Abstract
Aim
Anticoagulation prophylaxis for stroke is recommended for at-risk patients with either persistent or paroxysmal atrial fibrillation (AF). We compared outcomes in patients with persistent vs. paroxysmal AF receiving oral anticoagulation.
Methods and results
Patients randomized in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial (n = 14 264) were grouped by baseline AF category: paroxysmal or persistent. Multivariable adjustment was performed to compare thrombo-embolic events, bleeding, and death between groups, in high-risk subgroups, and across treatment assignment (rivaroxaban or warfarin). Of 14 062 patients, 11 548 (82%) had persistent AF and 2514 (18%) had paroxysmal AF. Patients with persistent AF were marginally older (73 vs. 72, P = 0.03), less likely female (39 vs. 45%, P < 0.0001), and more likely to have previously used vitamin K antagonists (64 vs. 56%, P < 0.0001) compared with patients with paroxysmal AF. In patients randomized to warfarin, time in therapeutic range was similar (58 vs. 57%, P = 0.94). Patients with persistent AF had higher adjusted rates of stroke or systemic embolism (2.18 vs. 1.73 events per 100-patient-years, P = 0.048) and all-cause mortality (4.78 vs. 3.52, P = 0.006). Rates of major bleeding were similar (3.55 vs. 3.31, P = 0.77). Rates of stroke or systemic embolism in both types of AF did not differ by treatment assignment (rivaroxaban vs. warfarin, Pinteraction = 0.6).
Conclusion
In patients with AF at moderate-to-high risk of stroke receiving anticoagulation, those with persistent AF have a higher risk of thrombo-embolic events and worse survival compared with paroxysmal AF.
Keywords: Atrial fibrillation, Paroxysmal, Persistent, Anticoagulation, Outcomes
Introduction
Atrial fibrillation (AF) is a progressive disorder, often transitioning from intermittent to continuous arrhythmia. Patients experiencing episodic AF, self-terminating within 7 days, are said to have paroxysmal AF; patients whose arrhythmia persists beyond 7 days (or requires intervention to terminate) are considered to have persistent AF. Several prior studies have documented symptomatic, physiologic, and anatomic differences between patients with paroxysmal and persistent AF.1,2 This categorization of AF can also have important implications for approaches to maintain sinus rhythm.3 All the patients with AF are at an increased risk of thrombo-embolism (stroke or systemic embolism) compared with patients without AF, and anticoagulation therapies are recommended in all patients with AF who are at moderate-to-high risk of stroke.4,5 The distinction between paroxysmal and persistent AF has not been used to guide choice of stroke prophylaxis, as it remains unclear whether patients with persistent AF are at higher risk compared with those with paroxysmal AF, particularly in patients with additional risk factors for stroke.6 The objectives of the current analysis were to (i) measure the differences, if any, in outcomes between anticoagulated patients with persistent vs. paroxysmal AF who had additional risk factors for stroke, and (ii) determine whether there was a difference in treatment effect between rivaroxaban and warfarin in these groups.
Methods
This was a post hoc analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism (ROCKET-AF) trial. The design of the ROCKET-AF study has been previously described (NCT00403767).7 In brief, ROCKET-AF was a prospective, randomized, double-blind, placebo-controlled trial of fixed-dose rivaroxaban vs. adjusted-dose warfarin for the prevention of stroke or systemic embolism in patients with non-valvular AF at a high risk of stroke. All the patients had to have electrocardiographic evidence of AF within 30 days prior to randomization; additionally, they had to have medical evidence of AF within the previous year. Patients were categorized by the enrolling physician at baseline as having either paroxysmal (lasting ≤7 days at any time) or persistent AF (>7 days at a time); no other AF types were provided as choices. Patients were subsequently assessed in clinic at least as frequently as every 4 weeks, and this included ascertainment of interval events.
The present study included all patients randomized in the ROCKET-AF trial [intention-to-treat (ITT)]. Patients were grouped according to the type AF at baseline enrolment (paroxysmal or persistent) according to pre-specified diagnostic criteria and prior to any analysis of the data. Patients with new-onset AF at baseline [1.4% (n = 202)] were excluded from this analysis. Baseline characteristics and outcomes were compared between these groups (paroxysmal or persistent). Outcomes were further stratified by subgroups of interest: CHADS2 scores (2 vs. ≥3),8 duration of AF diagnosis (≤6 vs. >6 months), baseline electrocardiogram (ECG) (AF or atrial flutter vs. other), presence of congestive heart failure (CHF), history of prior stroke, and presence of significant renal dysfunction (defined as creatinine clearance <60 mL/min).9
Outcomes
Pre-specified outcomes in the ROCKET-AF trial have been described previously.7,10 The present analysis compared outcomes between paroxysmal or persistent AF, as defined at baseline. The primary efficacy end-point for this analysis was stroke (ischaemic or haemorrhagic) or systemic embolism in the ITT population. Additional secondary outcomes included stroke only, transient ischaemic attack (TIA) only, stroke or TIA, all-cause mortality, and a composite of stroke, systemic embolism, or death. As in other ROCKET-AF analyses, 93 patients were excluded from the efficacy analyses due to violations of Good Clinical Practice at the enrolling centre. The safety outcome of major bleeding was assessed, and limited to the safety population (patients in the ITT population who received at least one dose of study medication).
