Abstract
Background
World Health Organization treatment guidelines recommend that adults with severe malaria be admitted to an intensive care unit (ICU). However, ICU facilities are limited in the resource-poor settings where most malaria occurs. Identification of patients at greater risk of complications may facilitate their triage and resource allocation.
Methods
With use of data from a trial conducted in Southeast Asia (n = 868), a logistic regression model was built to identify independent predictors of mortality among adults with severe malaria. A scoring system based on this model was tested in the original dataset and then validated in 2 series from Bangladesh (n = 188) and Vietnam (n = 292).
Results
Acidosis (base deficit) and cerebral malaria (measured as Glasgow Coma Score) were the main independent predictors of outcome. The 5-point Coma Acidosis Malaria (CAM) score was simply derived from these 2 variables. Mortality increased steadily with increasing score. A CAM score <2 predicted survival with a positive predictive value (PPV) of 95.8% (95% confidence interval [CI], 93%–97.7%). Of the 14 of 331 patients who died with a CAM score <2, 11 (79%) had renal failure and death occurred late after hospital admission (median, 108 h; range, 40–360 h). Substitution of plasma bicarbonate as the measure of acidosis only slightly reduced the prognostic value of the model. Use of respiratory rate was inferior, but a score <2 still predicted survival with a PPV of 92.2% (95% CI, 89.1%–94.7%).
Conclusions
Patients with a CAM score <2 at hospital admission may be safely treated in a general ward, provided that renal function can be monitored.
Severe Plasmodium falciparum malaria is associated with high mortality. It is usually defined according to World Health Organization (WHO) criteria [1], which have been derived from several large studies [2-4] and expert opinion [5]. The criteria were devised to identify patients who would benefit from intensive monitoring and parenteral antimalarial treatment; WHO guidelines recommend that patients satisfying these criteria should be treated in an intensive care unit (ICU) [6]. This is logical because the majority of deaths among patients with malaria occur within the first 48 h after their hospital admission, and prompt delivery of supportive care at this stage could be life saving [6, 7]. Precise fluid management and mechanical ventilation is best delivered in an ICU, and closer patient monitoring facilitates earlier renal replacement therapy [8] and the identification and treatment of complications, such as hypoglycemia and concomitant bacterial infection [9]. Indeed, mortality associated with severe adult malaria treated with parenteral quinine, which is no longer the recommended first-line treatment, was only 11% in a well equipped ICU in France [10], whereas in less-well–equipped hospitals in areas of endemicity, mortality is 15%–40% [5, 11]. Delays in referral to a hospital and, specifically, to the ICU of patients with malaria and other severe infections have been associated with increased mortality [12-14].
Although ICU facilities are becoming increasingly available in malarious regions, strict triage for admission to them must be applied because their capacity is usually severely limited. The inclusive WHO definition for severe malaria is too broad of a triage tool, because individual criteria differ substantially in their predictive value for mortality [15, 16]. Although mortality associated with severe malaria remains high, even with considerable improvements in antimalarial treatment [11], death is unusual among patients presenting with prostration, jaundice, or anemia as the sole defining severity criterion.
In resource-rich settings, tools have been developed to improve triage to ICU [17-19], and predictive scores are now a cornerstone of the management of a range of clinical conditions [20-24]. Efforts have been made to identify indicators of poor outcome of malaria, with some success among African children [25, 26]. However, noting that adults have different clinical manifestations and a greater risk of death, Mishra et al [27] derived an adult-specific predictive tool: the Malaria Severity Assessment (MSA) score. The MSA score was simple to use and had a reasonable predictive ability; however, it was derived at only a single location (Orissa, India), it was not applicable at admission to the hospital, and the authors expressed uncertainty with regard to its generalizability to other settings.
To address these issues, we reanalyzed data from the SEAQUAMAT study (the largest ever clinical trial involving adults with severe malaria) performed in southern and Southeast Asia [11]. Our aim was to derive a reliable, simple, and inexpensive predictive score that would assist clinicians in identifying adult patients with malaria who were at high risk of death and that might serve as an indicator for ICU referral. The derived scoring system was then tested on 2 other large datasets of adult patients with severe P. falciparum malaria.
