Abstract
Immunosuppression in the tumor microenvironment blunts vaccine induced immune effectors. PD-1/B7-H1 is an important inhibitory axis in the tumor microenvironment. Our goal in this study was to determine the effect of blocking this inhibitory axis during and following vaccination against breast cancer. We observed that using anti-PD-1 antibody and a multi-peptide vaccine (consisting of immunogenic peptides derived from breast cancer antigens, neu, legumain and β-catenin) as a combination therapy regimen for the treatment of breast cancer bearing mice prolonged the vaccine-induced progression-free survival period. This prolonged survival was associated with increase in number of Tc1 and Tc2 CD8 T cells with memory precursor phenotype, CD27+IL-7RhiT-betlo and decrease in number of PD-1+ dendritic cells (DCs) in regressing tumors and enhanced antigen reactivity of tumor-infiltrating CD8 T cells. It was also observed that blockade of PD-1 on tumor DCs enhanced IL-7R expression on CD8 T cells. Taken together, our results suggest that PD-1 blockade enhances breast cancer vaccine efficacy by altering both CD8 T cell and DC components of the tumor microenvironment. Given the recent success of anti-PD-1 monotherapy, our results are encouraging for developing combination therapies for the treatment of cancer patients in which anti-PD-1 monotherapy alone may be ineffective (i.e. PD-L1-negative tumors).
Keywords: PD-1, peptides, vaccines, breast cancer, memory T cells
INTRODUCTION
Vaccination, targeting self-antigens, to treat cancer has been evaluated for several years and the results overwhelmingly show that the approach, used alone, is not effective to any significant extent at mediating tumor regression. Immune regulatory networks, soluble inhibitory factors and regulatory cells that prevail throughout the body and in the tumor microenvironment (TME) mediate tolerance to self-antigens and inhibit vaccine-induced effector responses (1). Recent research suggests that much of the immune suppression in the TME of several diseases is mediated by the PD-1/PD-L1 axis (2-10). Extensive studies targeting this inhibitory axis using anti-PD-1 and anti-B7-H1 antibodies are being done in preclinical models and in the human clinical setting (11, 12). Results obtained from recent trials testing anti-PD-1 and anti-B7-H1 antibodies as monotherapy in patients with different types of cancers are encouraging (13, 14). In our recent study we have shown that PD-1 and B7-H1 both mediate immune suppression in a far more complex manner than engagement of B7-H1 on tumor cells with PD-1 on tumor-infiltrating T cells. For example, we found that both PD-1 and B7-H1 are expressed on infiltrating DCs during late tumor expansion (15). As emerging data suggest that there is more complexity associated with PD-1/B7-H1 axis (15, 16), additional studies are warranted to understand the unique contributions of PD-1 and B7-H1 to immune suppression in the TME and provide insight into how to target this axis therapeutically.
The generation of memory CD8 T cells is the major goal of active immunotherapy against cancer and blockade of suppressive pathways in the TME along with vaccination has the potential to induce long-lasting protection or result in complete cures (17). In the current study, we used a multi-peptide anti-tumor infrastructure vaccine targeting epithelial tumor cells with neu, breast cancer stem cells (BCSCs) and tumor associated macrophages (TAMs) with legumain, and both epithelial tumor cells and BCSCs with beta catenin. BCSCs are resistant to therapy (18) and targeting them is predicted to be associated with significant clinical benefit (19). TAMs (M2 macrophages) exhibit many pro-tumoral functions including promotion of angiogenesis, matrix remodeling, suppression of adaptive immunity (20) and their density in lesions correlates with poor prognosis (21). With an aim to develop a therapeutic strategy that can prevent the recurrence of breast cancer, in this study in addition to targeting neu expressing proliferating malignant epithelial cells, we targeted BCSCs and TAMs that are critical to tumor growth. We combined the multi-peptide vaccine with anti-PD-1 antibody as combination therapy against breast cancer in a mouse model observing enhanced survival, increased antigen-specific T cell responses, a substantial increase in the infiltration of CD8 T cells with a memory precursor effector cell (MPEC) phenotype into regressing tumors and a sharp decrease in the number of tumor-infiltrating immunosuppressive PD-1+ DC population. Lastly, we showed that blockade of PD-1 on tumor DCs resulted in increase in the expression of memory marker (IL-7R) on CD8 T cells.
MATERIALS AND METHODS
Mice
Female, 6-8 weeks old BALB/c mice were from Jackson Laboratories. Animal care and use were in accordance with institutional guidelines.
Cell lines
TUBO and P815 cell lines were provided by Dr. Larry Pease of the Mayo Clinic (Rochester, MN) in 2010 (22). The RAW 264.7 cell line was provided by Dr. Adam Schrum of the Mayo Clinic (Rochester, MN) in 2010. All cell lines were authenticated as mouse origin by IDEXX BioResearch (Columbia, MO).
Epitope prediction
Using epitope prediction programs BIMAS, ProPed-I and SYFPEITH, sequences of rat neu (neu), legumain and β-catenin were analyzed and three peptides from each protein with high affinity for MHC class I were identified. The peptide sequences were neu: TYVPANASL (N1), SYGVTVWEL (N2), PYNYLSTEV (N3); legumain: TYEHALRYL (L1), MYQKMVFYI (L2), RYLYVLANL (L3); β-catenin: SYLDSGIHS (C1), RAIPELTKL (C2), AYGNQESKL (C3).