Statistical methods
Baseline characteristics are presented as per cent (count) for categorical variables and as medians (25th, 75th percentiles) for continuous variables. Groups were compared using Wilcoxon rank-sum tests for continuous variables and Pearson chi-square tests for categorical variables.
For each of the end-points, event rates (events per 100 patient-years and total events) were generated. Comparisons were performed using Cox proportional hazards models. All models were adjusted for variables found to be predictive of efficacy and safety end-points in the full ROCKET-AF cohort.9,11 Efficacy end-point models were adjusted for the following (at baseline): age, sex, body mass index (BMI), region, diabetes, prior stroke/TIA, vascular disease [myocardial infarction (MI), peripheral artery disease (PAD), carotid occlusive disease], CHF, hypertension, chronic obstructive pulmonary disease, diastolic blood pressure (BP), creatinine clearance (calculated using the Cockcroft–Gault equation),12 heart rate, and abstinence from alcohol. Safety end-point models were adjusted for the following (at baseline): age; sex; region; prior stroke/TIA; anaemia; prior gastrointestinal bleed; chronic obstructive pulmonary disease; diastolic BP; creatinine clearance (Cockcroft–Gault equation);12 platelets; albumin; and prior aspirin, vitamin K antagonist, or thienopyridine use. Missingness was low overall –<0.1% for any efficacy model covariate, and <3% for any safety model covariate. Where missing, covariates were imputed using the median for continuous variables and the mode for categorical variables.13 The above efficacy and safety models also contained randomized treatment (rivaroxaban or warfarin). Hazard ratios (HRs) [with 95% confidence intervals (CI)] and P-values are presented.
In analyses of subgroups, a similar approach was used: event rates (events per 100 patient-years and total events) were generated for each combination of AF type (paroxysmal or persistent) and subgroup. The same Cox proportional hazards models were used, with the addition of a term for the subgroup (where not already in the model) and for the interaction between subgroup and AF type. Hazard ratios (with 95% CIs) for paroxysmal vs. persistent AF within each subgroup, along with the interaction P-value, were calculated.
To assess the difference in treatment effect, if any, between rivaroxaban and warfarin across AF type, the above models were used with the addition of a term for the interaction between treatment assignment and AF type. Hazard ratios (with 95% CIs) for rivaroxaban vs. warfarin within each AF type, along with the interaction P-value, are presented.
All the patients provided written, informed consent and all statistical analyses were performed by the Duke Clinical Research Institute (Durham, NC) using the SAS software (version 9.2, SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Characteristics of the patients, stratified by AF type at baseline, are shown in Table 1. Treatment assignment was balanced across AF types. Compared with patients with persistent AF at baseline, those with paroxysmal AF were slightly younger (median age 72 vs. 73 years, P = 0.03), more likely female (45 vs. 39%, P < 0.0001), with lower baseline heart rate (median 72 vs. 76 beats/min, P < 0.0001), and lower rates of diabetes (37 vs. 41%, P = 0.0003) and CHF (56 vs. 64%, P < 0.0001). However, mean CHADS2 (3.5 for each, P = 0.3) and CHA2DS2-VASc (4.9, P = 0.07) scores were both balanced between patients with paroxysmal and persistent AF, and rates of prior thrombo-embolic evens were higher in patients with paroxysmal AF (prior stroke, TIA, or systemic embolism 59 vs. 54%, P < 0.0001). There were also differences in prior vitamin K antagonist therapy (paroxysmal, 56 vs. 64% for persistent, P < 0.0001) and prior chronic aspirin use (paroxysmal, 41 vs. 35% for persistent, P < 0.0001).
Table 1.