METHODS
Patients
A logistic regression model was built to assess the probability of death with use of data from the SEAQUAMAT study, a trial that compared parenteral artesunate with quinine for the treatment of severe malaria [11]. The diagnosis of malaria was confirmed by examination of peripheral blood samples from patients, and 1050 patients fulfilled the modified WHO criteria for severe malaria: namely, cerebral malaria (Glasgow Coma Score [GCS] <11), shock (low blood pressure and cool peripheries), acidosis (plasma bicarbonate level <15 mmol/L), severe anemia (hematocrit, <20%; P. falciparum parasite level, >100,000 parasites/μL), visible jaundice and P. falciparum parasitemia (parasite level, >100,000 parasites/μL), renal failure (blood urea nitrogen level, >17 mmol/L), asexual P. falciparum parasitemia (parasite percentage, >10%), plasma glucose level <2.2 mmol/L, and respiratory distress. Because the clinical manifestations of severe malaria are different in children [5, 15], patients aged <16 years (n = 182) were excluded from our prediction model. The model’s predictive ability was subsequently validated using 2 independent datasets in which patients had been defined as having severe malaria with use of similar criteria to those used in the SEAQUAMAT trial. The first consisted of patients from Bangladesh who were enrolled in studies evaluating the efficacy of n-acetylcysteine [28] and levamisole (study ongoing; n = 193) as adjunctive therapy to intravenous artesunate. The second was from a trial from Vietnam (n = 549) that compared the efficacy of artemether with that of quinine [2].
Statistical methods
With use of fatal outcome as the dependent variable, the initial model was built from the SEAQUAMAT data, including all variables used by the WHO to identify severe malaria [1] as potential predictors (ie, independent variables): prostration, GCS, respiratory distress, convulsions, shock (systolic blood pressure, <80 mm Hg, plus cool extremities), acidosis (using base deficit), abnormal bleeding, jaundice, hemoglobinuria, hematocrit, glucose level, and (log) parasite level. Acute renal failure is defined by the WHO criteria as a serum creatinine level >3 mg/dL (250 μmol/L); however, because creatinine level was not measured in the study, blood urea nitrogen level >17 mmol/L was substituted, as was done in the SEAQUAMAT trial [11]. Hyperlactatemia and radiological evidence of pulmonary edema were not included in the model, because they were not recorded systematically in the trial. Pregnancy status, plasma sodium concentration, and age (as a continuous variable) were also included in the initial model, because these variables have been shown to be associated with poor outcomes [15, 29, 30]. Because the data were collected in 4 different countries, the dependency of the model mortality prediction on study site was also assessed. Antimalarial treatment with artesunate or quinine had a major impact on survival in the trial and was included in the model.
To determine which of the independent variables had significant prognostic value, we used a backward stepwise approach, specifying that only variables with P < .05 should be retained in the model. Appropriate fit of the final model was confirmed using the Hosmer-Lemeshow goodness-of-fit test after grouping the data by predicted probabilities of death into 10 approximately equal-sized groups [31]. After the remaining significant variables were identified, a simplified scoring system was devised. The score’s predictive ability was assessed using area under the receiver operating characteristic curve (AUROC) analysis and the positive predictive value (PPV) for survival. The scoring system was then validated in 2 independent datasets from Bangladesh [28] and Vietnam [2]. The score was compared with the previously published MSA score to determine its relative utility [27], although complete data regarding mechanical ventilation were not available for the validation datasets, precluding comparison in these groups. The MSA score is defined as 1 × (severe anemia [hemoglobin level, <5 g/dL]) + 2 × (acute renal failure [creatinine level, > 3mg/dL]) + 3 × (respiratory distress, requiring mechanical ventilation) + 4 × (cerebral malaria [GCS <11]), in which each variable was scored as 0 or 1, depending on its absence or presence, respectively.
Analyses were conducted with Stata software, version 10 (Stata). Ethical approval for all studies was obtained from the Oxford Tropical Medicine Research Ethical Committee and the respective national or institutional ethical committees in Bangladesh, India, Myanmar, Indonesia, and Vietnam.