Peptides, adjuvants and antibodies
Vaccination
Non-tumor-bearing BALB/c mice were given three doses (100μg peptides in CFA on day 0 and 100μg peptides in IFA on day 3 and day 6) of peptide vaccine subcutaneously (s.q.) at the base of the tail. To determine the effect of PD-1 blockade in enhancing the efficacy of peptide vaccine, mice were given 4 intraperitoneal (i.p.) injections (on days 0, 3, 6, 9) of PD-1 blocking antibody (200 μg/mouse/injection) along with the peptide vaccine that was given as per the schedule described above.
In vivo tumor studies
Mice were implanted with 1×105 TUBO cells s.q. on the right flank. To determine the effect of vaccine, mice were given a total of three injections of peptide vaccine in CFA (day 16) and IFA (days 19 and 21) post tumor challenge. For determining the combination effect of PD-1 blockade with peptide vaccine on tumor growth, mice were given 8 i.p. injections (on days 7, 10, 13, 16, 19, 22, 25, and 28) of PD-1 blocking antibody (200 μg) and three immunizations of peptide vaccine in CFA/IFA (on days 16, 19 and 21) as described above. The dose of anti-PD-1 was based on our previous studies which identified 200 μg as an optimal dose for treating ovarian cancer (15). Anti-PD-1 was started prior to vaccination to insure steady state concentrations (23). For in vivo cell depletion, anti-CD4 mAb (0.2 mg/dose) or anti-CD8 mAb (0.5 mg/dose) were injected i.p. (days 13, 14 and 15) post-tumor challenge followed by one dose on a weekly basis. For survival experiments, mice with the tumor size 300mm2 were considered moribund.
Measurement of immune responses
T cell responses and serum MCP-1 levels were measured by enzyme-linked immunosorbent spot assays (ELIspot), multiplexed cytokine assays and enzyme-linked immunosorbent assay (ELISA) respectively as described previously (15, 24). Methods are detailed in the supplementary methods section.
In vitro PD-1 blockade on tumor-infiltrating DCs
The effect of blockade of PD-1 on tumor DCs in inducing IL-7R and T-bet expression by splenic CD8 T cells was determined using in vitro co-culture. DCs and CD8 T cells from immunized mice were enriched from tumors and spleen, respectively using CD11c and CD8 microbeads (Miltenyi) (15). Tumor DCs were cultured overnight in the presence of anti-PD-1 or isotype control antibodies (10 μg/ml). Antibody was then washed from the culture, fresh media was added and the DCs were co-cultured with splenic CD8 T cells at 1:4 ratio for 24 hrs. Single cell suspensions obtained from culture wells were stained to determine the expression of IL-7R and T-bet by splenic CD8 T cells.
Flow cytometry
Cell surface molecule staining and flow cytometry were done as previously described (15, 25). Serum antitumor antibody analysis was done using flow cytometry and the methods are detailed in supplementary section.
Statistical-analysis
Two-tailed Mann-Whitney tests, one-way ANOVAs or student’s t-tests from GraphPad Instat or GraphPad Prism software were used to analyze the data unless otherwise stated. P < 0.05 was considered as significant. For survival analysis, Kaplan-Meier test was used.
RESULTS
A peptide vaccine targeting multiple tumor antigens is immunogenic and induces regression of breast cancer
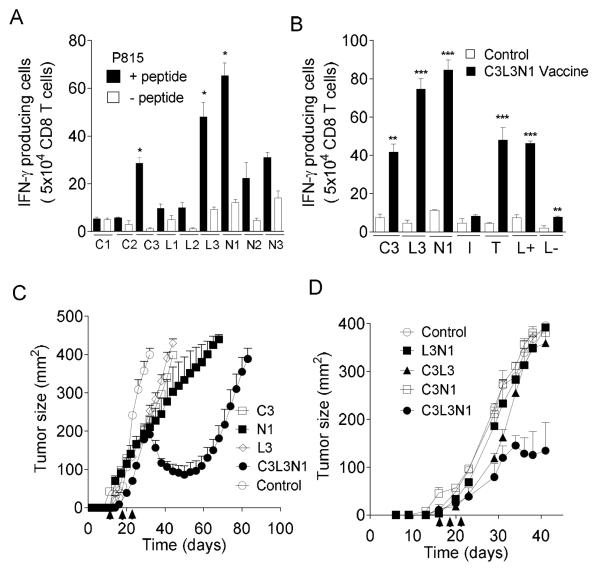
Immunogenicity of peptides of each of the antigens, identified by epitope prediction programs, was tested by immunizing mice as described in Materials and Methods. Three immunogenic peptides C3, L3 and N1 were identified (Fig. 1A). In the next step, the immunogenicity of these 3 peptides as a multi-peptide vaccine, C3L3N1, was tested. As shown in Fig. 1B, the multi-peptide vaccine induced IFN-γ responses against individual peptides and antigen-expressing TUBO and legumain+ RAW cells (26). IFN-γ responses against the TUBO cells (neu+ and β-catenin+) and legumain+ RAW macrophages (L+ cells) confirmed that C3, L3 and N1 are naturally processed peptides. The ability of the individual peptides and multi-peptide vaccine to reduce tumor burden in breast tumor (TUBO) bearing BALB/c mice was tested. As shown in Fig. 1C, the multi-peptide vaccine induced tumor regression, whereas individual peptides were ineffective. We then asked whether the combination of any two peptides can induce tumor regression. As shown in Fig. 1D, it was observed that having three peptides as a combination is essential to induce tumor regression. Collectively, these data suggest that the multi-peptide ‘infrastructure’ vaccine is immunogenic and is effective in reducing breast tumor burden in this model.
Figure 1. Multi-peptide vaccine induces CD8 T cell responses and reduces tumor burden.