Patient characteristics
| Paroxysmal AF (n = 2514) | Persistent AF (n = 11 548) | P-value | |
|---|---|---|---|
| Randomized to rivaroxaban, % (n) | 50% (1245) | 50% (5786) | 0.60 |
| Age | 72 (65, 78) | 73 (65, 78) | 0.033 |
| Female, % (n) | 45% (1121) | 39% (4459) | <0.0001 |
| CHADS2 score, mean (SD) | 3.5 (0.9) | 3.5 (0.9) | 0.32 |
| CHADS2 score, % (n) | |||
| 1 | 0 | <1% (3) | |
| 2 | 13% (334) | 13% (1510) | |
| 3 | 44% (1110) | 43% (4997) | |
| 4 | 28% (716) | 29% (3319) | |
| 5 | 12% (304) | 13% (1489) | |
| 6 | 2% (50) | 2% (230) | |
| CHA2DS2-VASc score, mean (SD) | 4.9 (1.3) | 4.9 (1.3) | 0.072 |
| CHA2DS2-VASc score, % (n) | |||
| 1 | 0 | <1% (2) | |
| 2 | 3% (65) | 3% (326) | |
| 3 | 12% (309) | 12% (1391) | |
| 4 | 24% (609) | 26% (2980) | |
| 5 | 29% (736) | 30% (3437) | |
| 6 | 20% (497) | 19% (2150) | |
| 7 | 9% (222) | 8% (941) | |
| 8 | 3% (67) | 2% (285) | |
| 9 | <1% (9) | <1% (34) | |
| Presenting characteristics | |||
| BMI, kg/m2 | 28 (25, 32) | 28 (25, 32) | 0.021 |
| Systolic blood pressure, mmHg | 130 (120, 140) | 130 (120, 140) | 0.99 |
| Diastolic blood pressure, mmHg | 80 (70, 85) | 80 (70, 86) | 0.021 |
| Heart rate, b.p.m. | 72 (63, 83) | 76 (68, 86) | <0.0001 |
| Creatinine clearance,a mL/min | 68 (53, 88) | 67 (52, 87) | 0.039 |
| Baseline comorbidities, % (n) | |||
| Prior stroke/TIA/SE | 59% (1493) | 54% (6207) | <0.0001 |
| Prior stroke | 36% (895) | 34% (3940) | 0.16 |
| Prior TIA | 27% (673) | 21% (2395) | <0.0001 |
| Significant valve disease | 13% (328) | 15% (1710) | 0.022 |
| PAD | 6% (150) | 6% (678) | 0.85 |
| Carotid occlusive disease | 5% (124) | 4% (459) | 0.032 |
| Hypertension | 91% (2280) | 91% (10 453) | 0.79 |
| Diabetes | 37% (922) | 41% (4684) | 0.0003 |
| Prior MI | 19% (478) | 17% (1954) | 0.013 |
| Heart failure | |||
| None | 44% (1097) | 36% (4159) | <0.0001 |
| NYHA class I | 8% (213) | 8% (962) | |
| NYHA class II | 32% (805) | 36% (4173) | |
| NYHA class III/IV | 16% (399) | 19% (2251) | |
| Chronic obstructive pulmonary disease | 10% (263) | 11% (1220) | 0.87 |
| Medications, % (n) | |||
| Prior VKA use | 56% (1410) | 64% (7431) | <0.0001 |
| Prior chronic aspirin use | 41% (1024) | 35% (4090) | <0.0001 |
| ACE inhibitor/ARB at baseline | 73% (1829) | 75% (8628) | 0.042 |
| β-Blocker at baseline | 67% (1685) | 64% (7447) | 0.015 |
| Digitalis at baseline | 24% (612) | 42% (4808) | <0.0001 |
| Diuretic at baseline | 52% (1308) | 61% (7076) | <0.0001 |
| Antiarrhythmic drug at baseline | 28% (714) | 9% (1009) | <0.0001 |
| TTR during follow-up, warfarin group, % | 57 (44, 70) | 58 (43, 71) | 0.94 |
Continuous variables are shown as median (25th, 75th percentiles) unless otherwise noted.
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; SD, standard deviation; SE, systemic embolism; TIA, transient ischaemic attack; TTR, time in therapeutic range; VKA, vitamin K antagonist.
aCreatinine clearance calculated according to the Cockcroft–Gault equation.
Treatments during the follow-up
There was no imbalance in allocation to rivaroxaban or warfarin by AF type; half of patients with paroxysmal and persistent AF were randomized to rivaroxaban and half were randomized to warfarin.
During the follow-up of patients allocated to warfarin, the TTR was similar between patients with paroxysmal and persistent AF (57 vs. 58%, P = 0.94).
Use of aspirin during the follow-up was balanced between patients with paroxysmal AF (21%) and those with persistent AF (20%). The mean duration of aspirin use during the trial was 19 months in both, and the mean dose was 90 mg for those with paroxysmal AF and 88 mg for those with persistent AF. Electrical cardioversion was performed infrequently–144 in total. There were 58 (2.3%) in the paroxysmal AF group and 86 (0.7%) in the persistent AF group.
Outcomes by atrial fibrillation type
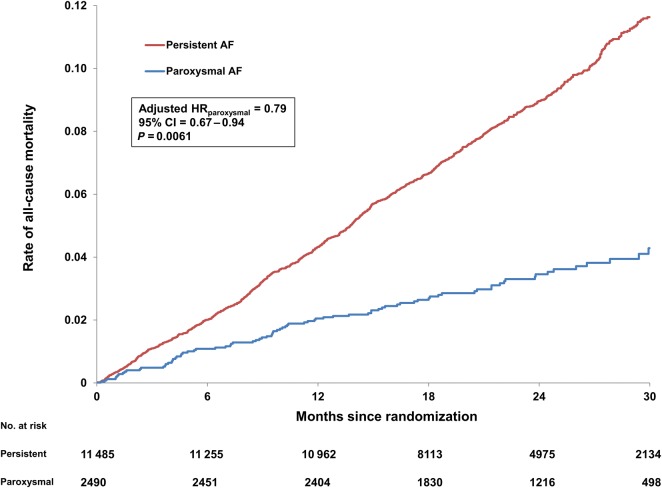
Adjusted efficacy and safety outcomes, by AF type, are shown in Table 2. Patients with paroxysmal AF had significantly lower rates of stroke (adjusted HR: 0.78, 95% CI: 0.61–0.99, P = 0.045), all-cause mortality (adjusted HR: 0.79, 95% CI: 0.67–0.94, P = 0.006), and the composite of stroke or systemic embolism or death (adjusted HR: 0.82, 95% CI: 0.71–0.94, P = 0.005). Kaplan–Meier curves for all-cause mortality, stratified by AF type, are shown in Figure 1.