RESULTS
The modified WHO criteria for severe malaria were fulfilled by 868 patients ≥16 years of age from the SEAQUAMAT study. Five independent predictors of death were identified by the model: artesunate therapy (odds ratio [OR], 0.46; 95% confidence interval [CI], 0.30–0.70), GCS (OR, 0.8; 95% CI, 0.75–0.85), base deficit (OR, 0.85; 95% CI, 0.82–0.88), hematocrit (OR, 1.03; 95% CI, 1.00–1.05), and blood urea nitrogen level (OR, 1.01; 95% CI, 1.00–1.01). Artesunate is now the recommended therapy for adults with severe malaria and was therefore not included in the prediction score.
For the 2 clinical variables with the largest effect on outcome (coma depth and acidosis), cutoff values were chosen on the basis of the reported literature and of the “roundness” of the numbers to facilitate recall. Cutoff values for coma depth were a GCS ≤14 (obtunded to deranged) and GCS ≤10 (comatose to very deranged). Cutoff values for acidosis were a base deficit >2 (deranged) and ≥10 (very deranged) [32]. A score of 0 for normal, 1 for deranged, or 2 for very deranged was ascribed to each of the 2 variables and then summed to give a simple Coma Acidosis Malaria (CAM) score ranging from 0 to 4 (Table 1).
Table 1. Derivation of the Coma Acidosis Malaria (CAM) Score, Bicarbonate-Based CAM Score and Respiratory Rate–Based CAM Score (Assessed at Hospital Admission).
| Variable | Score | ||
|---|---|---|---|
| 0 (normal) | 1 (deranged) | 2 (very deranged) | |
| Base deficit | <2 | 2 to <10 | ≥10 |
| GCS | 15 | >10 to 14 | ≤10 |
| Bicarbonate score | ≥24 | 15 to <24 | <15 |
| Respiratory rate score | <20 | 20 to <40 | ≥40 |
NOTE. CAM score (0–4) is calculated as the base deficit score (0–2) plus the Glasgow Coma Score (GCS; 0–2). Bicarbonate-based CAM score (0–4) is calculated as the bicarbonate score (0–2) plus the GCS (0–2). Respiratory rate–based CAM score (0–4) is calculated as the respiratory score (0–2) plus the GCS score (0–2).
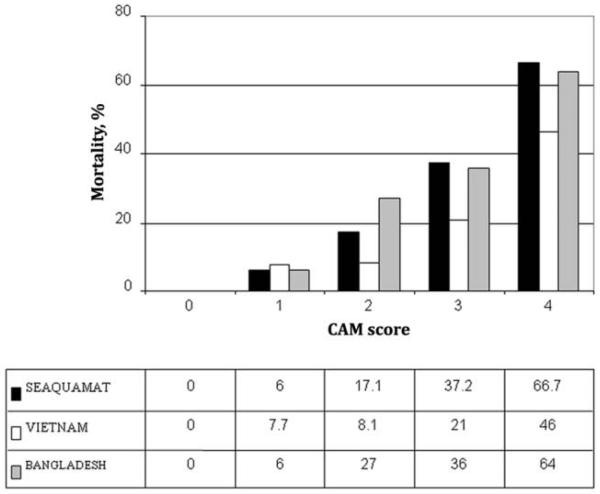
In the SEAQUAMAT dataset, a CAM score could be determined in 789 patients, because 79 patients did not have a base deficit measured at hospital admission. The final AUROC, using fatal outcome as the dependent variable, was 0.81 (95% CI, 0.77–0.84). If the analysis was restricted to only patients who received artesunate, the AUROC was 0.82 (95% CI, 0.78–0.87). As the CAM score increased, mortality steadily increased (P < .001) (Figure 1 and Table 2); this was similar at all 4 study sites. Of 225 patients with low CAM scores (<2), only 8 (3.6%) died (PPV for survival, 96.4%; 95% CI, 93.1–98.5) (Table 3). Results were similar at all study sites. The median time from hospital admission to death among these 8 patients was 96 h (range, 72–360 h). Of these 8 patients, 6 (75%) had renal failure, and only 1 of these 6 was able to receive dialysis. Of the 2 patients without renal failure, pregnancy was a risk factor for one, whereas the other patient had no additional risk factors identified at hospital admission.
Figure 1.
Mortality among adult patients with severe malaria who were enrolled in the 3 trials, by Coma Acidosis Malaria (CAM) score.