(A) Shown are the mean number (±s.e.m. n=3) of CD8 T cells producing IFN-γ, as assessed by ELIspot, in response to stimulation with (A) P815 cells alone or pulsed with predicted peptides and (B) C3, L3, N1 and control (I), legumain+ (L+) and legumain- (L-) RAW cells and neu+ and β-catenin+ TUBO (T) cells. (C-D) Tumor growth curves for BALB/c mice vaccinated with individual peptides compared with the C3L3N1 vaccine (C) and combinations of two peptides and C3L3N1 vaccine (D). Symbols show mean tumor size (n=4 per group, ±s.e.m.). Data are representative of one of three experiments yielding similar results. * = p<0.05; ** = p<0.005, ***p = p<0.0005.
Combination therapy enhances vaccine efficacy
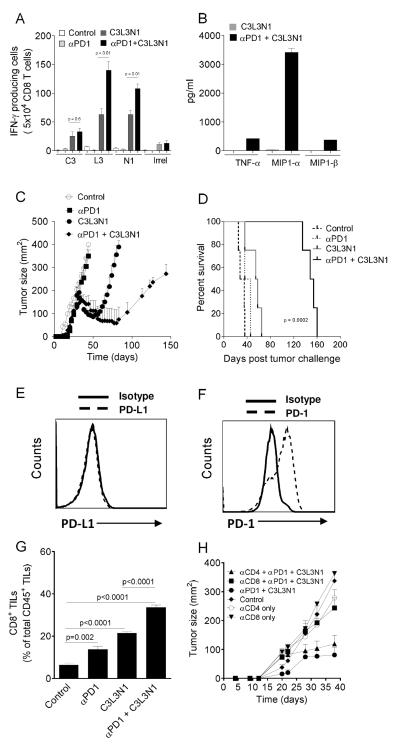
Even though it was observed that the multi-peptide vaccine induced tumor regression, complete cures were not observed. Given the fact that PD-1 and PD-L1 are reported to be expressed on activated lymphocytes including T cells (27) in addition to being well known that PD-1/PD-L1 is a major immunosuppressive pathway (2), first, we wanted to determine whether blockade of this inhibitory axis during the priming phase would enhance the efficacy of the multi-peptide vaccine. As depicted in Fig. 2A, anti-PD-1 antibody significantly augmented the numbers of antigen-specific effectors when combined with vaccine but had no impact when used alone. Additionally, the function of antigen specific effectors was improved as determined by the enhanced release of cytokines TNF-α, MIP1-α and MIP1-β (Fig. 2B). Next, we asked whether using anti-PD-1 therapy could improve the therapeutic efficacy of the multi-peptide vaccine. Tumor-bearing mice were treated with multi-peptide vaccine plus anti-PD1 antibody as described in Materials and Methods. As shown in Figs. 2C-D, this combination therapy induced regression in all tumors and led to sustained protection from progression, increasing median survival by nearly 3-fold when compared to vaccine alone. We also determined the expression of PD-1 and PD-L1 on TUBO tumors, observing that these tumors are PD-1 positive and PD-L1 negative (Figs. 2E-F). While it could be argued that the effects of anti-PD-1 could be mediated through blocking signaling of PD-1 on tumor cells, two pieces of data rule this out. First, anti-PD-1 alone had no impact on disease outcome as shown in Fig. 2C (p>0.05). Second, in vitro studies show that anti-PD-1 treatment of TUBO cells in culture has no impact on tumor cell growth (Supplementary Fig. S1). This suggests that the antibody was not acting by preventing interaction of immune effectors and the tumor. We also observed that combination treatment augmented the numbers of CD8 TILs by greater than two fold over the vaccine only group and 5-fold over the control group (Fig. 2G). To determine the role of CD4 and CD8 T cells in inducing sustained protection that was observed in combination therapy group, mice were treated with T cell-depleting anti-CD8 or anti-CD4 antibodies. Treatment with anti-CD8 mAb (Fig. 2H) reversed the effects of combination therapy and tumor size at Day 39 in the cohort of CD8 T cell-depleted that received both vaccine and anti-PD-1 as compared to untreated control mice (P>0.05). Overall, there was no statistically significant differences in tumors from control mice or those treated with anti-CD4 mAb alone, anti-CD8 mAb alone, or the combination of anti-CD8 mAb, anti-PD-1 mAb and vaccine (p>0.05). This shows that CD8 T cells are responsible for tumor regressions and sustained protection that was observed in combination therapy groups. We also observed that combination therapy did not result in significant increases in numbers of CD4 T cells compared to vaccine group (Supplementary Fig. S2A) and data from the T cell subset depletion studies shows that depletion of CD4 T cells in tumor-bearing mice treated with combination therapy resulted in protection that was observed in mice without CD4 T cell depletion. These results suggest that the combination acts independently of helper T cells. Lastly, as shown in Supplementary Fig. S2B, we observed that PD-1 blockade alone and combination resulted in two-fold decrease in number of tumor-infiltrating Tregs over control groups (28, 29) but no significant differences were observed when compared to vaccine only group suggesting that the improved protection observed in combination group, when compared to vaccine group, is not directly dependent on Tregs.
Figure 2. Anti-PD-1 treatment with multi-peptide vaccine enhances immune responses during priming phase and delays recurrence of disease.