Table 2.
Adjusted outcomes by type of atrial fibrillation
| Outcomes | Paroxysmal AF events/ 100 Pt-Yrs (total events) | Persistent AF events/ 100 Pt-Yrs (total events) | Paroxysmal vs. Persistent AF adjusted HR (95% CI) | P-value |
|---|---|---|---|---|
| Efficacy outcomes | ||||
| Stroke or SE | 1.73 (85) | 2.18 (480) | 0.79 (0.63, 1.00) | 0.048 |
| All-cause death | 3.52 (170) | 4.78 (1029) | 0.79 (0.67, 0.94) | 0.0061 |
| Stroke/SE/death | 4.91 (233) | 6.33 (1341) | 0.82 (0.71, 0.94) | 0.0047 |
| Stroke or TIA | 2.26 (110) | 2.55 (560) | 0.87 (0.71, 1.07) | 0.19 |
| Stroke | 1.59 (78) | 2.02 (446) | 0.78 (0.61, 0.99) | 0.045 |
| TIA | 0.67 (33) | 0.56 (125) | 1.13 (0.76, 1.67) | 0.55 |
| Safety outcomes | ||||
| Major bleeding | 3.31 (131) | 3.55 (638) | 0.97 (0.80, 1.17) | 0.77 |
Efficacy end-point models were adjusted for the following: age, sex, body mass index, region, diabetes, prior stroke/TIA, vascular disease (myocardial infarction, peripheral artery disease, carotid occlusive disease), congestive heart failure, hypertension, chronic obstructive pulmonary disease, diastolic blood pressure, creatinine clearance (calculated using Cockcroft–Gault equation), heart rate, and abstinence from alcohol. Safety end-point models were adjusted for the following: age; sex; region; prior stroke/TIA; anaemia; prior gastrointestinal bleed; chronic obstructive pulmonary disease; diastolic blood pressure; creatinine clearance (Cockcroft–Gault equation); platelets; albumin; and prior aspirin, vitamin K antagonist, or thienopyridine use.
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; SE, systemic embolism; TIA, transient ischaemic attack.
Figure 1.
Unadjusted Kaplan–Meier event curves for all-cause mortality, by atrial fibrillation type at baseline. AF, atrial fibrillation, HR, hazard ratio; CI, confidence interval.
The results were consistent throughout follow-up. Among patients with paroxysmal AF, there was not any initial greater excess of stroke or systemic embolism during the first month after randomization that could have been attributed to a lower prevalence of anticoagulation by VKA prior to randomization (Figure 1).
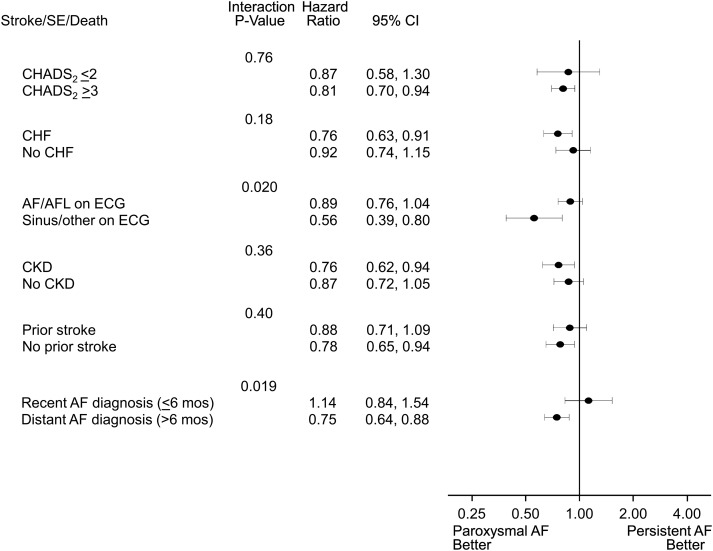
Lower hazards for patients with paroxysmal AF were consistent across subgroups of the CHADS2 score, CHF diagnosis, presence of chronic kidney disease, and history of stroke, for the composite end-point of stroke, systemic embolism, or death (Figure 2 and Supplementary material online). There was a significant interaction between AF type and (a) baseline rhythm (AF/atrial flutter vs. sinus/other, Pinteraction = 0.02), and (b) duration of AF diagnosis (≤6 vs. >6 months, Pinteraction = 0.02).
Figure 2.
Forest plot of the composite end-point of stroke, systemic embolism, or death for paroxysmal vs. persistent atrial fibrillation, stratified by high-risk subgroups. All strata assessed at baseline. AF, atrial fibrillation; AFL, atrial flutter; CHF, congestive heart failure; CKD, chronic kidney disease; ECG, electrocardiogram; SE, systemic embolism.
