Abstract
Background
Radiosurgery is increasingly used to treat vestibular schwannomas (VSs). Increasing the sensitivity of VS cells to irradiation (IR) could allow for lower and/or more effective doses of IR, improving safety and efficacy. Persistent JNK activity in VS cells reduces cell death by suppressing accumulation of reactive oxygen species (ROS) raising the possibility that JNK activity protects against IR-induced VS cell death, which is mediated by ROS.
Objective
Determine the extent to which JNK signaling contributes to VS cell radiosensitivity.
Methods
Primary human VS cultures, derived from acutely resected tumors, received single doses (5–40 Gy) of γ-irradiation. Histone 2AX phosphorylation, a marker of IR-induced DNA damage, was assayed by western blot and immunostaining. ROS levels were quantified by measuring 2',7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) fluorescence. Cell apoptosis was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling.
Results
The JNK inhibitors, SP6000125 and I-JIP, reduced H2AX phosphorylation following IR. They also increased H2DCFDA fluorescence in non-irradiated cultures and significantly increased IR-induced (5–10 Gy) H2DCFDA fluorescence 72 hours, but not 2 hours, after IR. Finally, I-JIP (50 µM) significantly increased VS cell apoptosis in cultures treated with 20–40 Gy. I-JIP (20 µM), SP600125 (20 µM), and JNK1/2 siRNA knock-down each increased VS cell apoptosis in cultures treated with 30–40 Gy, but not lower doses, of IR.
Conclusions
Inhibition of JNK signaling decreases H2AX phosphorylation and increases ROS and apoptosis in VS cells following γ-irradiation. These results raise the possibility of using JNK inhibitors to increase the effectiveness of radiosurgery for treatment of VSs.
Keywords: acoustic neuroma, cell death, histone H2AX, reactive oxygen species, radiosurgery, radiotherapy
INTRODUCTION
Vestibular schwannomas (VSs) comprise 8–10% of intracranial neoplasms. Contemporary options for tumor management depend on a variety of factors including tumor size, patient age and surgical candidacy, and hearing status. These options include observation with serial imaging, microsurgery, or irradiation (IR) in the form of stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (FSR).1–7 Recently there has been a dramatic increase in the number of VSs treated with SRS/FSR, yet the effects of IR on the schwannoma cells themselves remain largely unexplored. With the increase in the number of VSs treated with SRS/FSR and with longer follow-up periods, more treatment failures are being reported and tumors from patients with neurofibromatosis type 2 (NF2) appear particularly radioresistant.8–12 Further, there are few known reagents that enhance the response of human VS cells to IR.
Effects of irradiation (IR) on VS cells
SRS/FSR limit further tumor growth in the majority of VSs and can result in a reduction in tumor volume.11–14 It is not clear whether the response of VSs to SRS/FSR results from direct cytotoxic effects to the VS cells or whether it reflects indirect effects, for example, by decreasing tumor vascularity. Histopathological examination of 4 VSs resected for growth following SRS demonstrated viable, typical schwannoma cells with increased fibrosis of the tumor bed and surrounding tissues suggesting indirect effects.15 A separate study found reduced proliferation rate of 6 VSs resected for continued growth following SRS compared with the proliferation rate in 15 VSs resected for growth following incomplete microsurgical resection, although two irradiated VSs showed markedly increased proliferation.16 The effects of IR on the VS cells themselves, whether direct or indirect, are not well understood.
We recently evaluated the response of cultured human primary VS cells to increasing doses of IR and found that, compared to most neoplastic conditions, VS cells are relatively radioresistant in vitro, requiring over 20 Gy IR (e.g. 30–40 Gy) to induce apoptosis and cell cycle arrest.17 Anniko also noted that cultured VS cells required high doses of IR to induce cell death.18 The relative radioresistance of VS cells correlates with their low proliferative capacity. Increasing cell proliferation with exogenous mitogens enhances cell killing by IR while treatment with ErbB2 inhibitors, which reduce proliferation, further limits VS cell apoptosis following IR.17
JNK signaling contributes to VS cell redox status, proliferation, and cell survival
c-Jun N-terminal kinase (JNK) is a member of the mitogen-activated protein kinase (MAPK) family of protein kinases that regulates a variety of cellular responses including cell death.19, 20 Merlin, the protein product of the NF2 gene that is defective in NF2-associated and sporadic VSs, regulates JNK activity and loss of merlin function leads to persistent JNK activity in VS cells.21–24 This elevated JNK activity contributes to VS cell proliferation and to cell survival by limiting the accumulation of reactive oxygen species (ROS), particularly mitochondrial superoxides.24 The fact that IR induces cell death, at least in part, by increasing oxidative stress raises the possibility that elevated JNK activity confers a radioprotective effect to VS cells.25, 26 Alternatively, ultraviolet (UV) and ionizing IR activate JNK to promote cell death, and JNK activity may contribute to IR-induced VS cell death.25, 27, 28 This study sought to determine the extent to which JNK signaling modulates the response of cultured VS cells to IR.
METHODS
Vestibular schwannoma (VS) cultures
Primary human VS cultures were prepared from acutely resected tumors as previously described.17, 29 Briefly, acutely resected tumors were minced into ~1 mm3 fragments, treated with 0.25% trypsin and 0.1% collagenase for 30–40 min. at 37° C, and dissociated by tituration through narrow bore glass pipets. Cell suspensions were plated on 4-well plastic cultures slides (Nalge Nunc International, Rochester, NY) coated with poly-ornithine followed by laminin (20 µg/ml) in Dulbecco’s modified Eagle’s medium (DMEM) with N2 supplements (Sigma, St. Louis, MO), bovine insulin (Sigma, St. Louis, MO 10 µg/ml) and 10% fetal calf serum (FCS). The medium was exchanged 1–2 days later and the cells were subsequently maintained in serum free conditions until used for experiments, typically after 7–10 days. Cultures were maintained in a humidified incubator with 6.0% CO2 at 37° C. SP600125 (Calbiochem, San Diego, CA, 20 µM) or I-JIP (Calbiochem, 20 or 50 µM) was added to the indicated cultures 1–2 hr prior to irradiation and maintained throughout the duration of the experiment. siRNA-mediated knockdown of JNK1/2 was performed as previously described.24 A total of 12 VS cultures, each derived from separate patients, were used in these studies. All tumors represented sporadic, isolated VSs that had not received prior micro- or radiosurgery and were not derived from patients with NF2. None of the tumors were observed radiographically for a period of time prior to removal. All tumors were confirmed to be schwannomas by standard histopathology.
