all pediatric patients with HL have been ongoing since that time among the participating institutions, providing a cohort of patients followed prospectively in a standardized fashion. To address the question of optimal off-treatment follow-up, we examined the relapses that have occurred among our patient cohort. Specifically, we looked at outcome after relapse, timing and pattern of relapse, the presenting features of relapse, and the role of routine follow-up in detecting relapse. These findings are presented here.
METHODS
Patients
Between January 1990 and January 2006, patients were enrolled in our pediatric HL consortium clinical trials at the following institutions: St. Jude Children's Research Hospital, Stanford University Medical Center, Dana Farber Cancer Institute, Massachusetts General Hospital, and Maine Medical Center. Patients 21 years of age and younger with newly diagnosed, untreated HL were offered participation in a risk-adapted clinical trial. All trials were approved by the institutional review boards of the participating institutions and informed consent was obtained. The specific treatment regimens used over this time period and their outcomes have been published previously.5-8
All patients who experienced a relapse were included in this study. The records were reviewed to determine the patient's presentation at the time of relapse, and whether the relapse was detected at a routine, scheduled, off-treatment follow-up visit, or whether an extra visit was scheduled because of a patient complaint or symptom. In addition, the primary event that prompted the eventual diagnosis of relapse was classified as being a physical examination finding, an abnormal laboratory value, an imaging study finding, or a patient complaint. In cases where there were several conditions present simultaneously, the treating physician's notes were reviewed by authors A.F. and J.W. to ascertain the principal finding that prompted the subsequent evaluation. This additional review was also approved by the institutional review boards of each of the centers.
Off-treatment follow-up
Although the protocol guidelines evolved over the sixteen years of the various studies, the minimum required follow-up of patients who had completed therapy remained fairly standard during this time period. After completion of therapy, patients were followed regularly according to a recommended schedule of every 3 months for 1 year, every 4 months for the next 2 years, every 6 months for the fourth and fifth years, and annually thereafter. Follow-up examinations included physical examination, chest x-ray, and routine laboratory studies (complete blood count with differential, erythrocyte sedimentation rate, and lactate dehydrogenase). Additional surveillance studies included CT scans of the initially involved sites and gallium or FDG-PET scan at 1 and 2 years off therapy. Surveillance strategy did not differ according to patient risk group or stage. Other examinations were performed as clinically indicated in patients presenting with signs or symptoms suggestive of recurrent disease, and at the discretion of the treating physician.
Statistics
The exact Chi-square test was used to compare categorical variables between patients whose relapse was detected by diagnostic imaging studies and those whose relapse was detected by other findings. The Wilcoxon-Mann-Whitney test was used for comparison of age between the cohorts. Freedom from second failure (FF2F) was calculated from the date of first relapse to the date of second relapse or death; patients who did not suffer an event were censored at the last follow-up date. Overall survival (OS) after relapse was calculated from the date of relapse to the date of death from any cause. Living patients were censored at the last follow-up date. Survival was estimated using the Kaplan-Meier method.9 Log-rank tests were used to compare survival between groups. All analyses were performed in SAS software (version 9.1l SAS Institute, Inc., Cary, NC).
RESULTS
Four hundred and fifteen children and adolescents were enrolled onto the sequential clinical trials during the 16-year study period, and 77 patients (18.6%) were reported to have had recurrent disease at some point during the follow-up period. Upon review of the charts, 11 of these 77 patients (2.6% of the entire patient population) did not achieve an initial remission with front-line therapy and thus had primary refractory disease. These patients were excluded from the analysis of recurrent disease. Of the remaining 66 patients, 2 had insufficient data because their care was transferred early in their treatment course to another institution and follow-up information was not available. Thus, 64 patients with relapse were included in this study, with a median follow-up of 7.4 years (range, 0.6 to 18.3 years). Characteristics of this cohort are depicted in Table 1. The median time to relapse was 1.7 years (range 6.6 months to 7.9 years). All but 5 of the relapses were confirmed by biopsy. In these cases, the treating physicians decided that biopsy was unnecessary as the presenting features were diagnostic of recurrent HL.