Outcomes by treatment assignment
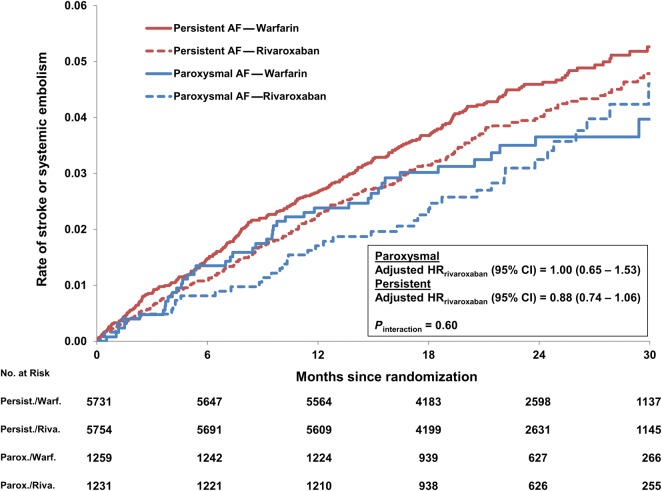
Adjusted outcomes comparing rivaroxaban vs. warfarin-assigned patients, stratified by AF type, are shown in Table 3. Corresponding Kaplan–Meier curves of the primary end-point, for each of the four groups, are shown in Figure 3. The relative treatment effects of rivaroxaban vs. warfarin were consistent among patients with persistent AF and paroxysmal AF. The number of stroke or systemic embolism events per 100 patient-years in those treated with rivaroxaban compared with warfarin was consistent among patients with paroxysmal AF (1.73% rivaroxaban vs. 1.74% warfarin; adjusted HR: 1.00, 95% CI: 0.65–1.53) and persistent AF (2.03 vs. 2.32%; adjusted HR: 0.88, 0.74–1.06, Pinteraction = 0.60). The number of major bleeding events per 100 patient-years in those treated with rivaroxaban compared with warfarin was consistent among patients with paroxysmal AF (3.43% rivaroxaban vs. 3.19% warfarin; adjusted HR: 1.06, 95% CI: 0.75–1.49) and persistent AF (3.61 vs. 3.49%; adjusted HR: 1.08, 0.92–1.26, Pinteraction = 0.94). All tests of interaction between treatment assignment and AF type were non-significant (Supplementary material online).
Table 3.
Adjusted outcomes of rivaroxaban vs. warfarin by atrial fibrillation type
| Paroxysmal AF |
Persistent AF |
Interaction P-value | |||||
|---|---|---|---|---|---|---|---|
| Rivaroxaban events/100 Pt-Yrs (total events) | Warfarin events/ 100 Pt-Yrs (total events) | Rivaroxaban vs. Warfarin adjusted HR (95% CI) | Rivaroxaban Events/100 Pt-Yrs (total events) | Warfarin events/100 Pt-Yrs (total events) | Rivaroxaban vs. Warfarin adjusted HR (95% CI) | ||
| Efficacy outcomes | |||||||
| Stroke or SE | 1.73 (42) | 1.74 (43) | 1.00 (0.65, 1.53) | 2.03 (225) | 2.32 (255) | 0.88 (0.74, 1.06) | 0.60 |
| All-cause death | 3.77 (90) | 3.28 (80) | 1.13 (0.83, 1.52) | 4.53 (490) | 5.02 (539) | 0.91 (0.80, 1.02) | 0.19 |
| Stroke/SE/death | 5.19 (122) | 4.63 (111) | 1.11 (0.86, 1.43) | 6.05 (643) | 6.62 (698) | 0.92 (0.82, 1.02) | 0.18 |
| Stroke or TIA | 2.19 (53) | 2.32 (57) | 0.95 (0.66, 1.39) | 2.47 (272) | 2.63 (288) | 0.94 (0.80, 1.11) | 0.96 |
| Stroke | 1.60 (39) | 1.58 (39) | 1.03 (0.66, 1.60) | 1.91 (212) | 2.13 (234) | 0.91 (0.75, 1.09) | 0.61 |
| TIA | 0.57 (14) | 0.76 (19) | 0.75 (0.37, 1.49) | 0.57 (64) | 0.54 (61) | 1.05 (0.74, 1.49) | 0.40 |
| Safety outcomes | |||||||
| Major bleeding | 3.43 (66) | 3.19 (65) | 1.06 (0.75, 1.49) | 3.61 (323) | 3.49 (315) | 1.08 (0.92, 1.26) | 0.94 |
Efficacy end-point models were adjusted for the following: age, sex, body mass index, region, diabetes, prior stroke/TIA, vascular disease (myocardial infarction, peripheral artery disease, carotid occlusive disease), congestive heart failure, hypertension, chronic obstructive pulmonary disease, diastolic blood pressure, creatinine clearance (calculated using the Cockcroft–Gault equation), heart rate, and abstinence from alcohol. Safety end-point models were adjusted for the following: age; sex; region; prior stroke/TIA; anaemia; prior gastrointestinal bleed; chronic obstructive pulmonary disease; diastolic blood pressure; creatinine clearance (Cockcroft–Gault equation); platelets; albumin; and prior aspirin, vitamin K antagonist, or thienopyridine use.