Irradiation of vestibular schwannoma cultures
VS cultures were irradiated as previously described using a cesium-137 γ-irradiation source set at dose rate of 0.84 Gy/min.17 Control cultures, receiving sham IR, were treated in an identical manner but were not exposed to irradiation.
Immunocytochemistry
Following fixation with 4% paraformaldehyde, the cultures were washed in phosphate buffered saline (PBS) and permeabilized with 0.8% Triton-X100 in PBS for 15 min. Nonspecific antibody binding was blocked with 5% goat serum, 2% bovine serum albumin, in PBS with 0.8% Triton-X100. The cultures were then treated with primary antibodies overnight at 4C and then rinsed 3 times in PBS with 0.8% Triton-X100. The following primary antibodies were used: Rabbit polyclonal anti-S100 antibody (Sigma, St. Louis, MO 1:800) and monoclonal anti-phosphorylated Ser139 histone H2AX (Upstate Cell Signaling Solutions, Charlottesville, VA, 1:500). Secondary detection of primary antibody labeling was accomplished using goat anti-rabbit and anti-mouse secondary antibodies conjugated to Alexa 488 or Alexa 568 (Invitrogen, Carlsbad, CA, 1:1000). Following immunostaining, nuclei were stained with DAPI.
Immunostaining was detected using an inverted Leica DMRIII microscope equipped with epifluorescence filters and digital images were captured with a CCD camera using MetaMorph software (Molecular Devices, Sunnyvale, CA). Images were analyzed in Image J (NIH, Bethesda. MD) and prepared for publication using Adobe Photoshop and Illustrator (Adobe, San Jose, CA). For quantification of γ-H2AX immunolabeling, the mean pixel intensity of a 5–7 µm diameter circular outline drawn on each nucleus was determined using the measurement tool in ImageJ. Background fluorescence/nonspecific labeling was subtracted from an identical circular selection positioned over the cytoplasm, taking advantage of the fact that H2AX is a nuclear protein so cytoplasmic fluorescence must be background. This background value was subtracted from the nuclear measurement to obtain a corrected value for γ-H2AX immunofluorescence.
Western blotting
Western blots of protein extracts prepared from VS culture lysates were preformed as previously described.24, 29, 30 Lysates were prepared two hours after radiation to assess H2AX phosphorylation. Blots were probed with anti-phosphorylated H2AX (γ-H2AX) or anti-JNK antibodies and then stripped and reprobed with non phospho-specific anti-H2AX or anti-β-actin antibodies. Goat anti-rabbit secondary antibodies (1:20,000–50,000; Santa Cruz, Santa Cruz, CA) were conjugated with horseradish-peroxidase. Blots were developed using Super Signal West Femto kit (Thermo Scientific, Rockford, IL) and exposed to film (Amersham Hyperfilm TM ECL, GE Healthcare Limited, Buckinghamshire, UK). Digital images of gels were captured on an Alpha Innotech gel imaging system (San Leandro, CA)
Determination of reactive oxygen species (ROS) status
VS cultures were labeled with 2',7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA, 25 µM, Invitrogen) as previously described.24 Digital epifluorescent images were captured from live cells using a Leica DMRIII inverted scope with exposure times set to a linear range based on cultures in control conditions and cells from cultures treated with I-JIP (100 µM). Fluorescent intensity was quantified from a circular selection within the cytoplasm of at least 30–50 cells using Image J software for each condition and the experiment was repeated on cultures from 3 separate tumors.24 Background fluorescence, determined from a similar sized circle placed outside cell boundaries, was subtracted from each image.
Determination of VS cell apoptosis
Seventy-two hours following IR, cultures were fixed and immunostained with anti-S100. Apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) with Alexa 568-labeled dUTP according to the manufacturer’s instructions.24, 31 Nuclei were labeled with DAPI. The percent of apoptotic VS cell (S100-positive) nuclei was determined by counting 10 randomly selected fields for each condition. Criteria for scoring were a TUNEL-positive nucleus with typical condensed morphology in an S100-positive cell. The percent of apoptotic VS cells was expressed as a percent of the control condition defined as 100%. Each condition was repeated on at least 3 VS cultures derived from separate patients.
Statistical analyses
Evaluation for statistical differences in mean percent apoptotic VS cells and mean fluorescence intensities among the various conditions was performed by ANOVA with post-hoc Hidak-Solm analysis using SigmaStat software (Systat Software, Richmond, CA).
RESULTS
Inhibition of JNK reduces H2AX phosphorylation in VS cells following IR
H2AX becomes phosphorylated following double stranded breaks in DNA and is a sensitive indicator of IR-induced DNA damage.32 Sublethal doses of IR (5–10 Gy) strongly induce H2AX phosphorylation in cultured VS cells.17 To explore the possibility that JNK signaling modulates the effect of IR in VS cells we compared H2AX phosphorylation status in primary VS cultures irradiated in the presence or absence of JNK inhibitors. Two different inhibitors were used: I-JIP is a cell permeable peptide that competitively disrupts JNK binding to scaffolding proteins necessary for activation and SP600125 is a small molecule inhibitor of JNK activity.33–35 We have previously shown that I-JIP and SP600125 specifically and effectively block JNK signaling in primary VS cells.24 These cultures contain over 95% schwannoma cells based on S100 immunoreactivity. VS cultures were immunostained with an antibody that specifically detects phosphorylated H2AX (γ-H2AX) and the extent of γ-H2AX was quantified by measuring fluorescence intensity in the nucleus. I-JIP and SP600125 each significantly reduced γ-H2AX immunofluorescence intensity 2 h following IR (Fig. 1A–D). To confirm that inhibition of JNK reduces H2AX phosphorylation following IR we immunoblotted protein lysates from VS cultures 2 h after IR with the anti-γ-H2AX antibody. I-JIP and SP600125 each reduced IR-induced H2AX phosphorylation (Fig. 1E). These results suggest that JNK activity is necessary for IR-induced H2AX phosphorylation.