(A) Shown are mean ELIspot numbers (±s.e.m. n=3) of IFN-γ producing CD8 T cells, in response to P815 cells pulsed with C3, L3, N1 and control (Irrel) peptides). (B) Shown are the mean (±s.e.m., n=2) cytokine concentrations detected in supernatants of CD8 T cells incubated with P815 cells pulsed with N1 peptide from vaccinated mice. (C) Shown are the tumor growth curves for BALB/c mice (n=4 per group) vaccinated with multi-peptide vaccine and multi-peptide vaccine plus anti-PD-1 antibody. Points, average tumor size; bars, s.e.m. (D) Shown is the kaplan meier survival curve representing different groups of TUBO tumor-bearing BALB/c mice. (E) and (F) Shown are the histograms (gated on R1) representative of PD-L1 and PD-1 expression by TUBO tumor cells. (G) Mean (± s.e.m., n=4) levels of infiltrating CD8 T cells in treated and control animals. (H) Tumor growth curves for different groups of TUBO tumor-bearing BALB/c mice (n=5 per group) treated with or without anti-CD4 or anti-CD8 monoclonal antibodies. Points, average tumor size; bars, SD. Data are representative of one of three (A-F, H) experiments with similar results.
Combination therapy enhances the generation of antigen loss variants
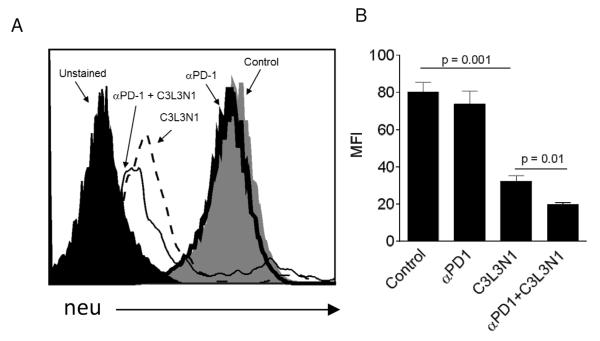
Even though the multi-peptide vaccine with or without anti-PD-1 antibody was able to induce tumor regression, the tumors ultimately recurred. As it was known that immune pressure in tumors results in the development of antigen-negative variants (25, 30), we wanted to determine the cell surface expression of neu antigen on the recurring tumors. As shown in Figs. 3A-B, a significant reduction in neu expression was observed in recurring tumors obtained from vaccinated groups whereas no loss in neu antigen expression was observed in tumors that were obtained from PBS and anti-PD-1 groups. Importantly, the inclusion of anti-PD-1 further decreased neu expression as compared with the vaccine alone group suggesting that T cells generated with combination therapy recognized lower levels of peptide:MHC class I complexes.
Figure 3. PD-1 blockade enhances the generation of antigen loss variants.
(A) Shown is a histogram representative of neu expression on tumors obtained from different treatment groups. (B) Shown is the bar graphs representative of mean neu-specific fluorescence intensity (MFI) of the TUBO tumors. Data is shown as mean (± s.e.m). and from three independent experiments.
Combination therapy enhances the numbers of antigen-specific effectors at the effector phase
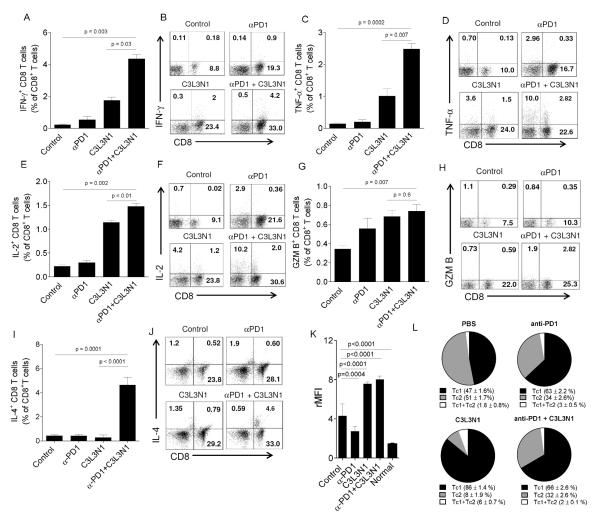
As we observed that combination therapy resulted in a significant increase in the survival of tumor bearing mice and also given the fact that blockade of tumor associated immunosuppression is known to enhance function of vaccine induced effectors (31), next, we wanted to determine whether combination therapy would increase the effector function of TILs. For this, lymphocytes obtained from tumors on day 35-38 (the time point at which tumor regression began in the different groups of vaccinated mice) were analyzed for effector function. As shown in Figs. 4A-F, TILs from combination groups when stimulated with N1 peptide showed a significant increase in IFN-γ+, TNF-α+ and IL-2+ CD8 TILs compared to other groups. No significant difference in granzyme B+ CD8 TILs obtained from combination therapy groups and vaccine groups was observed (Figs. 4G-H). Similar results were obtained when evaluating IFN-γ responses to the β-catenin (C3) and legumain peptide (L3), although the overall frequencies of antigen-specific T cells were considerably lower (Figs. S3A-B). As shown in Figs. 4I-J, combination therapy also resulted in a significant increase in IL-4+ CD8 T cells as compared to vaccine or anti-PD-1 therapy alone, demonstrating the generation of Tc2 CD8 T cells in addition to Tc1. The generation of Tc2 in combination therapy was also confirmed using multiplexed cytokine assays for IL-4 and IL-5 (Fig. S4). Considering the role of IL-4 and IL-5 in driving antibody responses, we evaluated mice for the presence of anti-tumor antibodies demonstrating that while anti-PD-1 therapy reduces the natural antibody response to tumor, this suppression is mitigated by combining anti-PD-1 with the vaccine (Fig. 4K). Lastly, we evaluated the proportional distribution of Tc1 and Tc2 TILs in the tumors (Fig. 4L). As shown, tumors from untreated animals had roughly similar amounts of Tc1 and Tc2 effectors which was slightly skewed in favor of Tc1 effectors by anti-PD-1 alone. The use of vaccine alone favored a local accumulation of Tc1 over Tc2, whereas, the addition of anti-PD-1 with vaccine increased Tc2 effectors, 4-fold, from 8 ± 1.9% (vaccine alone) of the total CD8 TILs to 32 ± 2.6% in the combination treatment group (p<0.0001). Collectively, these data suggest that PD-1 blockade during vaccination against breast cancer not only enhances effector responses during the priming phase but also at the effector phase.