Table 2. Case Fatality Rate among Patients with Severe Malaria, by Coma Acidosis Malaria (CAM) Score, Bicarbonate-Based CAM (BCAM) Score and Respiratory Rate–Based CAM (RCAM) Score at Hospital Admission in the Validation and Derivation Series.
| Score, study, outcome | No. (%) of patients, by score | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Total | |
| CAM | ||||||
| SEAQUAMAT | ||||||
| Survived | 91 | 126 (94) | 209 (83) | 113 (63) | 44 (33) | 583 (74) |
| Died | 0 | 8 (6) | 43 (17) | 67 (37) | 88 (67) | 206 (26) |
| Vietnam | ||||||
| Survived | 21 | 60 (92) | 79 (92) | 64 (79) | 21 (54) | 245 (84) |
| Died | 0 | 5 (7.7) | 7 (8.1) | 17 (21) | 18 (46) | 47 (16) |
| Bangladesh | ||||||
| Survived | 4 | 15 (94) | 29 (73) | 45 (64) | 21 (36) | 114 (61) |
| Died | 0 | 1 (6) | 11 (27) | 25 (36) | 37 (64) | 74 (39) |
| All | ||||||
| Survived | 116 | 201 (89) | 317 (84) | 222 (67) | 86 (38) | 942 (74) |
| Died | 0 | 14 (11) | 61 (16) | 109 (33) | 143 (62) | 327 (26) |
| BCAM scorea | ||||||
| SEAQUAMAT | ||||||
| Survived | 52 (98) | 149 (95) | 172 (85) | 158 (67) | 44 (34) | 575 |
| Died | 1 (2) | 8 (5) | 31 (15) | 77 (33) | 86 (66) | 203 |
| Bangladesh | ||||||
| Survived | 1 | 18 (95) | 18 (69) | 58 (70) | 23 (36) | 118 |
| Died | 0 | 1 (5) | 8 (31) | 25 (30) | 41 (64) | 75 |
| RCAM score | ||||||
| SEAQUAMAT | ||||||
| Survived | 26 (93) | 170 (92) | 157 (77) | 260 (66) | 24 (44) | 637 |
| Died | 2 (7) | 15 (8) | 48 (23) | 134 (34) | 31 (56) | 230 |
| Vietnam | ||||||
| Survived | 1 | 131 (92) | 95 (83) | 216 (85) | 25 (71) | 468 |
| Died | 0 | 11 (8) | 20 (17) | 39 (15) | 10 (29) | 80 |
| Bangladesh | ||||||
| Survived | 1 | 16 (94) | 23 (82) | 65 (57) | 12 (43) | 117 |
| Died | 0 | 1 (6) | 5 (18) | 50 (43) | 16 (57) | 72 |
Table 3. Predictive Value of a Low Coma Acidosis Malaria (CAM) Score, Bicarbonate-Based CAM (BCAM) Score and Respiratory Rate–Based CAM (RCAM) Score (<2) for Survival in the Derivation Series by Country and in the Validation Series.
| Score, study | PPV, % (95% CI) | No. of deaths/no. of patients with a low score |
|---|---|---|
| CAM | ||
| SEAQUAMAT | ||
| Myanmar | 98.1 (90–100) | 1/53 |
| Bangladesh | 93 (83–98) | 4/57 |
| India | 100 (87.2–100) | 0/27 |
| Indonesia | 96.6 (90.4–99.3) | 3/88 |
| Total | 96.4 (93.1–98.5) | 8/225 |
| Vietnam | 94.2 (87–98.1) | 5/86 |
| Bangladesh | 95 (75–99.9) | 1/20 |
| BCAMa | ||
| SEAQUAMAT | 95.7 (92–98) | 9/210 |
| Bangladesh | 95 (75.1–99.9) | 1/20 |
| RCAM | ||
| SEAQUAMAT | 92.6 (88.4–95.6) | 17/213 |
| Vietnam | 92.3 (86.7–96.1) | 11/143 |
| Bangladesh | 94.4 (72.7–99.9) | 1/18 |
For comparison, we calculated an MSA score, which was possible for 805 of the 868 patients in the SEAQUAMAT dataset (Table 4). Because serum creatinine level was not measured, we used the adapted definition of acute renal failure at hospital admission (blood urea nitrogen level, >17 mmol/L) [11]. Among patients who had both scores calculated (n = 772), with use of ROC analysis, the AUROC for the MSA score in predicting death was 0.75 (95% CI, 0.72–0.79), which was significantly lower than that for the CAM score (0.81; 95% CI, 0.77–0.84; P = .006)
Table 4. Prognostic Capacity of the Coma Acidosis Malaria (CAM) Score for Mortality among Patients with Severe Malaria in the Derivation Series, Compared with the Malaria Severity Assessment (MSA) Score.