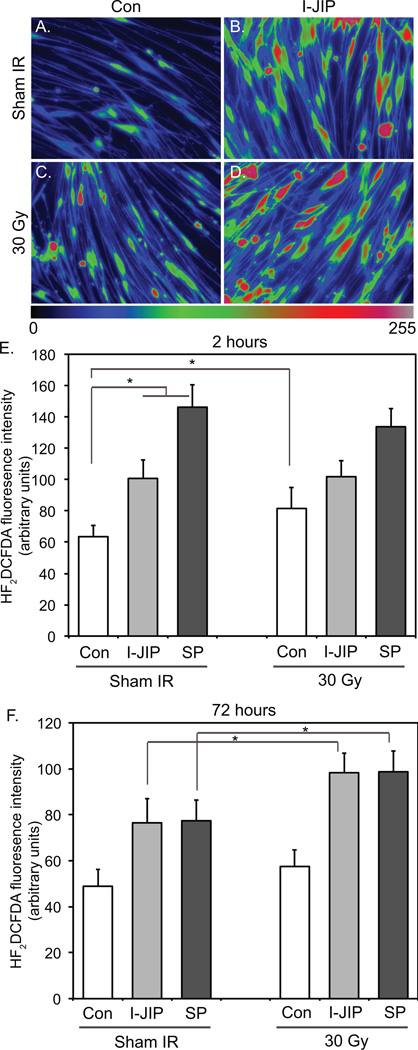
Figure 1.
Inhibition of JNK reduces histone 2A phosphorylation (γ-H2AX) following γ-irradiation (IR) in vestibular schwannoma (VS) cells. A–C. Representative images of primary VS cultures fixed 2 hr after IR and immunostained with anti-γ-H2AX and anti-S100 antibodies followed by Alexa 488 secondary antibody (green) and Alexa 568 secondary antibody (red), respectively. Nuclei were identified with DAPI (blue). A. Control cultures received sham IR. B. Cultures irradiated with 10 Gy. C. Cultures irradiated with 10 Gy in the presence of I-JIP (20 µM). Scale bar=50 µm. D. Quantification of anti-γ-H2AX immunofluorescence in cultures receiving sham or 10 Gy IR and maintained in the absence (Control, Con) or presence of I-JIP (20 µM) or SP600125 (SP 20 µM). *p<0.05 by one way ANOVA with post hoc Holm-Sidak method. E. Western blots of VS cell culture lysates receiving sham or 5 Gy IR in the presence or absence of SP600125 (SP, 20 µM) or I-JIP (20 µM) probed with anti-γ-H2AX antibodies and then stripped and reprobed with non phospho-specific anti-H2AX antibodies.
JNK activity limits ROS accumulation in VS cells following irradiation
To further explore the effects of JNK activity on VS cell responses to IR, we analyzed the oxidative state of VS cells 2 h and 72 h following IR in the presence and absence of JNK inhibitors since IR has been shown to cause both an early and a late increase in ROS.36 Overall ROS levels were determined by quantifying H2DCFDA fluorescence intensity. As previously shown, I-JIP and SP600125 each increased H2DCFDA fluorescence intensity in control cultures (Fig. 2A–F).24 IR (30 Gy) induced an increase in H2DCFDA fluorescence that was significantly higher than cultures receiving sham IR 2 h (p=0.021) following IR but this increase did not reach statistical significance 72 h following IR (p=0.13) (Fig. 2E,F). The increase in H2DCFDA fluorescence following 30 Gy IR was significantly less than the increase due to JNK inhibitors (p<0.05). Irradiated cultures treated with I-JIP or SP600125 displayed significantly increased H2DCFDA fluorescence 72 h, but not 2 h, following IR compared to non-irradiated cultures maintained in the presence of the JNK inhibitors (p<0.05) (Fig. 2E,F). These results suggest that JNK activity reduces oxidative stress in VS cells and that inhibition of JNK sensitizes VS cells to IR-induced late increases in ROS.
Figure 2.
Inhibition of JNK increases reactive oxygen species (ROS) following γ-irradiation (IR). A–D. Representative scaled intensity images of H2DCFDA fluorescence in cultures treated with sham or 30 Gy IR and maintained in the absence (Con) or presence of I-JIP (20 µM). E and F. Mean H2DCFDA fluorescence in cultures treated with sham or 30 Gy IR and maintained in the absence (Con) or presence of I-JIP (20 µM) or SP600125 (SP, 20 µM) and imaged 2 hours (E) or 72 hours (F) following IR. Results are averaged from measurements from 3 cultures derived from separate tumors. Error bars present standard error of the mean (SEM).
Inhibition of JNK increases IR-induced VS cell apoptosis
We next assessed the ability of JNK signaling to protect cells from IR-induced cell death. The clonogenic assay represents the standard assay for IR-induced cell death.37 However, VS cells are not transformed and do not form clones making this assay impossible. Therefore, we used apoptosis as a marker for IR-induced cell death.17, 37 IR also induces non-apoptotic cell death, however, JNK signaling regulates apoptosis in VS cells,24 and we focused on defining the apoptotic response of VS cells to IR.