Table I.
Patient characteristics, with comparison of patients with relapse detected by diagnostic imaging (DI) versus other finding (patient complaint or physical examination finding)
| Number of patients All N=64 (%) | Relapse detected by DI N=23 (%) | Relapse detected other finding N=41 (%) | P-value* | |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| Median | 15.6 | 15.9 | 15.3 | 0.16† |
| Range | 3.9 to 19.8 | 9.3 to 19.0 | 3.9 to 19.8 | |
| Age at first treatment failure (years) | ||||
| Median | 17.3 | 18.9 | 17.1 | 0.41† |
| Range | 7.1 to 24.6 | 13.2 to 22.2 | 7.1 to 24.6 | |
| Race | ||||
| White | 42 (66) | 14 (61) | 28 (68) | 0.46 |
| Black | 12 (19) | 3 (13) | 9 (22) | |
| Hispanic | 7 (11) | 3 (13) | 4 (10) | |
| Asian | 1 (2) | 1 (4) | 0 | |
| Not reported | 2 (3) | 2 (9) | -- | |
| Sex | ||||
| Male | 41 (64) | 13 (57) | 28 (68) | 0.35 |
| Female | 23 (36) | 10 (43) | 13 (32) | |
| Histology | ||||
| Nodular sclerosing | 53 (83) | 19 (83) | 34 (83) | 0.55 |
| Mixed cellularity | 7 (11) | 2 (9) | 5 (12) | |
| Lymphocyte predominant | 2 (3) | 0 | 2 (5) | |
| Not reported | 2 (3) | 2 (9) | -- | |
| Ann Arbor stage at original diagnosis | ||||
| I | 1 (2) | 0 | 1 (2) | 0.12 |
| II | 28 (44) | 6 (26) | 22(54) | |
| III | 14 (22) | 6 (26) | 8 (20) | |
| IV | 21 (33) | 11(48) | 10(24) | |
| Ann Arbor stage at time of relapse | ||||
| I | 14 (22) | 4 (17) | 10 (24) | 0.17 |
| II | 17 (27) | 3 (13) | 14 (34) | |
| III | 6 (9) | 3 (13) | 3 (7) | |
| IV | 27 (42) | 13(57) | 14 (34) | |
| Type of treatment failure | ||||
| Early relapse | 13 (20) | 5 (22) | 8 (20) | 0.83 |
| Late relapse | 51 (80) | 18 (78) | 33 (80) |
P-value obtained from χ2-test
P-value obtained from Wilcoxon-Mann-Whitney test
Outcome after relapse
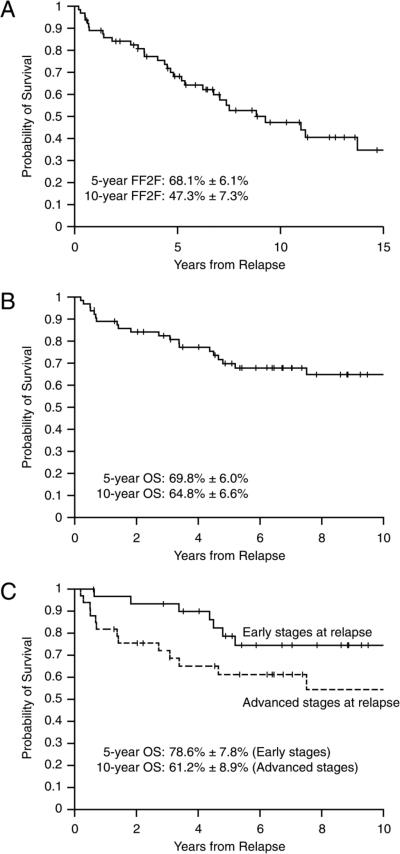
After relapse, the choice of second-line treatment was left to the discretion of the treating physician. The FF2F was 68.1% at 5 years and 47.3% at 10 years, while overall survival from the time of first relapse was 69.8% at 5 years and 64.8% at 10 years. Survival curves are shown in figures 1A and 1B. Patients with early stage disease (stages I and II) at relapse tended to be diagnosed earlier (median 1.5 years; range 0.5-5.2 years) compared to patients with more advanced stage at relapse (median 1.8 years; range 0.6-7.9) though this difference was not statistically significant and had no impact on survival (p=0.062, Figure 1C). Forty four of 64 patients (68.7%) were treated at some time point with an autologous hematopoietic cell transplant as part of their additional therapy. Of the 21 deaths among the 64 patients with recurrent disease, 18 were related to HL; one patient died in remission of an arrhythmia and two patients died of transplant-related complications.