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; SE, systemic embolism; TIA, transient ischaemic attack.
Figure 3.
Unadjusted Kaplan–Meier event curves for stroke or systemic embolism, by atrial fibrillation type and treatment assignment. AF, atrial fibrillation; HR, hazard ratio, CI, confidence interval.
Discussion
Of the 14 264 patients randomized in the ROCKET-AF trial, a sizable minority (18%) had paroxysmal AF at baseline. While patients with persistent AF had some higher-risk features, compared with those with paroxysmal AF, CHADS2 and CHA2DS2-VASc scores were equivalent. After adjustment, thrombo-embolic and mortality outcomes were consistently higher in patients with persistent AF, and this association endured across high-risk subgroups (including patients with prior stroke). There did not appear to be significant differences in event rates between rivaroxaban and dose-adjusted warfarin, across AF type.
Several prior cohorts have suggested no difference in outcomes between patients with paroxysmal or persistent AF.14–16 However, there are important distinctions of these studies. For example, in the GISSI-AF post hoc analysis, a total of 1234 patients were studied, and antithrombotic rates varied significantly between paroxysmal and persistent (76 vs. 96%, P < 0.0001).14 Furthermore, mean CHADS2 scores were dramatically lower compared with those in ROCKET-AF (1.4 vs. 3.5). Similarly, a post hoc analysis of the ACTIVE-W trial included patients with mean CHADS2 scores of 1.8–2.0; and while anticoagulation was not uniform by design, there were imbalances of the randomization scheme between paroxysmal and persistent AF patients (65% warfarin for paroxysmal vs. 85% for sustained AF, P < 0.0001).15 Lastly, in an analysis of 5533 patients in the Euro Heart Survey, the authors identified dramatic differences in the use of oral anticoagulation rates across several AF types (ranging from 45 to 79%, P < 0.001), and highly dynamic subsequent management in these patients.16 Overall, these studies have been significantly smaller than the present analysis (limiting their relative power); they frequently have lower risk and more heterogeneous populations (confounding comparisons); and most importantly, none of these previously reported analyses included consistently anticoagulated patients. The use of anticoagulation in all patients likely had a significant impact on our findings, as historical stroke/TIA events and anticoagulation use trended in opposite directions in paroxysmal AF patients in our cohort—they were less likely to be previously exposed to anticoagulation but more likely to have had prior stroke or TIA. Following uniform anticoagulation between the groups (at randomization), our data demonstrate that patients with persistent AF have worse outcomes, including thrombo-embolic events and mortality.
Our findings extend observations from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) and Apixaban for Reduction in Stroke and Other Thrombo-embolic Events in Atrial Fibrillation (ARISTOTLE) trials—patients enrolled in ROCKET-AF had substantially higher CHADS2 scores than either of those trials (mean 3.5 in ROCKET-AF vs. 2.1 in RE-LY and ARISTOTLE).17,18 In our analysis, CHADS2 scores, CHA2DS2-VASc scores, and the intensity of anticoagulation were all balanced between patients with paroxysmal AF and those with persistent AF. Even in patients at such high stroke risk, those with persistent AF still demonstrated worse survival and higher risk of thrombo-embolic events. Furthermore, the effect on mortality appears to be a sustained phenomenon, as event curves continue to separate out to 2.5 years of follow-up.
These data provide important insights into the risks associated with more advanced forms of AF (i.e. those with persistent AF). While the risk of stroke has been clear and remains present in patients with paroxysmal AF, it was not clear that outcomes are worse in patients with persistent AF once stroke risk is treated with oral anticoagulation. Our data demonstrate that, in the setting of adequate anticoagulation with either dose-adjusted warfarin or rivaroxaban, persistent AF is associated with worse outcomes, and this finding has important implications for overall AF treatment strategies aimed at improving outcomes, including survival. It suggests that the worse outcomes associated with advanced AF are unlikely to be attributable to stroke risk alone, and may instead be related to electromechanical or haemodynamic sequelae of the rhythm. Notable prior studies have failed to demonstrate a substantive survival benefit to maintaining sinus rhythm; however, several concerns have been raised with these data and they were not specific to advanced AF.19–21 Our analysis suggests there is an opportunity for improving outcomes of patients with advanced AF, potentially through disease-state modification. Rhythm control strategies such as catheter ablation have been shown to slow progression, and may provide an opportunity to improve clinical outcomes.22 There is also emerging evidence supporting the use of such procedures in patients with persistent AF.23 However, strategies such as risk factor modification may also provide additional opportunities to slow the disease progression.24
The lower risk of thrombo-embolic events and death in patients with paroxysmal AF, compared with persistent AF, was particularly pronounced in two subgroups—patients with the diagnosis of AF >6 months prior to baseline, and those with rhythms other than AF or atrial flutter on baseline ECG (Pinteraction <0.05 for each). While these remain hypothesis-generating results only, there are potential explanations that deserve further study. The effect of AF duration may reflect diagnostic biases in patients with more recent diagnoses, but both AF duration and ECG rhythm may also reflect measures of burden of AF arrhythmia. Patients not in AF or atrial flutter at baseline represent those with AF less likely to be captured on any single ECG. Burden of AF has been an intensely studied topic, and one of great debate. The Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) study suggested as little as 6 min of AF increased stroke risk,25 and additional studies have demonstrated some dose–response effect—that is, more AF portends worse thrombo-embolic outcomes.26 Yet to date, neither AF type nor AF burden is included in guideline-recommended risk stratification for thrombo-embolism prophylaxis. This is likely due in part to the difficulties of defining and measuring paroxysmal episodes of AF, and additional studies of the implications of AF burden for treatment decisions are required. However, patients with paroxysmal AF remain at significant risk of stroke, relative to patients without AF, and current evidence does not support withholding anticoagulation in these patients. In contrast, our data suggest that the use of more aggressive stroke prevention in patients with persistent AF, who are otherwise at lower risk, deserves further study.