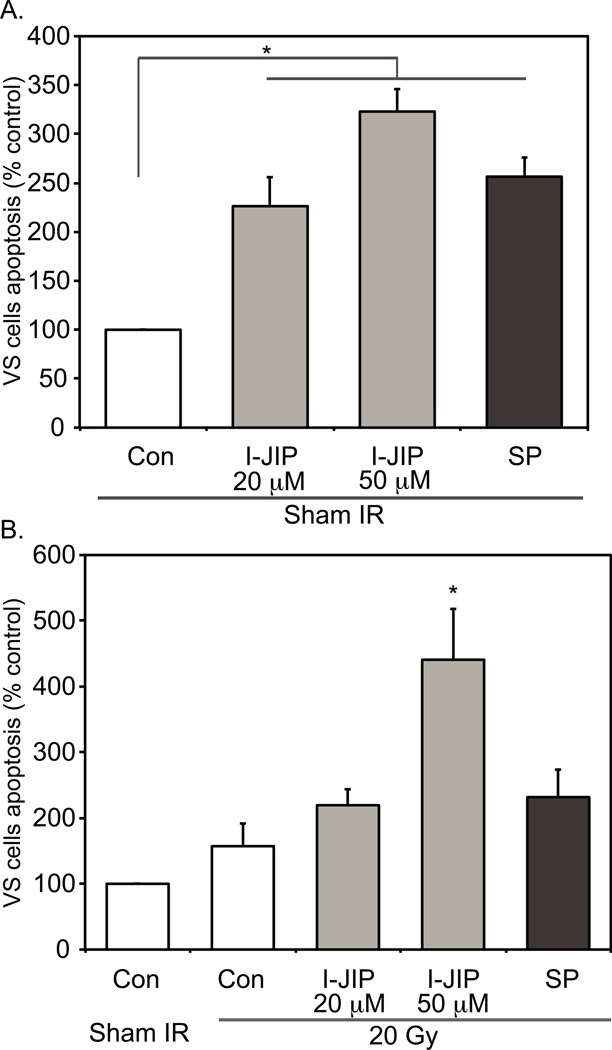
Seventy-two hours following IR, primary VS cultures were fixed and immunostained with anti-S100 antibodies followed by an Alexa 488 conjugated secondary antibody to specifically identify VS cells. The cultures were also labeled with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) which identifies apoptotic cells in situ by using terminal deoxynucleotidyl transferase (TdT) to transfer Alexa 568-labeled dUTP to strand breaks of cleaved DNA. Nuclei were identified with DAPI labeling. All TUNEL-positive nuclei were condensed typical of apoptotic cell death. We first asked whether inhibition of JNK would sensitize VS cells to sub-lethal doses of IR. We have previously shown that 20 Gy IR fails to significantly increase VS cell apoptosis compared with sham IR and therefore assessed VS cell apoptosis following 20 Gy IR in the absence or presence of JNK inhibitors.17 The average percent of TUNEL-positive VS cells in control conditions was 3.23±0.92 (mean±SEM) across the 9 cultures that were used in these studies. SP600125 and I-JIP significantly increased VS cell apoptosis in cultures receiving sham IR (p<0.05) consistent with prior results demonstrating that inhibition of JNK increases VS cell apoptosis (Fig. 3A). VS cultures irradiated with 20 Gy and treated with 50 µM I-JIP (p<0.05), but not 20 µM I-JIP or SP600125 (20 µM), displayed significantly increased VS cell apoptosis compared to cultures receiving sham IR and treated with I-JIP or SP600125 (Fig. 3B). Thus, at the higher concentration I-JIP sensitized VS cells to IR-induced cell death.
Figure 3.
Effect of JNK inhibitors on γ-irradiation-induced VS cell apoptosis at sublethal dose. A and B. Average relative percent of TUNEL-positive VS cell nuclei (apoptotic) with control (Con) cultures receiving sham IR defined as 100%. The average percent of TUNEL-positive VS cells in control conditions was 3.23±0.92 (mean±SEM). A. SP600125 (SP, 20 µM) and I-JIP (20 or 50 µM) each significantly increased VS cell apoptosis as previously shown in cultures receiving sham IR.24 *p<0.05 by one way ANOVA with post hoc Holm-Sidak method. B. I-JIP (50 µM) significantly (*p<0.05 by one way ANOVA with post hoc Holm-Sidak method) increased VS cell apoptosis in cultures treated with 20 Gy compared with cultures treated with 20 Gy and maintained in the absence of JNK inhibitors or cultures treated with I-JIP (50 µM) and receiving sham IR (see A). Each condition was performed on a minimum of 4 cultures from tumors derived from separate patients. Error bars present standard error of the mean (SEM).
We next examined the influence of JNK signaling on VS cell responses to cytolethal doses of IR. VS cultures were maintained in the presence or absence of I-JIP or SP600125 and irradiated with 30 Gy or 40 Gy. Apoptosis was determined by TUNEL (Fig. 4A–F). Each 30 Gy (p=0.034) and 40 Gy (p=0.027) IR significantly increased VS cell apoptosis compared with sham IR, as previously shown (Fig. 4G,H).17 In cultures irradiated with 30 Gy, 50 µM I-JIP significantly increased cell death compared to cultures receiving sham IR and maintained in 50 µM I-JIP or irradiated with 30 Gy in the absence of I-JIP (p<0.05), similar to the results seen with 20 Gy IR (Fig. 4G). In cultures treated with 40 Gy, I-JIP (20 µM and 50 µM) and SP600125 each significantly increased VS cell apoptosis compared with cultures maintained in the JNK inhibitors and receiving sham IR or with cultures treated with 40 Gy and maintained in the absence of JNK inhibitors (p<0.05) (Fig. 4H). Thus, inhibition of JNK signaling increased VS cell death in response to cytotoxic levels of ionizing radiation.
Figure 4.
Inhibition of JNK increases γ-irradiation-induced VS cell apoptosis. A–C. Representative images of primary VS cultures labeled with TUNEL (red) and immunostained with anti-S100 antibodies (green). Nuclei were identified with DAPI (blue). Arrows indicate TUNEL-positive nuclei. A. Control cultures received sham IR. B. Cultures irradiated with 30 Gy. C. Cultures irradiated with 30 Gy in the presence of I-JIP (20 µM). Scale bar=50 µm. D–E. Magnified images of DAPI (D), TUNEL (E) and DAPI and TUNEL combined (E) staining from the rectangular region outlined in (C). G and H. Average relative percent of TUNEL-positive VS cell nuclei (apoptotic) with control (Con) cultures receiving sham IR defined as 100%. The average percent of TUNEL-positive VS cells in control conditions was 3.23±0.92 (mean±SEM). G. I-JIP (50 µM) significantly (*p<0.05 by one way ANOVA with post hoc Holm-Sidak method) increased VS cell apoptosis in cultures treated with 30 Gy compared with cultures treated with 30 Gy and maintained in the absence of JNK inhibitors or cultures treated with I-JIP (50 µM) and receiving sham IR (see Fig 3A). H. SP600125 (SP, 20 µM) and I-JIP (20 or 50 µM) each significantly (*p<0.05 by one way ANOVA with post hoc Holm-Sidak method) increased VS cell apoptosis in cultures treated with 30 Gy compared with cultures treated with 30 Gy and maintained in the absence of JNK inhibitors or cultures treated with SP (20 µM) or I-JIP (20 µM or 50 µM) and receiving sham IR (see Fig 3A). Each condition was performed on a minimum of 4 cultures from tumors derived from separate patients. Error bars present standard error of the mean (SEM).