| Study location, score | AUROC (95% CI) | No. of patients |
|---|---|---|
| Myanmar | ||
| CAM | 0.81 (0.74–0.87) | 199 |
| MSA | 0.75 (0.68–0.82) | 220 |
| Bangladesh | ||
| CAM | 0.77 (0.72–0.82) | 306 |
| MSA | 0.71 (0.65–0.77) | 301 |
| India | ||
| CAM | 0.8 (0.72–0.88) | 119 |
| MSA | 0.78 (0.69–0.87) | 119 |
| Indonesia | ||
| CAM | 0.82 (0.73–0.91) | 165 |
| MSA | 0.75 (0.64–0.86) | 165 |
| Alla | ||
| CAM | 0.81 (0.77–0.84) | 789 |
| MSA | 0.75 (0.72–0.79) | 805 |
Validation series
Mortality was lower in the Vietnamese series than in the other series, likely because of the better level of ICU care available at this site. The CAM score could be calculated for 292 of 549 patients at this site; 257 did not have a base deficit recorded at hospital admission (there was no statistically significant difference in the age, sex, or outcome between the patients who did and did not have a base deficit recorded). The CAM score had an AUROC of 0.74 (95% CI, 0.67–0.82) for predicting mortality. Mortality steadily increased as the score increased (Table 2 and Figure 1). The PPV for survival with a CAM score <2 was 94.2% (95% CI, 87.0%–98.1%) (Table 3). A total of 5 (5.8%) of 86 patients with a low CAM score died a median of 120 h (range, 40–151 h) after hospital admission; of whom 4 (80%) of these 5 patients had renal failure (one of whom could not receive dialysis). The remaining patient developed gastrointestinal bleeding.
In the Bangladeshi series, 188 of 193 patients had a CAM score calculated (5 lacked a base deficit measurement at hospital admission). The mortality rate for each tier of the CAM score was similar to the rates in the SEAQUAMAT series (Table 2 and Figure 1). The PPV of a CAM score <2 for survival was 95% (95% CI, 75.1%–99.9%) (Table 3). Only 1 (5%) of 20 patients with a low CAM score died; this patient presented with renal failure, developed pulmonary edema, and died 96 h after hospital admission. The patient was not able to receive dialysis.
Bicarbonate and respiratory CAM scores
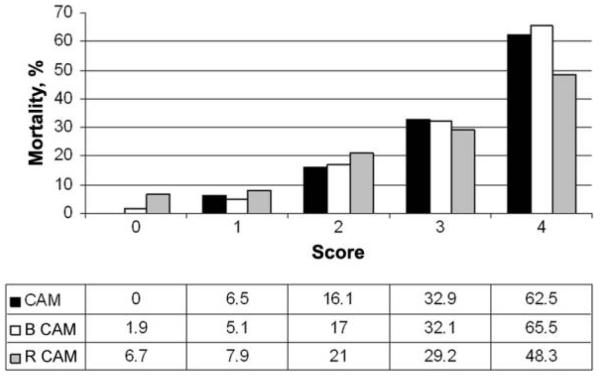
Because a base deficit measurement is not always available, we assessed the usefulness of a modified CAM score using plasma bicarbonate level as the marker of acidosis (BCAM) for patients in SEAQUAMAT (n = 778); we used cutoff values of ≥24 mmol/L (score, 0) for normal, <24 mmol/L but ≥15 mmol/L (score 1) for deranged, and <15 mmol/L (score, 2) for very deranged to generate the acidosis component of the CAM score (Table 1). As the BCAM score increased, mortality increased (Table 3 and Figure 2). The AUROC for the BCAM score as a predictor for mortality was 0.79 (95% CI, 0.76–0.82), which was similar although mildly inferior to the AUROC obtained for the CAM score (P = .04). A BCAM score <2 predicted 201 of 210 surviving patients in the SEAQUAMAT dataset (PPV, 95.7%; 95% CI, 92.0%–98.0%) (Table 3). When the SEAQUAMAT data were pooled with data from the validation series, a BCAM score <2 predicted 220 of 230 surviving patients (PPV, 95.7%; 95% CI, 92.1%–97.9%), very similar to the CAM score using base deficit (Table 5).