The treatment effects of rivaroxaban vs. adjusted-dose warfarin were not different between patients with paroxysmal vs. persistent AF. This finding is consistent with prior data, suggesting both groups benefit from oral anticoagulation, particularly among patients at high risk of stroke at baseline.15,27 However, those patients at the highest risk of adverse outcome often derive the greatest benefit from aggressive therapies; in our analysis, patients with persistent AF were at substantially higher risk of thrombo-embolic events and death. This may explain, in part, the variance in the hazards of rivaroxaban vs. dose-adjusted warfarin by AF type. However, none of the differences was statistically significant and wider confidence intervals indicate relatively under-powered assessments.
Limitations
The current study represents a post hoc analysis of the ROCKET-AF trial, and is largely hypothesis generating. The classification of AF type was made at the site level at baseline and there may be variation (despite pre-specified definitions) or changes in AF type or burden over time that are not captured. Additionally, patients with paroxysmal AF represented a minority of the overall cohort, and power to detect differences in outcomes by treatment may be limited relative to the overall trial population. This is particularly pronounced for patients with low CHA2DS2-VASc scores—low numbers of patients and events limits additional analyses at this low-risk end of the spectrum. Furthermore, although treatment assignment was balanced and multivariable adjustment was performed, we cannot exclude significant, unmeasured confounding in the comparison between paroxysmal and persistent AF patients. Lastly, generalizability may be limited: these data are derived from a randomized trial population; few warfarin-treated patients had TTR >70%; and patients were generally of high stroke risk.
Conclusions
Among patients at a high risk of stroke who are receiving anticoagulation, those with persistent AF have a higher risk of thrombo-embolic events and death compared with those with paroxysmal AF. This effect is consistent across subgroups, and outcomes in both AF types did not differ between patients treated with rivaroxaban vs. dose-adjusted warfarin. These data have important implications for the management of patients with advanced AF, and additional data are needed regarding the potential benefit of therapies aimed at reducing AF persistence.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work and the ROCKET-AF trial were supported by Janssen Research & Development LLC, Raritan, NJ; and Bayer HealthCare AG, Leverkusen, Germany. B.A.S. was funded by the National Institutes of Health (NIH T-32 training grant #5 T32 HL 7101-38). Funding to pay the Open Access publication charges for this article was provided by Janssen Research & Development LLC, Raritan, NJ; and Bayer HealthCare AG, Leverkusen, Germany.
Conflict of interest: B.A.S., A.S.H., and Y.L.: none. N.R.P.: honoraria from Johnson & Johnson and Bayer HealthCare for serving on the executive committee of the ROCKET-AF trial; consulting fees from Ortho McNeil Janssen, and Bayer HealthCare; and advisory board fees from Genzyme. G.B.: honoraria from Johnson & Johnson and Bayer; and advisory board fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Sanofi-Aventis. G.J.H.: honoraria for serving on executive committees for Johnson & Johnson and Bayer. R.C.B.: consulting fees/honoraria and/or research grant support from Johnson & Johnson, Regado, Boehringer Ingelheim, AstraZeneca, Daiichi-Sankyo. D.E.S.: supported, in part, by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA, USA); has served as a consultant and/or member of a scientific advisory board for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Johnson & Johnson, and Pfizer; has an institutional research support contract with Johnson & Johnson; served as an unpaid ROCKET-AF executive committee member. J.L.H.: consulting fees/honoraria and/or research grant support from AstraZeneca, Bayer AG HealthCare, Boehringer Ingelheim, Daiichi-Sankyo, Johnson & Johnson, Pfizer, Sanofi-Aventis, Biotronik, Inc. Hacke: consulting fees/honoraria and/or research grant support from Bayer, Johnson & Johnson, and Boehringer Ingelheim. C.C.N.: employee of Janssen Research & Development LLC. S.D.B.: employee of Bayer. K.W.M: consulting fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Forest, Johnson & Johnson, WebMD. K.A.A.F.: grant funding and honoraria from Bayer and Johnson & Johnson. R.M.C.: available at https://www.dcri.org/about-us/conflict-of-interest/Califf-COI_2–3–2014.pdf. J.P.P.: research grants from ARCA Pharmaceuticals, Janssen, GE Healthcare, and ResMed and provides consulting to Forest Laboratories, Janssen, Medtronic, and Spectranetics.