To confirm that JNK contributes to VS cell radiosensitivity we transfected VS cells with scrambled or JNK1/2 siRNA oligonucleotides. Western blots of protein lysates confirmed decreased JNK expression following siRNA transfection (Fig. 5A). Similar to the results with pharmacologic and peptide inhibitors of JNK, siRNA-mediated JNK knockdown resulted in an significant increase in VS cell apoptosis (Fig. 5B). Further, transfection with JNK-targeted oligonucleotides significantly increased radiation-induced (30–40 Gy) cell death compared to cultures transfected with scrambled oligonucleotides (Fig. 5B). These results further confirm that JNK signaling contributes to VS cell radiosensitivity.
Figure 5.
siRNA knockdown of JNK1/2 increases γ-irradiation-induced VS cell apoptosis. A. Western blot of lysates from primary VS cultures prepared 24 h after transfection with scrambled (Scr) or JNK1/2 targeted siRNA oligonucleotides and probed with anti-JNK antibody. The membrane was stripped and re-probed with anti-β-actin antibody to verify protein loading. B. Average relative percent of TUNEL-positive VS cell nuclei (apoptotic) with control cultures transfected with scrambled (Scr) oligonucleotides and receiving sham IR defined as 100%. Cultures were irradiated with 30 or 40 Gy 24 h after transfection with scrambled or JNK1/2 targeted siRNA oligonucleotides. *p<0.05 by one way ANOVA with post hoc Holm-Sidak method. Error bars present standard error of the mean (SEM).
DISCUSSION
Effects of irradiation on VS cells
The number of VSs treated with SRS/FSR has increased dramatically during the past two decades,5 however the effects of IR on the VS cells themselves are not well understood. VS cells in vitro are relatively radioresistant to single doses of IR, requiring over 20 Gy IR (e.g. 30–40 Gy) to induce apoptosis and cell cycle arrest.17, 18 By comparison, most current SRS protocols deliver 12–15 Gy at the 50–80% isodense line.38–40 The lack of VS cell death in response to <20 Gy IR in vitro raises the possibility that the ability of SRS to limit further growth of the majority of VSs results from indirect effects (e.g. decreased tumor vascularity) rather than direct cytotoxicity to the VS cells. Alternatively, VS cells in vivo may be more susceptible to IR due to the tumor microenvironment or other factors not recapitulated in cultures. This study used primary VS cultures to explore the apoptotic response of the VS cells themselves to IR and the molecular mechanisms accounting for these responses. It does not address other potential mechanisms (e.g. vascular compromise) that contribute to tumor responses to IR in vivo. Further, our study was limited to single doses of IR, similar to SRS. To date, the response of VS cells to multiple fractionated doses of IR, akin to FSR, remains unknown and may involve additional mechanisms not explored here.
The low proliferation rate of VS cells likely contributes to their limited radiosensitivity.17 Treatment of cultured VS cells with ErbB2 inhibitors, which reduces their proliferative capacity, decreases IR-induced cell death whereas treatment with mitogens increases cell death following IR.17 Sublethal doses of IR (5–10 Gy) rapidly induce DNA damage, evidenced by H2AX phosphorylation.17 Thus, VS cells suffer DNA damage with doses of IR much lower than those required to induce apoptotic cell death. Since cell death following IR typically requires re-entry into the cell cycle, the limited proliferative capacity of VS cells likely allows for DNA repair mechanisms to occur prior to cell cycle entry and subsequent death.
Even though the sensitivity of VS cells to IR depends on their proliferation rate, several reports indicate that VSs in patients with NF2 are more likely to grow following SRS/FSR than sporadic VSs.8–10, 41 Whether this reflects decreased radiosensitivity of VSs from NF2 patients compared with sporadic VSs or whether it simply reflects the greater growth potential of the remaining viable tumor cells in NF2-associated VSs requires further investigation.
JNK signaling in VS cells
JNK is activated by dual phosphorylation of threonine and tyrosine residues by two MAPK kinases, MKK4 and MKK7, typically in response to cellular stress.20 JNK activity influences diverse cellular processes including cell motility and axon growth, cell death, and cell proliferation.19, 20, 42–45 Several studies indicate that merlin, the product of the NF2 tumor suppressor gene defective in VSs, suppresses JNK activity.24, 46, 47 Correspondingly, JNK remains persistently phosphorylated (active) in VS cells, which lack merlin expression, and replacement of functional merlin in VS cells reduces JNK activity.24 A recent study demonstrated that JNK activity promotes VS cell proliferation and survival.24 By contrast, increased JNK activity in normal SCs and many other cells (e.g. neurons, fibroblasts) increases apoptosis.{Cheng, 2001 #10550;Parkinson, 2004 #9119;Hochedlinger, 2002 #9212;Borsello, 2007 #918348 Since inhibition of JNK increases VS cell death and reduces proliferation, but prevents apoptosis of normal glial cells and neurons, JNK inhibitors represent potential therapeutic targets that, theoretically, would be relatively specific to VS cells. Future in vivo studies using xenografts in mice or transgenic animals are needed to further establish the therapeutic potential of JNK inhibitors for VSs. One orally available JNK inhibitor, PGL5001, is currently being investigated in a Phase II trial for inflammatory endometriosis (clinicaltrials.gov ID: NCT01630252).
Inhibition of JNK activity increases VS cell radiosensitivity
Current SRS protocols effectively limit tumor progression in the majority of sporadic VSs. Nevertheless, large tumors are generally considered to be radioresistant. Likewise, NF2-associated tumors are less sensitive to SRS compared with sporadic VSs.8–10, 41 Identification of radiosensitizing agents may allow for expansion of SRS criteria to include large and/or NF-2 associated tumors that are not considered good candidates for current protocols. Radiosensitizing agents may also increase the efficacy of current SRS protocols so that the IR results in a tumoricidal response with reduced tumor volume rather than a tumorostatic response. Further, radiosensitizing agents may allow for lower radiation doses to be used to achieve similar control rates thus limiting the risks of side effects (e.g. hearing loss). Thus, while current SRS protocols are highly effective, use of radiosensitizing agents may improve SRS efficacy, particularly for tumors that are currently deemed radioresistant.