Figure 2.
Mortality among adult patients with severe malaria according to the base deficit based Coma Acidosis Malaria (CAM) score and the bicarbonate-based (BCAM) and respiratory rate–based (RCAM) score. Data were pooled from all 3 studies.
Table 5. Overall Performance of the Three Scores as a Predictor of Survival (Data from Derivation and Validation Series Pooled).
| Score | PPV, % (95% CI) | No. of deaths/no. of patients with a low score |
|---|---|---|
| CAM | 95.8 (93–97.7) | 14/331 |
| BCAM | 95.7 (92.1–97.9) | 10/230 |
| RCAM | 92.2 (89.1–94.7) | 29/374 |
NOTE. BCAM, bicarbonate-based Coma Acidosis Malaria (CAM); CI, confidence interval; PPV, positive predictive value; RCAM, respiratory rate–based CAM.
Respiratory rate in the 3 datasets showed a weak correlation with plasma base deficit (rs = −0.2; P < .001). Using respiratory rate as a surrogate marker for acidosis, a respiratory rate–based CAM (RCAM) score was calculated for patients in the SEAQUAMAT dataset (n = 867). The cutoffs values were <20 breaths/min (score, 0) for normal, 20–39 breaths/min (score, 1) for deranged, and ≥40 breaths/min (score, 2) for very deranged (Table 1). As the RCAM score increased, mortality increased (Table 3 and Figure 2). The AUROC for the RCAM score as a predictor for mortality was 0.68 (95% CI, 0.64–0.71), which was significantly inferior to the CAM score (P < .001). An RCAM score <2 predicted 196 of 213 surviving patients in the SEAQUAMAT dataset (PPV, 92.6%; 95% CI, 88.4%–95.6%) (Table 3). When the SEAQUAMAT data were pooled with data from the validation series, a low RCAM score predicted 345 of 374 surviving patients (PPV, 92.4%; 95% CI, 89.3%–94.9%) (Table 4).
DISCUSSION
The present study describes a simple prediction score for adult patients with severe P. falciparum malaria who are derived from the largest available series of prospectively studied patients with severe malaria. The score was developed using the multinational SEAQUAMAT trial conducted in Asia and was validated in 2 additional, large prospectively gathered datasets from Vietnam and Bangladesh. The 5-point CAM score uses only a patient’s GCS and the plasma base deficit and has strong predictive value for mortality. The CAM score is especially useful for identifying patients with a good prognosis who, therefore, do not require ICU care if this resource is scarce. In the absence of good ICU facilities, as at the SEAQUAMAT trial sites, a CAM score <2 identified 96.4% (95% CI, 93.1%–98.5%) of the surviving patients.
Of the 14 patients who died despite having a low CAM score, 11 (79%) had acute renal failure. Because of limited availability in the settings where they were treated, only 4 patients (36%) received dialysis. This emphasizes the importance of dialysis in malaria-associated renal failure; without dialysis, mortality is ~70% [8]. In all 3 series, only 1 patient (0.08%) with a CAM score <2 died during the first 3 days of hospitalization. This implies that, if patients with a low CAM score are admitted to a general ward, there is time to identify renal failure by either measurement of urine output or, clearly preferable because nonoliguric renal failure may also occur, biochemical testing of sequential blood samples.
Base deficit has been identified as the single best clinical or laboratory of fatal outcome in adults with severe malaria [16]; however, its measurement requires appropriate laboratory facilities. Point-of-care biochemical analyzers that require a minimum of maintenance and can be used at costs comparable to those for commercial laboratories are now available. However, if the base deficit is unavailable, the substitution of less specific markers for metabolic acidosis—namely, BCAM score or RCAM score—provide useful information. Plasma bicarbonate level is routinely measured by automated analyzers; however, although the BCAM score was slightly inferior to the CAM score, its clinical usefulness in identifying patients at low risk was similar (Table 3). The RCAM score was inferior in its ability to predict outcome, but a score <2 still had a PPV for survival of 92.2% (95% CI, 89.3%–94.9%). The entirely clinical and rapidly calculable RCAM score may complement more rigorous assessment by the treating physician in a resource-poor setting. Evidently, an elevated respiratory rate may indicate not only acidosis but also pulmonary complications.