References
- 1.Suzuki T, Yamazaki T, Ogawa S, Nagai R, Yamashita T. Echocardiographic predictors of frequency of paroxysmal atrial fibrillation (AF) and its progression to persistent AF in hypertensive patients with paroxysmal AF: Results from the Japanese Rhythm Management Trial II for Atrial Fibrillation (J-RHYTHM II Study) Heart Rhythm. 2011;8:1831–1836. doi: 10.1016/j.hrthm.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O'Neill J, Silva-Cardoso J, Zharinov O, Gamra H, Alam S, Ponikowski P, Lewalter T, Rosenqvist M, Steg PG. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5:632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 3.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA, ThermoCool AFTI. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, Lip GY, Manning WJ. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwlaat R, Dinh T, Olsson SB, Camm AJ, Capucci A, Tieleman RG, Lip GY, Crijns HJ. Should we abandon the common practice of withholding oral anticoagulation in paroxysmal atrial fibrillation? Eur Heart J. 2008;29:915–922. doi: 10.1093/eurheartj/ehn101. [DOI] [PubMed] [Google Scholar]
- 7.ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–347 e1. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 9.Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 10.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 11.Goodman SG, Wojdyla DM, Piccini JP, White HD, Paolini JF, Nessel CC, Berkowitz SD, Mahaffey KW, Patel MR, Sherwood MW, Becker RC, Halperin JL, Hacke W, Singer DE, Hankey GJ, Breithardt G, Fox KA, Califf RM Investigators RA. Factors Associated with Major Bleeding Events: Insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) J Am Coll Cardiol. 2014;63:891–900. doi: 10.1016/j.jacc.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 14.Disertori M, Franzosi MG, Barlera S, Cosmi F, Quintarelli S, Favero C, Cappellini G, Fabbri G, Maggioni AP, Staszewsky L, Moroni LA, Latini R. Thromboembolic event rate in paroxysmal and persistent atrial fibrillation: data from the GISSI-AF trial. BMC Cardiovasc Disord. 2013;13:28. doi: 10.1186/1471-2261-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Pajitnev D, Pogue J, Healey JS, Pfeffer MA, Yusuf S, Connolly SJ. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–2161. doi: 10.1016/j.jacc.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 16.Nieuwlaat R, Prins MH, Le Heuzey JY, Vardas PE, Aliot E, Santini M, Cobbe SM, Widdershoven JW, Baur LH, Levy S, Crijns HJ. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29:1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 17.Flaker G, Ezekowitz M, Yusuf S, Wallentin L, Noack H, Brueckmann M, Reilly P, Hohnloser SH, Connolly S. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study. J Am Coll Cardiol. 2012;59:854–855. doi: 10.1016/j.jacc.2011.10.896. [DOI] [PubMed] [Google Scholar]
- 18.Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, Ezekowitz J, Alings M, Yang H, Alexander JH, Flaker G, Hanna M, Granger CB. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. doi: 10.1093/eurheartj/eht135. [DOI] [PubMed] [Google Scholar]
- 19.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 20.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, Josephson RA, Kellen JC, Klein RC, Krahn AD, Mickel M, Mitchell LB, Nelson JD, Rosenberg Y, Schron E, Shemanski L, Waldo AL, Wyse DG. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 21.Saksena S, Slee A, Waldo AL, Freemantle N, Reynolds M, Rosenberg Y, Rathod S, Grant S, Thomas E, Wyse DG. Cardiovascular Outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management): an Assessment of Individual Antiarrhythmic Drug Therapies Compared With Rate Control With Propensity Score-Matched Analyses. J Am Coll Cardiol. 2011;58:1975–1985. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A. Radiofrequency Ablation vs Antiarrhythmic Drugs as First-Line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2) JAMA. 2014;311:692. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 23.Mont L, Bisbal F, Hernandez-Madrid A, Perez-Castellano N, Vinolas X, Arenal A, Arribas F, Fernandez-Lozano I, Bodegas A, Cobos A, Matia R, Perez-Villacastin J, Guerra JM, Avila P, Lopez-Gil M, Castro V, Arana JI, Brugada J. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study) Eur Heart J. 2014;35:501–507. doi: 10.1093/eurheartj/eht457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naruse Y, Tada H, Satoh M, Yanagihara M, Tsuneoka H, Hirata Y, Ito Y, Kuroki K, Machino T, Yamasaki H, Igarashi M, Sekiguchi Y, Sato A, Aonuma K. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331–337. doi: 10.1016/j.hrthm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 26.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 27.Lip GY, Frison L, Grind M. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med. 2008;264:50–61. doi: 10.1111/j.1365-2796.2007.01909.x. [DOI] [PubMed] [Google Scholar]