Most radiosensitizing agents such as cis-platin or 5-fluorouracil (5-FU) do not specifically target tumor cells and carry the potential of increasing IR-induced damage to normal, non-target tissues.49 An alternative strategy is to identify reagents that specifically target VS cells for use in combination with IR in an effort to enhance the response of the tumor, but not adjacent normal tissues (e.g. facial nerve), to IR. Here we evaluated JNK signaling as one potential target to enhance VS cell radiosensitivity. We employed three separate mechanisms to reduce JNK signaling: a small molecule kinase inhibitor (SP600125), a peptide inhibitor that disrupts JNK binding to scaffolding proteins (I-JIP), and siRNA-mediated knockdown of JNK expression. Each yielded qualitatively similar results indicating that JNK mediates VS cell radiosensitivity in vitro.
Typically activation of JNK results in increased ROS levels; however, in VS cells, JNK activity reduces oxidative stress by limiting the accumulation of mitochondrial superoxides.24 As increased oxidative stress represents a primary mechanism contributing to IR-induced cell death,25, 26, 36 we hypothesized that the ability of JNK to suppress ROS contributes to the relative radioresistance of VS cells. Alternatively, JNK activity, which promotes VS cell proliferation, may increase VS cell radiosensitivity, similar to the effects of mitogenic ErbB2 ligands. Here we found that two distinct JNK inhibitors, I-JIP and SP600125, increased H2DCFDA fluorescence 72 h after IR. Ionizing radiation is known to cause both an early (within millisecs) and a late (2–8 days) rise in ROS in other cells (e.g. glioma cells).36 This later rise is related to the so-called “metabolic redox response” and, in addition to the ROS generated within milliseconds of IR exposure, provides an additional regulatory mechanism controlling the fate irradiated cells.36 Our results suggest that JNK activity reduces the late accumulation of ROS following IR and is consistent with the ability of JNK to limit oxidative stress in non-irradiated VS cells. This ability of JNK to limit oxidative stress likely contributes to the relative resistance of VS cells to IR-induced cell death since I-JIP and SP600125 each significantly increased VS apoptotic cell death following IR. By contrast, activation of JNK in response to UV or ionizing radiation promotes apoptosis in many cell types and, in these cases, JNK inhibitors protect cells from IR-induced death.25, 27, 28 Here our study focused on apoptotic cell death; given the limited number of primary VS cells available we did not assay other forms of radiation-induced cell death (e.g. mitotic catastrophe, necrosis, autophagy). Whether inhibition of JNK likewise increases VS cell death by these alternative pathways following IR requires further investigation.
H2AX becomes phosphorylated on serine 139 following double stranded DNA breaks, including those induced by IR. Ataxia telangiectasia mutated (ATM) and other members of the phosphatidylinositol (PI) 3-kinase family, including AT and Rad3-related protein (ATR) and DNA-dependent protein kinase (DNA-PK), have been shown to mediate H2AX phosphorylation.32, 50–54. The extent to which ATM kinases are active in VS cells remains unkown. Subsequent studies raised the possibility that other kinases also mediate H2AX phosphorylation. For example, H2AX was phosphorylated in cells expressing kinase-dead ATM, ATR, or DNA-PK mutants and Stiff, et. al., found that ATM did not contribute to IR-induced H2AX phosphorylation in fibroblasts.51, 55 Lu, et. al., demonstrated that JNK also phosphorylates H2AX following ultraviolet A irradiation and our data suggest that JNK activity is necessary for H2AX phosphorylation following γ-irradiation in VS cells.56 It is not clear whether H2AX phosphorylation is necessary for repair of IR-induced damage.55–59 If it is, inhibition of this repair process represents another mechanism whereby JNK inhibitors could potentiate VS cell radiosensitivity, in addition to increasing oxidative stress.
Taken together with recent studies, these results support a model whereby loss of merlin function leads to persistent JNK activity, which in turn suppresses VS cell apoptosis, including IR-induced apoptosis, likely by limiting oxidative stress. Thus, JNK inhibitors represent potential therapeutic compounds to treat VSs that are not amenable to microsurgery or SRS/FRS. Further, for VSs treated with SRS/FRS, concurrent treatment with JNK inhibitors may augment IR-induced cytotoxicity and increase efficacy. Whether inhibitors of other signaling cascades (e.g. Akt, mTOR, ErbBs, histone deacetylase) that are being explored as potential therapies for NF2-associated VSs likewise modulate VS cell radiosensitivity requires further exploration.60–70
CONCLUSIONS
Persistent JNK activity in VS cells promotes cell proliferation and cell survival and inhibition JNK signaling represents a potential therapeutic approach to manage VSs that are not amenable for current therapies. Here we show that JNK activity in VS cells mediates IR-induced H2AX phosphorylation and reduces IR-induced oxidative stress and cell apoptosis. Thus, treatment with JNK inhibitors concurrently with SRS may increase VS radiosensitivity, particularly in selected tumors that are deemed resistant to current SRS protocols.