Figure 1.
A: Freedom from second failure (FF2F); B: Overall survival (OS) after relapse; C: Overall survival by early stage (I and II) versus advanced stage (III and IV) at the time of relapse
Presentation of relapse
Table 2 depicts the clinical features at the time of relapse. The majority of relapses (60%) were diagnosed at the time of a routine follow-up visit (38 of 63 relapses for which there were sufficient data), while 40% of the patients scheduled an early visit because of a complaint. The most common primary event leading to a diagnosis of recurrent disease was a patient complaint (30 of 64 patients, 47%), with the most frequent complaint being an enlarged lymph node (26 of 64 patients, 41%), followed by pain in 12 patients (19%). Other common presenting complaints were weight loss, pruritus, night sweats, and fevers. Many patients had multiple complaints (15 of 64 patients, 23%). An abnormality on an imaging study in an asymptomatic patient without complaints led to the diagnosis of relapse in 23 (36%) of patients. CT scans detected the recurrence in 14 patients, while chest X-rays and nuclear medicine studies were diagnostic in 5 and 4 patients, respectively. An abnormal physical exam finding prompted the diagnosis of relapse in 11 (17%) patients. Abnormal laboratory values were never the primary finding that led to the evaluation for relapse, though they were frequently present at the time of relapse. The most common lab abnormalities were lymphopenia (present in 70% of patients at the time of relapse), elevated lactate dehydrogenase (59%), elevated ESR (53%), and anemia (25%). Leukocytosis was present in 13% of cases and leukopenia in 8%.
Table II.
Presentation of Relapse
| Number of Patients (n=64) | % | |
|---|---|---|
| Type of visit leading to diagnosis of relapse | ||
| Routine follow-up visit | 38 | 59 |
| Unscheduled, early visit | 25 | 39 |
| Unknown | 1 | 2 |
| Primary event leading to diagnosis of relapse | ||
| Patient symptom/complaint | 30 | 47 |
| Enlarged lymph node | 26 | 41 |
| Pain | 12 | 19 |
| Pruritus | 6 | 9 |
| Weight loss | 5 | 8 |
| Night sweats | 4 | 6 |
| Fever | 4 | 6 |
| Multiple complaints (including other non-specific) | 15 | 23 |
| Imaging study abnormality | 23 | 36 |
| CT scan | 14 | 22 |
| Chest X-ray | 5 | 8 |
| Nuclear study (PET or gallium scan) | 4 | 6 |
| Physical exam finding | 11 | 17 |
| Laboratory value | 0 | 0 |
Survival did not differ for patients whose relapse was diagnosed at the time of a scheduled visit compared to those who had an extra or early visit because of a concern (p=0.477, Figure 2). Furthermore, the percentage of patients with advanced stage (stages III or IV) disease at the time of relapse did not differ for patients diagnosed with relapse at a scheduled visit compared to those diagnosed at an unscheduled visit (55% advanced stage disease at scheduled visit versus 48% advanced stage at unscheduled visit). Also, the outcome was similar for patients who were diagnosed with relapse because of a symptom they experienced or an abnormality on physical examination to those who were diagnosed with relapse because of a finding on any type of imaging study (p=0.186, Figure 3).
Figure 2.
Overall survival according to whether the relapse was detected at a scheduled visit or unscheduled visit
Figure 3.