Figure 4. Combination therapy enhances the antigen-specific function of tumor-infiltrating lymphocytes.
Shown are intracellular cytokine staining for IFN-γ (A-B), TNF-α (CD), IL-2 (E-F), granzyme B (G-H), and IL-4 (I-J) in CD8+ TIL following peptide stimulation. Shown are means (±s.e.m) of the triplicate samples pooled from 3 mice (A, C, E, G, and I) and representative flow cytometry dot plots (B, D, F, H, and J). Data is from three experiments with similar results. (K) Shown are the mean (±s.e.m, n=12) relative mean fluorescent intensity values (rMFIs) obtained from anti-tumor antibody binding experiments. ‘Normal’ serum was obtained from similarly aged but non-tumor bearing mice. (L) Shown are pie charts representing proportions of Tc1 (IFN-γ+) and Tc2 (IL-4+) T cells amongst total CD8+ TIL from mice that received vaccine alone, anti-PD-1 alone, nothing, or the combination. Each slice (shown are means ±s.e.m) is calculated from 6 replicates using flow cytometry.
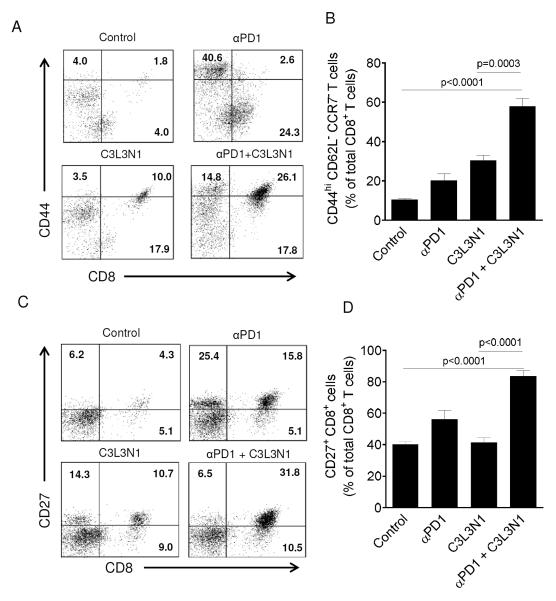
Combination therapy increases the infiltration of CD8 T cells with memory precursor effector cell (MPEC) phenotype into regressing tumors
Given that the role of PD-1/B7-H1 axis in regulating the expression of memory markers on CD8 T cells had been demonstrated in recent studies (32-34), we hypothesized that PD-1 blockade during vaccination increases the infiltration of T cells with memory phenotype into regressing tumors. To test this hypothesis, we analyzed the expression of memory markers on CD8 TILs. As shown in Figs. 5A-B, we observed a significant increase in infiltration of CD8 T cells with activated effector memory phenotype, CD44hiCD62L−CCR7−, into regressing tumors of mice treated with combination therapy. As it is widely reported that CD27 is critical for the generation and long-term maintenance of effector T cell pool (35, 36) and as our results show that combination therapy prolonged the survival of tumor bearing mice by 3 fold, in the next step we analyzed the expression of CD27 on the CD8 TILs in different groups. As shown in Figs. 5C-D, we observed a significant increase in the number CD8 TILs that are CD27+ in regressing tumors in combination therapy groups. From these results, it is evident that PD-1 blockade during the vaccination results in increase in infiltration of tumors with CD8 T cells that have memory phenotype and enhanced persistence.
Figure 5. Combination therapy enhanced the infiltration of CD8 T cells with memory phenotype into regressing tumors.
TILs were analyzed for the expression of CD44, CD62L, CCR7 and CD27 on CD8 T cells. (A) Shown are the cytometry dotplots representative of CD8+CD44+ lymphocytes in tumors of different treatment groups. All cells were gated on CD62L− and CCR7−. Quadrants were established with isotype controls and inset values are the percent of CD62L−CCR7− lymphocytes that fall in that quadrant. Results shown are representative of one of the three independent experiments with similar results. (B) Shown are the mean numbers of tumor-infiltrating CD8+CD44hiCD62L−CCR7− T cells in different treatment groups. Data shown is the mean of the (±s.e.m) six samples pooled from three independent experiments. (C) Shown are the cytometry dot plots representative of CD27+CD8+ TILs from the different treatment groups. Inset values are the percent of R1 gated lymphocytes that fall in that quadrant. (D) Shown are the mean numbers of CD27+CD8+ T cells in different treatment groups. Data is shown as mean of the (±s.e.m) six samples pooled from three independent experiments.