Acknowledgments
Support: NIDCD R01 DC009801 and P30 DC010362
RFERENCES
- 1.Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005 Mar;115(3):450–454. doi: 10.1097/00005537-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia A, Mastrodimos B, Cueva R. Comparison of growth patterns of acoustic neuromas with and without radiosurgery. Otol Neurotol. 2006 Aug;27(5):705–712. doi: 10.1097/01.mao.0000226302.59198.87. [DOI] [PubMed] [Google Scholar]
- 3.Wackym PA. Stereotactic radiosurgery, microsurgery, and expectant management of acoustic neuroma: basis for informed consent. Otolaryngol Clin North Am. 2005 Aug;38(4):653–670. doi: 10.1016/j.otc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Pollock BE. Vestibular schwannoma management: an evidence-based comparison of stereotactic radiosurgery and microsurgical resection. Prog Neurol Surg. 2008;21:222–227. doi: 10.1159/000157170. [DOI] [PubMed] [Google Scholar]
- 5.Pollock BE, Lunsford LD, Noren G. Vestibular schwannoma management in the next century: a radiosurgical perspective. Neurosurgery. 1998 Sep;43(3):475–481. doi: 10.1097/00006123-199809000-00041. discussion 481-473. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford SA, King AT. Vestibular schwannoma management: What is the 'best' option? Br J Neurosurg. 2005 Aug;19(4):309–316. doi: 10.1080/02688690500305399. [DOI] [PubMed] [Google Scholar]
- 7.Whitmore RG, Urban C, Church E, Ruckenstein M, Stein SC, Lee JY. Decision analysis of treatment options for vestibular schwannoma. J Neurosurg. 2011 Feb;114(2):400–413. doi: 10.3171/2010.3.JNS091802. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu D, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007 Mar;60(3):460–468. doi: 10.1227/01.NEU.0000255340.26027.53. discussion 468–470. [DOI] [PubMed] [Google Scholar]
- 9.Rowe JG, Radatz M, Walton L, Kemeny AA. Stereotactic radiosurgery for type 2 neurofibromatosis acoustic neuromas: patient selection and tumour size. Stereotact Funct Neurosurg. 2002;79(2):107–116. doi: 10.1159/000070106. [DOI] [PubMed] [Google Scholar]
- 10.Wowra B, Muacevic A, Jess-Hempen A, Hempel JM, Muller-Schunk S, Tonn JC. Outpatient gamma knife surgery for vestibular schwannoma: definition of the therapeutic profile based on a 10-year experience. J Neurosurg. 2005 Jan;102(Suppl):114–118. [PubMed] [Google Scholar]
- 11.Friedman RA, Berliner KI, Bassim M, et al. A paradigm shift in salvage surgery for radiated vestibular schwannoma. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2011 Oct;32(8):1322–1328. doi: 10.1097/MAO.0b013e31822e5b76. [DOI] [PubMed] [Google Scholar]
- 12.Friedman RA, Brackmann DE, Hitselberger WE, Schwartz MS, Iqbal Z, Berliner KI. Surgical salvage after failed irradiation for vestibular schwannoma. Laryngoscope. 2005 Oct;115(10):1827–1832. doi: 10.1097/01.mlg.0000175063.76945.75. [DOI] [PubMed] [Google Scholar]
- 13.Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005 Jan;102(Suppl):195–199. [PubMed] [Google Scholar]
- 14.Hasegawa T, Kida Y, Kobayashi T, Yoshimoto M, Mori Y, Yoshida J. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005 Jan;102(1):10–16. doi: 10.3171/jns.2005.102.1.0010. [DOI] [PubMed] [Google Scholar]
- 15.Lee DJ, Westra WH, Staecker H, Long D, Niparko JK, Slattery WH., 3rd Clinical and histopathologic features of recurrent vestibular schwannoma (acoustic neuroma) after stereotactic radiosurgery. Otol Neurotol. 2003 Jul;24(4):650–660. doi: 10.1097/00129492-200307000-00020. discussion 660. [DOI] [PubMed] [Google Scholar]
- 16.Lee F, Linthicum F, Jr, Hung G. Proliferation potential in recurrent acoustic schwannoma following gamma knife radiosurgery versus microsurgery. Laryngoscope. 2002;112(6):948–950. doi: 10.1097/00005537-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hansen MR, Clark JJ, Gantz BJ, Goswami PC. Effects of ErbB2 signaling on the response of vestibular schwannoma cells to gamma-irradiation. Laryngoscope. 2008 Jun;118(6):1023–1030. doi: 10.1097/MLG.0b013e318163f920. [DOI] [PubMed] [Google Scholar]
- 18.Anniko M. Early morphological changes following gamma irradiation. A comparison of human pituitary tumours and human acoustic neurinomas (schwannomas) Acta Pathol Microbiol Scand [A] 1981 Mar;89(2):113–124. [PubMed] [Google Scholar]
- 19.Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007 Aug;46(8):591–598. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007 Apr;19(2):142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Chadee DN, Xu D, Hung G, et al. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4463–4468. doi: 10.1073/pnas.0510651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilton DA, Ristic N, Hanemann CO. Activation of ERK, AKT and JNK signalling pathways in human schwannomas in situ. Histopathology. 2009 Dec;55(6):744–749. doi: 10.1111/j.1365-2559.2009.03440.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003 Jun 1;12(11):1211–1221. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- 24.Yue WY, Clark JJ, Fernando A, Domann F, Hansen MR. Contribution of persistent C-Jun N-terminal kinase activity to the survival of human vestibular schwannoma cells by suppression of accumulation of mitochondrial superoxides. Neuro-oncology. 2011 Sep;13(9):961–973. doi: 10.1093/neuonc/nor068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valerie K, Yacoub A, Hagan MP, et al. Radiation-induced cell signaling: inside-out and outside-in. Molecular cancer therapeutics. 2007 Mar;6(3):789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 26.Fisher CJ, Goswami PC. Mitochondria-targeted antioxidant enzyme activity regulates radioresistance in human pancreatic cancer cells. Cancer biology & therapy. 2008 Aug;7(8):1271–1279. doi: 10.4161/cbt.7.8.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim CH, Won M, Choi CH, et al. Increase of RhoB in gamma-radiation-induced apoptosis is regulated by c-Jun N-terminal kinase in Jurkat T cells. Biochemical and biophysical research communications. 2010 Jan 8;391(2):1182–1186. doi: 10.1016/j.bbrc.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Wang Y, Xu HT, et al. X-radiation induces non-small-cell lung cancer apoptosis by upregulation of Axin expression. International journal of radiation oncology, biology, physics. 2009 Oct 1;75(2):518–526. doi: 10.1016/j.ijrobp.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006 Apr 15;53(6):593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 30.Brown KD, Hansen MR. Lipid raft localization of erbB2 in vestibular schwannoma and Schwann cells. Otol Neurotol. 2008;29(1):79–85. doi: 10.1097/mao.0b013e31815dbb11. [DOI] [PubMed] [Google Scholar]
- 31.Provenzano MJ, Minner SA, Zander K, et al. p75(NTR) expression and nuclear localization of p75(NTR) intracellular domain in spiral ganglion Schwann cells following deafness correlate with cell proliferation. Molecular and cellular neurosciences. 2011 Aug;47(4):306–315. doi: 10.1016/j.mcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001 Nov 9;276(45):42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 33.Barr RK, Kendrick TS, Bogoyevitch MA. Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem. 2002 Mar 29;277(13):10987–10997. doi: 10.1074/jbc.M107565200. [DOI] [PubMed] [Google Scholar]