Overall survival according to whether relapse was detected on imaging study (DI) versus other finding (patient complaint or physical examination finding)
The proportion of patients whose relapse was detected on clinical grounds (by physical exam or symptom) rather than by imaging study remained relatively constant over the time period that the study was conducted. Of the relapses diagnosed between 1991 and 1995, 16 of 22 (73%) were detected by physical exam or symptom, while 11 of 20 (55%) and 14 of 23 (61%) were detected clinically between 1996-2000, and 2001-2005, respectively. Similarly, the proportion of relapses detected by imaging study remained relatively constant over time from original diagnosis. 23 of the 64 relapses were detected by imaging. 9 of these 23 imaging diagnoses were made in the first 12 months off treatment, while 8 were made between 12 and 24 months, and another 6 were made at later time points. CT scan was the imaging test that detected relapse in the majority of these cases (14 of 23), while it was PET or PET-CT in 3 cases, gallium scan in 1 case, and CXR in the remaining 5 cases. Three of the patients with relapse diagnosed primarily by imaging also had concerning symptoms or new physical exam abnormalities at the time of relapse, while 2 others had concerning lab abnormalities that contributed to the suspicion of relapse. Thus, there were18 patients with diagnosis of relapse made solely on the basis of an imaging abnormality.
Relapses occurred at a median of 20 months from original diagnosis (range, 7 months to 7.9 years). For patients with early stage disease at diagnosis, the median time to relapse was 18 months from diagnosis (range, 7 months to 5.2 years) and it was 21 months (range, 7 months to 7.9 years) for patients with advanced stage disease. Seventy-eight percent of the relapses occurred within 3 years of initial diagnosis. Regardless of whether relapse occurred early or late, or with localized versus advanced stage of disease, the method of relapse diagnosis did not impact FF2F.
DISCUSSION
Our study provides data on the presentation and outcome of relapse in a large, well-characterized cohort of pediatric patients with HL treated with contemporary, risk-adapted therapy in the era of CT and FDG-PET imaging and followed prospectively. It is the largest study focused specifically on relapse of pediatric HL and demonstrates the effectiveness of off-treatment surveillance that is primarily based on clinical history and examination. Ongoing clinical research efforts in our consortium are aimed at reducing or eliminating toxicity in a risk-adapted manner, and thus it is useful to note that radiation exposure in the form of frequent imaging studies can be minimized. In addition, this report is timely given the continued rapid escalation of health-care costs and the widespread availability and use of advanced imaging technologies which are frequently employed without careful examination of their clinical or economic effectiveness.
Our findings support those of other studies that have examined the role of active follow-up for the early diagnosis of relapse after cancer treatment. Biasotti et al. studied 101 children with recurrent cancer of all types.10 In this large series, half of the relapses were detected at a scheduled visit while half were detected at an unscheduled visit. The majority (3/4) of the relapses were first suspected on clinical grounds (e.g. because of symptoms or physical exam findings). Detection of relapse at a surveillance visit did not impact survival after relapse. Since this study included all cancer diagnoses, there would be less power to detect a survival advantage for more aggressive surveillance given the lower salvage rates for pediatric cancers other than HL.
Torrey et al. looked at the detection of relapse in early-stage HL in adult patients treated between 1969 and 1994 with radiation therapy alone.11 This study examined the relative costs of various tests as well as how relapse was detected. The combination of history and physical examination yielded the highest rate of relapse detection and was more cost-effective when compared with chest radiograph, abdominal radiograph, and labs. As in our series, the most common presenting symptom reported by the patient was a new, enlarged lymph node. There was no improvement in survival for patients in whom relapse was detected by radiographs. The advantage of our study in comparison is that those patients were adults treated in a very different manner and era.
Radford et al. studied the 37 relapses of 210 adult patients treated for HL between 1984 and 1990.12 The majority of patients (81%) were discovered to have relapsed because of symptoms they described, half of which prompted an earlier appointment. Only 11% of relapses were detected during a routine examination or investigation of an asymptomatic patient. As with the other studies, a new lump was the most common presenting symptom. This was a smaller series of patients also treated in an earlier era.