As a triage system, this simple scoring system has a significant advantage over the WHO definition of severe malaria, which comprises 9 clinical and 6 laboratory markers [1]. Admission of all patients fulfilling one of these criteria to an ICU is not feasible in any country where malaria is endemic. Some of the criteria have relatively little prognostic significance and may divert the attention of the clinician from assessing for metabolic acidosis, which is the single strongest prognostic indicator [16] and is frequently overlooked. The CAM score can provide a reliable triage mechanism for patients with malaria. When applied to our series, it would have led to a 26% (range, 11%–29%) absolute reduction in unnecessary, expensive ICU admissions.
The CAM score improves on the previously described MSA score, which also has the disadvantage that 1 of the score’s 4 variables, mechanical ventilation, cannot be assessed at hospital admission. Despite its simplicity, the AUROCs achieved using the CAM score (0.8–0.83 in the derivation series and 0.71–0.74 in the validation series) were similar to or better than the AUROCs generated by predictive scores that are widely used for community-acquired pneumonia (0.68–0.75) [33-35], ischemic heart disease (0.63–0.66) [23], atrial fibrillation (0.63–0.7) [24], cerebrovascular disease (0.6–0.79) [25], and pulmonary embolism (0.75) [36]. The CAM score also performs well when compared with a scoring system devised for general medical admissions (0.67–0.72) [37]. Our results are supported by other studies describing prognostic variables in severe malaria. Coma and acidosis (or manifestations related to acidosis, such as elevated lactate level, respiratory distress, and renal impairment) have been previously reported as prominent risk factors for death associated with severe malaria [25-27, 38].
The CAM score should not be used in isolation from clinical evaluation of the patient. Independent indications for ICU admission, such as refractory shock, respiratory insufficiency, or persistent hypoglycemia may arise, although in our large dataset, these complications were not present in the absence of acidosis or coma. Although the central underlying pathophysiology in pediatric and adult severe malaria is probably similar, overall mortality in pediatric severe malaria is lower (~10%) [5, 15]. Although acidosis, respiratory distress, and impaired consciousness have been established as the strongest prognostic factors in series of children suffering from severe malaria in Africa [25, 26], the CAM score was derived for adults in a low-transmission setting in Asia. It is therefore not validated for pediatric severe malaria in high-transmission settings.
In conclusion, the CAM score provides a simple scoring system that can identify adults with a good prognosis with severe malaria. The score can be used as a triage tool for ICU admission.
Acknowledgments
We thank the directors and staff of the trial hospitals and the doctors, research nurses, and research assistants, for their help; Dr Marlar Than, who coordinated the SEAQUAMAT study in Myanmar; and Dr Richard Maude and Dr Sam Douthwaite, who collected and collated data in Bangladesh.