- 34.Kuan CY, Burke RE. Targeting the JNK signaling pathway for stroke and Parkinson's diseases therapy. Curr Drug Targets CNS Neurol Disord. 2005 Feb;4(1):63–67. doi: 10.2174/1568007053005145. [DOI] [PubMed] [Google Scholar]
- 35.Dickens M, Rogers JS, Cavanagh J, et al. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997 Aug 1;277(5326):693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z, Sarsour EH, Kalen AL, Li L, Kumar MG, Goswami PC. Late ROS accumulation and radiosensitivity in SOD1-overexpressing human glioma cells. Free Radic Biol Med. 2008 Aug 14; doi: 10.1016/j.freeradbiomed.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004 Jul 15;59(4):928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Dutta D, Balaji Subramanian S, Murli V, Sudahar H, Gopalakrishna Kurup PG, Potharaju M. Dosimetric comparison of Linac-based (BrainLAB(R)) and robotic radiosurgery (CyberKnife (R)) stereotactic system plans for acoustic schwannoma. Journal of neuro-oncology. 2012 Feb;106(3):637–642. doi: 10.1007/s11060-011-0703-5. [DOI] [PubMed] [Google Scholar]
- 39.Massager N, Lonneville S, Delbrouck C, Benmebarek N, Desmedt F, Devriendt D. Dosimetric and clinical analysis of spatial distribution of the radiation dose in gamma knife radiosurgery for vestibular schwannoma. International journal of radiation oncology, biology, physics. 2011 Nov 15;81(4):e511–e518. doi: 10.1016/j.ijrobp.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 40.Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. International journal of radiation oncology, biology, physics. 2011 Mar 15;79(4):985–997. doi: 10.1016/j.ijrobp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Dirks MS, Butman JA, Kim HJ, et al. Long-term natural history of neurofibromatosis Type 2-associated intracranial tumors. J Neurosurg. 2012 Jul;117(1):109–117. doi: 10.3171/2012.3.JNS111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkinson PJ, Cho CH, Hansen MR, Green SH. Activity of all JNK isoforms contributes to neurite growth in spiral ganglion neurons. Hearing research. 2011 May 1; doi: 10.1016/j.heares.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET. All JNKs can kill, but nuclear localization is critical for neuronal death. J Biol Chem. 2008 Jul 11;283(28):19704–19713. doi: 10.1074/jbc.M707744200. [DOI] [PubMed] [Google Scholar]
- 44.Tararuk T, Ostman N, Li W, et al. JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length. The Journal of cell biology. 2006 Apr 24;173(2):265–277. doi: 10.1083/jcb.200511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M, Liu NY, Wang XE, et al. Activin B promotes epithelial wound healing in vivo through RhoA-JNK signaling pathway. PloS one. 2011;6(9):e25143. doi: 10.1371/journal.pone.0025143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol. 2004 Aug;6(8):770–776. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- 47.Zhan Y, Modi N, Stewart AM, et al. Regulation of mixed lineage kinase 3 is required for Neurofibromatosis-2-mediated growth suppression in human cancer. Oncogene. 2011 Feb 17;30(7):781–789. doi: 10.1038/onc.2010.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. !!! INVALID CITATION !!! [Google Scholar]
- 49.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Seminars in radiation oncology. 2003 Jan;13(1):13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 50.Park EJ, Chan DW, Park JH, Oettinger MA, Kwon J. DNA-PK is activated by nucleosomes and phosphorylates H2AX within the nucleosomes in an acetylation-dependent manner. Nucleic acids research. 2003 Dec 1;31(23):6819–6827. doi: 10.1093/nar/gkg921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004 Apr 1;64(7):2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 52.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001 Dec 21;276(51):47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 53.Ward IM, Minn K, Chen J. UV-induced ataxia-telangiectasia-mutated and Rad3-related (ATR) activation requires replication stress. J Biol Chem. 2004 Mar 12;279(11):9677–9680. doi: 10.1074/jbc.C300554200. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-Capetillo O, Chen HT, Celeste A, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nature cell biology. 2002 Dec;4(12):993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA repair. 2004 Aug-Sep;3(8–9):959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Lu C, Zhu F, Cho YY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006 Jul 7;23(1):121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell research. 2008 Jan;18(1):134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 58.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nature cell biology. 2003 Jul;5(7):675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 59.Celeste A, Petersen S, Romanienko PJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002 May 3;296(5569):922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee TX, Packer MD, Huang J, et al. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. European journal of cancer. 2009 Jun;45(9):1709–1720. doi: 10.1016/j.ejca.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009 Aug;29(15):4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008 Jul 1;68(13):5236–5245. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- 63.Subbiah V, Slopis J, Hong DS, et al. Treatment of patients with advanced neurofibromatosis type 2 with novel molecularly targeted therapies: from bench to bedside. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Feb 10;30(5):e64–e68. doi: 10.1200/JCO.2011.38.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ammoun S, Cunliffe CH, Allen JC, et al. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro-oncology. 2010 Aug;12(8):834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ammoun S, Hanemann CO. Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nature reviews. Neurology. 2011 Jul;7(7):392–399. doi: 10.1038/nrneurol.2011.82. [DOI] [PubMed] [Google Scholar]
- 66.Bush ML, Burns SS, Oblinger J, et al. Treatment of vestibular schwannoma cells with ErbB inhibitors. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012 Feb;33(2):244–257. doi: 10.1097/MAO.0b013e31823e287f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bush ML, Oblinger J, Brendel V, et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro-oncology. 2011 Sep;13(9):983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacob A, Oblinger J, Bush ML, et al. Preclinical validation of AR42, a novel histone deacetylase inhibitor, as treatment for vestibular schwannomas. The Laryngoscope. 2012 Jan;122(1):174–189. doi: 10.1002/lary.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark JJ, Provenzano M, Diggelmann HR, Xu N, Hansen SS, Hansen MR. The ErbB inhibitors trastuzumab and erlotinib inhibit growth of vestibular schwannoma xenografts in nude mice: a preliminary study. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008 Sep;29(6):846–853. doi: 10.1097/MAO.0b013e31817f7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doherty JK, Ongkeko W, Crawley B, Andalibi A, Ryan AF. ErbB and Nrg: Potential Molecular Targets for Vestibular Schwannoma Pharmacotherapy. Otol Neurotol. 2008 Jan;29(1):50–57. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]