Finally, Voss et al. recently reported the experience on POG9425, examining the relapses of 25 pediatric patients with intermediate and advanced stage HL.13 76% of relapses were detected on the basis of symptoms, laboratory tests, or physical examination. Only 2 relapses (8%) were detected by surveillance imaging within the first year after treatment, and 4 (16%) after the first year. The authors suggested that the use of surveillance CT scans should be limited to the first year off treatment. The smaller size of this study limited its ability to detect an impact of surveillance imaging on survival, though again, there did not appear to be one.
The optimal follow-up strategy will depend on the specific disease. For a disease like HL with a relatively high salvage rate, one can make an argument that frequent, regular follow-up during the time period when relapse is most common is sensible. It is interesting to note that a patient's complaint of a new lump is consistently the most common presenting clinical feature of relapse. Careful history and physical examination together are the most valuable tools which will detect between 2/3 and 3/4 of the relapses.
FDG-PET is now widely available and considered the standard of care in HL for initial staging and assessment of response. The fact that it is a functional imaging modality and highly sensitive for detecting viable HL makes it very attractive for routine use in surveillance after treatment. Several studies have demonstrated its validity in detecting relapse. Zinzani et al. performed scans at 6, 12, 18, 24 months, and then annually in adult patients with lymphoma, 160 of whom had HL.14 FDG-PET was positive more often than either CT or clinical findings in patients with relapse, and it was clearly useful at detecting unsuspected relapse. However, there were numerous false positive scans and 3 patients without relapse who underwent biopsies because of false positive FDG-PET scans.
Another study in 36 adults with HL performed FDG-PET scans every 4 to 6 months for 2 to 3 years.15 While five patients with persistent or recurrent disease were identified before other testing indicated the presence of disease, another six patients had false positive PET scans that led to anxiety and further testing and procedures.
Although FDG-PET is effective at detecting pre-clinical relapse of HL, it is important to note that we cannot conclude from the available data that earlier detection either changes the clinical outcome or is cost-effective. These are much more challenging questions to answer. To our knowledge, no studies to date demonstrate that survival, the most important outcome, is affected by the mode or timing of detection of relapse. Goldschmidt et al. examined the role of routine imaging procedures in the detection of relapse of adult patients with HL and aggressive non-Hodgkin lymphoma.16 Their findings mirror our own with most relapses diagnosed clinically and no impact of mode of detection on survival. They did observe an increase in the number of radiologically-detected relapses after 2001, corresponding to increased FDG-PET/CT use and availability.
The relative frequency of relapse detection by FDG-PET or FDGPET/CT depends heavily on how often these scans are being performed. The more these tests are obtained, the more “useful” they seem to be. It is very likely that detection of relapse by imaging in our own study was rather infrequent precisely because we chose to use CT and/or FDG-PET scans sparingly in our surveillance strategy. In a patient population at relatively low risk of recurrence, where the major research efforts are to reduce or eliminate unnecessary radiation exposure, and where relapse is approached with systemic therapy, we do not currently believe there is a rationale for frequent FDGPET or CT imaging after treatment. This view is supported by the literature.17-18 For our current and upcoming consortium trials, we plan to eliminate surveillance FDG-PET images altogether. We will continue our practice of regular clinical follow-up, chest radiographs, and very limited use of CT scans, at the one and two-year time-points.