Financial support. Wellcome Trust of Great Britain to the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.World Health Organization . Guidelines for the treatment of malaria. World Health Organization; Geneva: 2006. [Google Scholar]
- 2.Tran TH, Day N, Nguyen H, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 3.Warrell DA, Looareesuwan S, Warrell MJ, et al. Dexamethasone proves deleterious in cerebral malaria: a double blind trial in 100 comatose patients. N Engl J Med. 1982;306:313–319. doi: 10.1056/NEJM198202113060601. [DOI] [PubMed] [Google Scholar]
- 4.Hien TT, Phu NH, Mai NTH, et al. An open randomized comparison of intravenous and intramuscular artesunate in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:584–585. doi: 10.1016/0035-9203(92)90138-3. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- 6.World Health Organization . Management of severe malaria: a practical handbook. 2nd ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 7.Dondorp AM, Day NP. The treatment of severe malaria. Trans R Soc Trop Med Hyg. 2007;101:633–634. doi: 10.1016/j.trstmh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15:874–880. doi: 10.1093/clind/15.5.874. [DOI] [PubMed] [Google Scholar]
- 9.Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: severe malaria. Crit Care. 2003;7:315–323. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruneel F, Hocqueloux L, Alberti C, et al. The clinical spectrum of severe imported falciparum malaria in the intensive care unit. Am J Respir Crit Care Med. 2003;167:684–689. doi: 10.1164/rccm.200206-631OC. [DOI] [PubMed] [Google Scholar]
- 11.Dondorp A, Nosten F, Stepniewska K, et al. Artesunate versus quinine for the treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 12.Woodhead M, Welch CA, Harrison DA, et al. Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care. 2006;10(Suppl 2):S1. doi: 10.1186/cc4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapoport J, Teres D, Lemeshow S, Harris D. Timing of intensive care unit admission in relation to ICU outcome. Crit Care Med. 1990;18:1231–1235. doi: 10.1097/00003246-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Webb S, Evans M, Batson S, et al. Intensive care for severe falciparum malaria: a retrospective study of clinical course and outcome of 53 cases managed in a London teaching hospital. BJA. 2000;84:664. [Google Scholar]
- 15.Dondorp AM, Lee SJ, Faiz MA, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47:151–157. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 16.Day NPJ, Phu NH, Mai NT, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000;28:1833–1840. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Mirghani HM, Hamed M, Ezimokhai M, Weerasinghe DS. Pregnancy-related admissions to the intensive care unit. Int J Obstet Anesth. 2004;13:82–85. doi: 10.1016/j.ijoa.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Hudson S, Boyd O. Criteria for admission to the ICU and scoring systems for severity of illness. Surgery. 2007;25:117–121. [Google Scholar]
- 19.Hargrove J, Nguyen HB. Bench-to-bedside review: outcome predictions for critically ill patients in the emergency department. Crit Care. 2005;9:376–383. doi: 10.1186/cc3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WS, Van Der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 22.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 23.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 25.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 26.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–459. [PubMed] [Google Scholar]
- 27.Mishra SK, Panigrahi P, Mishra R, Mohanty S. Prediction of outcome in adults with severe falciparum malaria: a new scoring system. Malar J. 2007;6:24. doi: 10.1186/1475-2875-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charunwatthana P, Abul Faiz M, Ruangveerayut R, et al. N-acetylcysteine as adjunctive treatment in severe malaria: a randomized, double-blinded placebo-controlled clinical trial. Crit Care Med. 2009;37:516–522. doi: 10.1097/CCM.0b013e3181958dfd. gelatinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anya SE. Seasonal variation in the risk and causes of maternal death in the Gambia: malaria appears to be an important factor. Am J Trop Med Hyg. 2004;70:510–513. [PubMed] [Google Scholar]
- 30.Hanson J, Hossain A, Charunwatthana P, et al. Hyponatremia in severe malaria: evidence for an appropriate anti-diuretic hormone response to hypovolemia. Am J Trop Med Hyg. 2009;80:141–145. doi: 10.4269/ajtmh.2009.08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stata manual. StataCorp; College Station, TX: 2009. [Google Scholar]
- 32.Abbott I-Stat product information. Abbott Laboratories; Princeton, NJ: 2004. [Google Scholar]
- 33.Man SY, Lee N, Ip M, et al. Prospective comparison of three predictive rules for assessing severity of community-acquired pneumonia in Hong Kong. Thorax. 2007;62:348–353. doi: 10.1136/thx.2006.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angus DC, Marrie TJ, Obrosky DS, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society diagnostic criteria. Am J Respir Crit Care Med. 2002;166:717–723. doi: 10.1164/rccm.2102084. [DOI] [PubMed] [Google Scholar]
- 35.Escobar GJ, Fireman BH, Palen TE, et al. Risk adjusting community-acquired pneumonia hospital outcomes using automated databases. Am J Manag Care. 2008;14:158–166. [PubMed] [Google Scholar]
- 36.Klok FA, Mos ICM, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168:2131–2136. doi: 10.1001/archinte.168.19.2131. [DOI] [PubMed] [Google Scholar]
- 37.Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 38.Krishna S, Waller DW, ter Kuile F, et al. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg. 1994;88:67–73. doi: 10.1016/0035-9203(94)90504-5. [DOI] [PubMed] [Google Scholar]