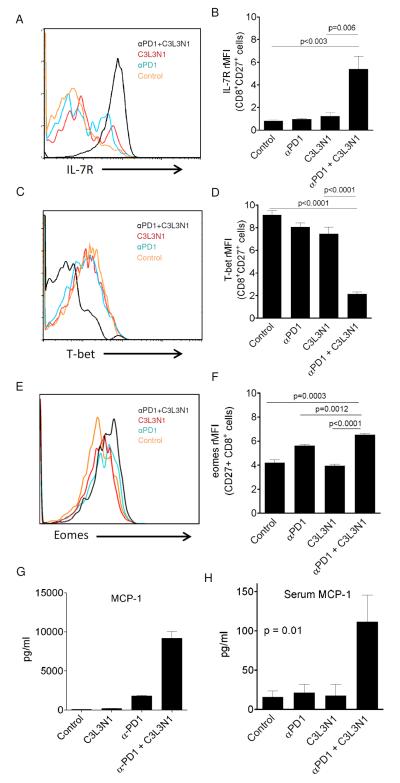
CD8 T cells differentiate into two different cell types upon interaction with target antigen (1) short-lived effector cells (SLECs) and (2) memory precursor effector cells (MPECs). SLECs are terminally differentiated effector T cells with little capacity to survive whereas MPECs are the activated effector T cell pool that have a greater capacity for survival (37). Thus, in the next step, we asked whether CD8 TILs infiltrating into regressing tumors in combination therapy group have either SLEC or MPEC phenotype. As shown in Figs. 6A-F, combination therapy resulted in increased infiltration of regressing tumors with CD27+CD8+ T cells which express high levels of IL-7R, low levels of T-bet, and high levels of Eomes, suggesting that infiltrating CD8 TILs have MPEC phenotype. We also observed that antigen-specific T cells in the TME of mice, treated with combination therapy, demonstrated highly elevated levels of MCP-1 production (Fig. 6E), and this correlated with a high level of serum MCP-1 in mice that received combination therapy and were actively rejecting tumors, suggesting its potential use as a serum biomarker for anti-tumor response (Fig. 6H).
Figure 6. Combination therapy enhanced IL-7R expression, eomes expression and decreased T-bet expression by CD8 TILs.
Histograms show expression of IL-7R (A), T-bet (C), and Eomes (E) on/in CD8+CD27+ TILs from different treatment groups. Shown are the mean relative MFI (rMFI) values representing the expression of IL-7R (B), T-bet (D), and Eomes (F) by CD8+CD27+ TILs from different treatment groups. Data is the mean (±s.e.m) from three independent experiments. P-values in B, D, and F were calculated using a Student’s T test. (G) Shown are the mean (±s.e.m) supernatant levels of MCP-1 released by purified CD8 TILs. Data is representative of duplicate samples pooled from 3 mice and from one of three experiments with similar results. (H) Shown are the mean (±s.e.m) serum levels of MCP-1 in treated or untreated mice. Data is representative of duplicate samples pooled from 3 mice and from one of three experiments with similar results. P-values in G and H were calculated using a one-way ANOVA.
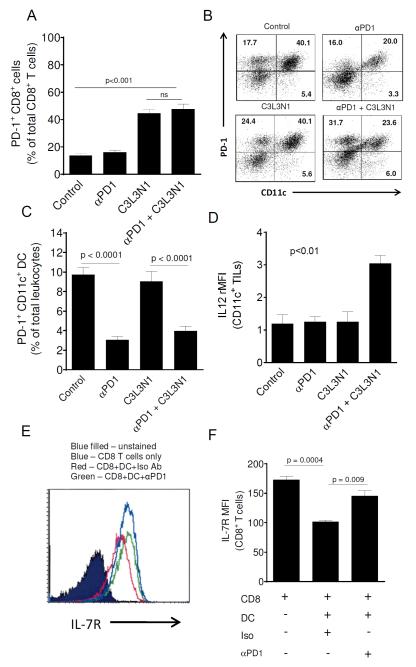
Combination therapy affects PD-1+ DC population in the TME and tumor-associated PD-1+ DCs regulate IL-7R expression on CD8 T cells
Based on prior results which suggest that PD-1, PD-L1 and CTLA-4 blockade during vaccination against melanoma increases PD-1 expression by CD4 and CD8 TILs (28), next, we wanted to determine whether PD-1 blockade during vaccination alters numbers of tumor-infiltrating PD-1+ CD8 T cells. We did not observe any effect on the levels of PD-1+ CD8 T cells (Fig. 7A) but we observed that breast tumors are infiltrated with PD-1+ DCs similar to our prior observations in ovarian tumor model (15). Also, as shown in Fig. 7B-C, we observed that both combination therapy as well as anti-PD-1 antibody treatment alone resulted in significant decrease in the number of PD-1+ DCs in the tumors. This suggests that PD-1 blockade during vaccination also modifies the myeloid compartment in the TME and consistent with this, we found that DCs in the TME of mice treated with combination therapy had elevated levels of IL-12 (Fig. 7D). Additionally, we also found that the combination treatment resulted in a significant downregulation of MDSCs (Fig. S5). Interestingly, treatment with anti-PD-1, when used alone, led to nearly a doubling of the numbers of the MDSCs (as a % of total leukocytes).
Figure 7. Combination therapy decreased the number of tumor-infiltrating PD-1+ CD11c+ cells and PD-1 blockade on tumor-infiltrating DCs increases IL-7R expression on antigen-primed CD8 T cells.
(A) (B) PD-1+ CD8+ and PD-1+ CD11c+ cells in tumors of different treatment groups was analyzed. (A) Shown are the mean numbers of tumor-infiltrating PD-1+ CD8+ T cells in different treatment groups. (B) Shown are the cytometry dot plots (gated on R1) representative of tumor-infiltrating PD-1+, CD11c+ cells in the different treatment groups. Quadrants were established with isotype controls and the inset values are the percent of R1 gated lymphocytes that fall in that quadrant. Dot plots shown are the representative of one of three separate experiments with similar results. (C) Shown are the mean numbers of tumor-infiltrating PD-1+ CD11c+ cells of total leukocytes in different treatment groups. Data is shown as mean (± s.e.m) and is from three independent experiments. (D) Shown are the mean (±s.e.m., n=3) rMFI values obtained from staining tumor-infiltrating DCs for IL-12. DCs were obtained from tumors between days 35-38. (E) Shown is the histogram representative of IL-7R expression on splenic CD8 T cells co-cultured with tumor derived PD-1+ DCs pretreated with anti-PD-1 antibody or isotype antibody. (F) Shown are the bar graphs representative of IL-7R expression on splenic CD8 T cells. Data is shown as mean (±s.e.m) and is from three independent experiments.