Acknowledgments
Funding support for this work comes from the National Institutes of Health Cancer Support Core Grant (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.El-Galaly TC, Mylam KJ, Brown P, et al. Positron emission tomography/computed tomography surveillance in patients with Hodgkin lymphoma in first remission has a low positive predictive value and high costs. Haematologica. 2012;97:931–936. doi: 10.3324/haematol.2011.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine JM, Weiner M, Kelly KM. Routine use of PET scans after completion of therapy in pediatric Hodgkin disease results in a high false positive rate. Journal of Pediatric Hematology Oncology. 2006;11:711–714. doi: 10.1097/01.mph.0000243648.66734.eb. [DOI] [PubMed] [Google Scholar]
- 3.Meany HJ, Gidvani VK, Minniti CP. Utility of PET scans to predict disease relapse in pediatric patients with Hodgkin lymphoma. Pediatric Blood and Cancer. 2007;48:399–402. doi: 10.1002/pbc.20797. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes MM, Delbeke D, Whitlock JA, et al. Utility of FDG-PET/CT in follow-up of children treated for Hodgkin and non-Hodgkin lymphoma. Journal of Pediatric Hematology Oncology. 2006;28:300–306. doi: 10.1097/01.mph.0000212912.37512.b1. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SS, Hudson MM, Lamborn KR, et al. VAMP and low-dose, involved-field radiation for children and adolescents with favorable, early-stage Hodgkin's disease: results of a prospective clinical trial. Journal of Clinical Oncology. 2002;20:3081–3087. doi: 10.1200/JCO.2002.12.101. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin's disease. Journal of Clinical Oncology. 2007;25:332–337. doi: 10.1200/JCO.2006.08.4772. [DOI] [PubMed] [Google Scholar]
- 7.Friedmann AM, Hudson MM, Weinstein HJ, et al. Treatment of unfavorable childhood Hodgkin's disease with VEPA and low-dose, involved-field radiation. Journal of Clinical Oncology. 2002;20:3088–3094. doi: 10.1200/JCO.2002.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin's disease. Journal of Clinical Oncology. 2004;22:4541–4550. doi: 10.1200/JCO.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Assocication. 1958;53:457–481.11. [Google Scholar]
- 10.Biasotti S, Garaventa A, Padovani P, et al. Role of active follow-up for early diagnosis of relapse after elective end of therapies. Pediatric Blood and Cancer. 2005;45:781–786. doi: 10.1002/pbc.20356. [DOI] [PubMed] [Google Scholar]
- 11.Torrey MJ, Poen JC, Hoppe RT. Detection of relapse in early-stage Hodgkin's disease: role of routine follow-up studies. Journal of Clinical Oncology. 1997;15:1123–1130. doi: 10.1200/JCO.1997.15.3.1123. [DOI] [PubMed] [Google Scholar]
- 12.Radford JA, Eardley A, Woodman C, et al. Follow up policy after treatment for Hodgkin's disease: too many clinic visits and routine tests? A review of hospital records. British Medical Journal. 1997;314:343–346. doi: 10.1136/bmj.314.7077.343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss SD, Lu Chen, Constine LS, et al. Surveillance computed tomography imaging and detection of relapse in intermediate- and advanced-stage pediatric Hodgkin lymphoma: A report from the Children's Oncology Group. J Clin Oncol. 2012:30. doi: 10.1200/JCO.2011.40.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinzani PL, Stefoni V, Tani M, et al. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. Journal of Clinical Oncology. 2009;27:1781–1787. doi: 10.1200/JCO.2008.16.1513. [DOI] [PubMed] [Google Scholar]
- 15.Jerusalem G, Beguin Y, Fassotte MF, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin's disease. Annals of Oncology. 2003;14:123–130. doi: 10.1093/annonc/mdg011. [DOI] [PubMed] [Google Scholar]
- 16.Goldschmidt N, Or O, Klein M, et al. The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Annals of Hematology. 2011;90:165–171. doi: 10.1007/s00277-010-1044-8. [DOI] [PubMed] [Google Scholar]
- 17.Cheson B. The case against heavy PETing. J Clin Oncol. 2009;27:1742–1743. doi: 10.1200/JCO.2008.20.1665. [DOI] [PubMed] [Google Scholar]
- 18.Dryver ET, Jernstrom H, Tompkins K, et al. Follow-up of patients with Hodgkin's disease following curative treatment: the routine CT scan is of little value. British Journal of Cancer. 2003;89:482–486. doi: 10.1038/sj.bjc.6601052. [DOI] [PMC free article] [PubMed] [Google Scholar]