As our results suggest that PD-1 blockade during vaccination alters the expression of IL-7R and T-bet by tumor-infiltrating CD8 T cells, we wanted to determine whether the blockade of PD-1 on tumor-infiltrating DCs had any direct effect on CD8 T cells in terms of acquiring the MPEC phenotype. For this, tumor PD-1+ DCs were co-cultured with splenic CD8 T cells and following co-culture CD8 T cells were analyzed for the expression of IL-7R and T-bet as described in the Materials and Methods. Our data shows that in this co-culture system, PD-1 is expressed only on tumor-derived DCs but not on splenic CD8 T cells (supplementary Fig. S6). As shown in Figs. 7E-7F, it was observed that blockade of PD-1 on tumor-associated DCs rescued IL-7R expression on CD8 T cells. We did not observe any effect of PD-1 blockade on DCs on T-bet expression by T cells. These results suggest that PD-1 expressed on DCs controls the expression of IL-7R which is a marker of MPECs (38).
DISCUSSION
In summary, in this study we show that the combination of anti-PD-1 with peptide vaccine greatly enhanced vaccine-induced immune responses and resulted in delayed tumor regrowth following vaccine-induced regression. Key findings from our study are that PD-1 blockade during cancer vaccination (1) generates CD8 Tc1 and Tc2 TILs that have memory precursor effector cell (MPEC) phenotype, (2) decreases the PD-1+ DC population (but not the T cells) in tumors, and (3) regresses B7-H1-negative tumors and greatly enhances progression free survival.
In addition to confirming previous studies which show that PD-1 blockade during vaccination results in increased infiltration of effector T cells into tumors and increases their functionality (28, 29), in this study we identified that PD-1 blockade during vaccination induces the tumor-infiltrating CD8 T cells to develop into MPECs i.e. IL7RhiT-betlo CD8 TILs. MPECs are the effector CD8 T cells that serve as precursors to a stable pool of memory T cells (17, 37, 38) and T-bet gradient during inflammation directs the differentiation of effector CD8 T cells into MPECs or SLECs (37). Our observation that combination therapy results in decrease in T-bet expression in CD8 TILs suggests that blockade of PD-1/B7-H1 inhibitory axis not only enhances tumor-infiltrating effector cell functions as reported extensively but also alters (reduces) the T-bet gradient in CD8 TILs. In addition, low levels of T-bet in CD8 TILs suggest that these effector cells are poised to preserve their proliferative capacity and are not directed towards terminal differentiation (39). We also observed that MPECs are CD27+, supporting the fact that MPECs induced by our combination strategy have enhanced persistence (35). Furthermore, given the role of MCP-1, a chemokine previously identified to enhance the recruitment of CD8 memory T cells to sites of infection in a paracrine fashion (40, 41), increase in production of MCP-1 by TILs from combination group describes the reason behind increase in the number of MPECs in the regressing tumors. Lastly, despite the observation that combination therapy strategy also enhanced Tc2 cytokine production by TILs, depletion studies underscores the fact that MPECs induced sustained protection is independent of CD4 T cell help. Overall, the ability of combination therapy to enhance the survival of tumor-bearing mice observed in this study can be attributed to the induction of MPECs.
Despite the fact that DCs are the major cell type that generate anti-tumor immunity in the TME, emerging data shows that tumor associated DCs are immunosuppressive and express PD-1 (15, 42). Similar to our ovarian cancer model (15), tumor associated PD-1+ DCs were also observed in this study. Role of PD-1 blockade in altering the number of tumor-infiltrating regulatory cells, such as Tregs and myeloid derived suppressor cells (MDSCs), was shown in prior studies (28, 29). Recently, Duraiswamy and colleagues showed that these changes (decrease) in the numbers of tumor-infiltrating Tregs are not due to increase in the apoptosis of Tregs (29). Thus, it is plausible to speculate that the decrease in numbers of tumor-infiltrating regulatory cells upon blockade of immune checkpoints could be due to the alteration in their migratory properties. In fact, this speculation can be supported by the recently published results from Peng and colleagues which show that PD-1 blockade during adoptive T cell transfer increases the infiltration of transferred T cells into the tumor site (43). Thus, in this study, the decrease in numbers of PD-1+ DCs observed in tumors of mice treated with anti-PD-1 antibody with or without vaccine could be due to increase in migration of DCs from tumors probably into the regional lymph nodes. Also, our results from in vitro experiments show that PD-1 on tumor-associated DCs regulates the expression of IL-7R, a marker for MPECs, on CD8 T cells. These results provide valuable insight that PD-1 blockade during vaccination against cancer targets the potential antigen-presenting cells (APCs) of the TME. In addition to prior studies which show that anti-PD1 therapy modulates T cells and MDSCs (28, 29), here we show that tumor DCs are also modulated. Studies aimed at understanding the complete mechanics of anti-PD-1 antibody modulation of tumor-associated DC population which in turn affects the infiltrating CD8 T cell population seem like a logical next step.
We observed that treatment of tumor bearing mice with anti-PD-1 antibody alone was not effective and this can be attributed to the lack of PD-L1 expression on the TUBO tumors. Our data is consistent with the recent results from phase I clinical trial which shows that anti-PD1 antibody as monotherapy is ineffective in patients with PD-L1 negative tumors (13) and our study shows that combination therapy is an alternative strategy that would be effective against PD-L1 negative tumors. Despite the fact that our combination therapy strategy induced sustained protection against breast cancer, we observed the recurrence of the disease. This may be attributable to the widely accepted cancer immune editing phenomenon (44). It could be possible that in our model, PD-1 blockade during vaccination would have prolonged the equilibrium phase which is evident from the observed sustained protection (25, 45). However, an alternative explanation is that tolerance mechanisms may have resulted in more rapid decline and elimination of the legumain and β-catenin T cells since these are self-antigens with a more limited T cell repertoire which is in contrast to rat neu which is foreign. Regardless, while developing strategies to vaccinate against cancer with simultaneous blockade of tumor-associated immunosuppression, continued concern should be taken to avoid the recurrence of disease which might occur due to cancer immunoediting processes.
Our study does not reveal the antigen-specificity of the majority of the infiltrating CD8 T cells. While one possibility is that all of the CD8 T cells were responsive to one or more of the antigens included in the vaccine, our flow cytometry studies indicate that only between 5-6% of the infiltrating CD8 T cells are specific for the vaccine. Another possibility is that anti-PD-1 treatment may lead to expansion of memory T cells that kill by innate mechanism as recently described (46). In that study, Tietze and colleagues found that cytokine and non-specific immunotherapy with anti-CD40 led to bystander expansion of memory CD8 T cells that appeared to kill tumor by innate mechanisms including NKG2D ligation. Lastly, it is possible that in our model the combination therapy with vaccine and anti-PD-1 would have induced CD8 T cells reactive to tumor antigens other than the ones that are targeted using our vaccine attributable to PD-1 blockade-induced epitope spreading as suggested by Dhodapkar and colleagues (47).
Anti-PD-1 and anti-PD-L1 antibodies are moving towards advance phase clinical trials after being successfully tested in phase I trial (13, 14). We expect that data from this study which suggests that combination therapy strategy can provide protection against PD-L1 negative tumors and induce MPECs, can serve to leverage the designing of clinical trials that would use peptide or other vaccines in combination with PD-1 antagonism for the treatment of cancer.
Supplementary Material
Acknowledgments
Grant support: A gift from Martha and Bruce Atwater (KLK), a Susan G Komen Grant (KG101333, KLK and LK), NIH/NCI Grants R01 CA152045 and P50-CA136393 (KLK) and support from the Mayo Graduate School (PL)
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flemming A. Cancer: PD1 makes waves in anticancer immunotherapy. Nat Rev Drug Discov. 2012;11:601. doi: 10.1038/nrd3806. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–36. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–52. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–15s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 9.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 11.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology. 2012;1:1223–5. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotti G. Blocking PD-1 in cancer immunotherapy. Blood. 2009;114:1457–8. doi: 10.1182/blood-2009-05-223412. [DOI] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol. 2011;186:6905–13. doi: 10.4049/jimmunol.1100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–8. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellavance EC, Kohlhapp FJ, Zloza A, O’Sullivan JA, McCracken J, Jagoda MC, et al. Development of tumor-infiltrating CD8+ T cell memory precursor effector cells and antimelanoma memory responses are the result of vaccination and TGF-beta blockade during the perioperative period of tumor resection. J Immunol. 2011;186:3309–16. doi: 10.4049/jimmunol.1002549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saifo MS, Rempinski DR, Jr., Rustum YM, Azrak RG. Targeting the oncogenic protein beta-catenin to enhance chemotherapy outcome against solid human cancers. Mol Cancer. 2010;9:310. doi: 10.1186/1476-4598-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korkaya H, Wicha MS. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013;73:3489–93. doi: 10.1158/0008-5472.CAN-13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 21.Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55:861–7. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 22.Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589–96. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12:33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–34. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–51. doi: 10.1158/0008-5472.can-03-0173. [DOI] [PubMed] [Google Scholar]
- 26.Lewen S, Zhou H, Hu HD, Cheng T, Markowitz D, Reisfeld RA, et al. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–15. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulko V, Harris KJ, Liu X, Gibbons RM, Harrington SM, Krco CJ, et al. B7-h1 expressed by activated CD8 T cells is essential for their survival. J Immunol. 2011;187:5606–14. doi: 10.4049/jimmunol.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Perez L, Kottke T, Diaz RM, Ahmed A, Thompson J, Chong H, et al. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res. 2005;65:2009–17. doi: 10.1158/0008-5472.CAN-04-3216. [DOI] [PubMed] [Google Scholar]
- 31.Shiao SL, Ganesan AP, Rugo HS, Coussens LM. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev. 2011;25:2559–72. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–77. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–15. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charlton JJ, Chatzidakis I, Tsoukatou D, Boumpas DT, Garinis GA, Mamalaki C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190:6104–14. doi: 10.4049/jimmunol.1201617. [DOI] [PubMed] [Google Scholar]
- 35.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–80. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong H, Franklin NA, Roberts DJ, Yagita H, Glennie MJ, Bullock TN. CD27 stimulation promotes the frequency of IL-7 receptor-expressing memory precursors and prevents IL-12-mediated loss of CD8(+) T cell memory in the absence of CD4(+) T cell help. J Immunol. 2012;188:3829–38. doi: 10.4049/jimmunol.1103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 39.Joshi NS, Cui W, Dominguez CX, Chen JH, Hand TW, Kaech SM. Increased numbers of preexisting memory CD8 T cells and decreased T-bet expression can restrain terminal differentiation of secondary effector and memory CD8 T cells. J Immunol. 2011;187:4068–76. doi: 10.4049/jimmunol.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JJ, Nottingham LK, Sin JI, Tsai A, Morrison L, Oh J, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–24. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–95. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, et al. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–83. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV. SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology. 2013;2:e25205. doi: 10.4161/onci.25205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.