Abstract
Voltage-gated sodium (Nav) channels are intrinsic plasma membrane proteins that initiate the action potential in electrically excitable cells. They are a major focus of research in neurobiology, structural biology, membrane biology and pharmacology. Mutations in Nav channels are implicated in a wide variety of inherited pathologies, including cardiac conduction diseases, myotonic conditions, epilepsy and chronic pain syndromes. Drugs active against Nav channels are used as local anaesthetics, anti-arrhythmics, analgesics and anti-convulsants. The Nav channels are composed of a pore-forming α subunit and associated β subunits. The β subunits are members of the immunoglobulin (Ig) domain family of cell-adhesion molecules. They modulate multiple aspects of Nav channel behaviour and play critical roles in controlling neuronal excitability. The recently published atomic resolution structures of the human β3 and β4 subunit Ig domains open a new chapter in the study of these molecules. In particular, the discovery that β3 subunits form trimers suggests that Nav channel oligomerization may contribute to the functional properties of some β subunits.
Keywords: sodium channel β subunits, X-ray crystallography, ion channelopathies
2. Introduction
Electrically excitable cells such as neurons and myocytes communicate via action potentials, and voltage-gated sodium (Nav) channels play an essential role in this process (figure 1). The vertebrate Nav channel α subunit is a single polypeptide chain (molecular mass approx. 260 kDa) that contains the ion-selective component. There are 10 mammalian α subunit genes encoding the proteins Nav1.1–Nav1.9, and an atypical channel, Nax. Separate α subunit isoforms are expressed in tissue-specific patterns and exhibit differences in gating behaviour that tailor them for distinct physiological roles [1]. Each vertebrate Nav α subunit contains four homologous but non-identical domains (I–IV), each of which contains six transmembrane helical segments (S1–S6) (figure 2a). The domains assemble around the central ion-selective pore [2]. Evidence based on µ conotoxin GIIIA binding suggests that the domains may be organized in a clockwise orientation as viewed from above the extracellular surface [3].
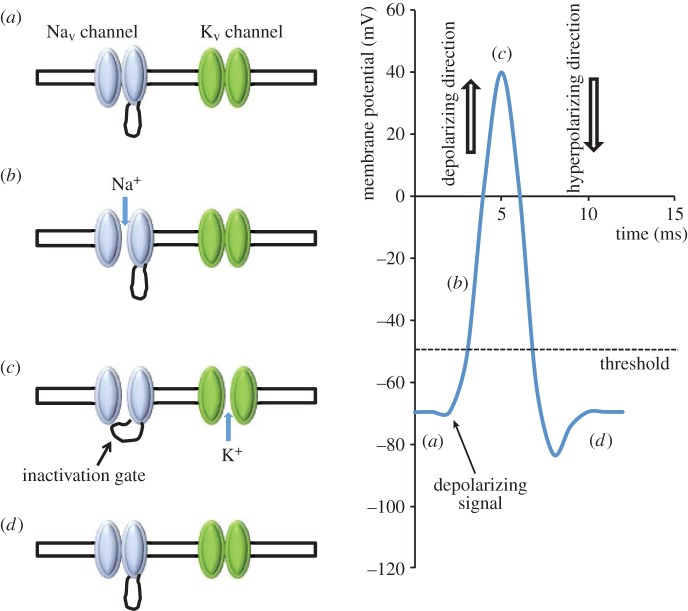
Figure 1.
A simplified cartoon showing the main events underlying the action potential. (a) Neurons and other electrically excitable cells maintain a plasma membrane resting potential of about −70 mV (the membrane potential is defined relative to the extracellular medium; a potential of −70 mV implies that the cell interior is negative relative to the exterior). The negative resting membrane potential is largely set by the greater membrane permeability of potassium ions compared with that of sodium ions. This occurs in the presence of a high intracellular potassium ion concentration relative to the extracellular media, and a high extracellular to intracellular sodium ion concentration generated by sodium potassium ATPase activity. Under resting conditions, both voltage-gated sodium (Nav) and voltage-gated potassium (Kv) channels are closed. The Nav channels begin to open in response to local membrane depolarization (generated for example by the action of an ionotrophic neurotransmitter on its receptor). (b) The net inward flow of sodium ions causes further depolarization that opens more Nav channels, which in turn causes even greater sodium ion entry and further depolarization. This positive feedback ensures the action potential generates a rapid ‘all or nothing’ response, whenever the initial stimulus is above a threshold value (here set at −50 mV). (c) In the ‘fast inactivation pathway’, sodium channels enter an inactive state after a few milliseconds, whereby they cannot respond to any further membrane depolarization signals. Fast inactivation is driven by a conformational change in which a hydrophobic sequence (the ‘inactivation gate’) lying within the cytoplasmic loop between domains III and IV moves to block the inner mouth of the pore (see text). As a result, the action potential can only be propagated in the forward direction. The subsequent opening of voltage-gated potassium (Kv) channels allows potassium ions to move out of the cell, down their electrochemical gradient. (d) This moves the membrane potential in the hyperpolarizing direction, restores the membrane to its resting potential and enables the Nav channels to recover from inactivation by returning to their closed conformation.
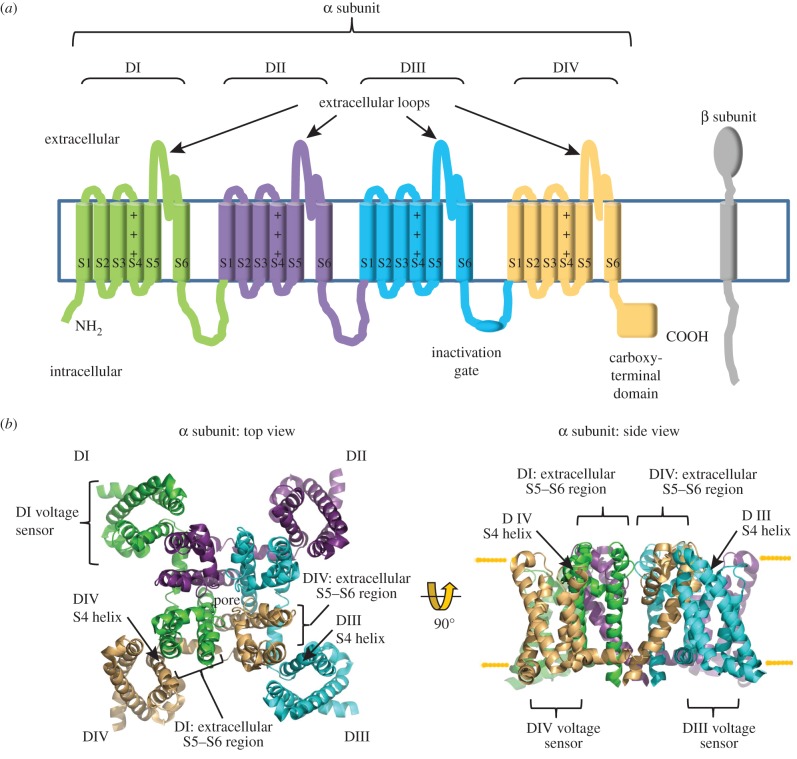
Figure 2.
The subunit organization of the voltage-gated Nav channel. (a) Cartoon topology of the α and β subunits. Within the α subunit, domains I–IV, the amphipathic helix S4 of the voltage sensors, the extracellular region between helices S5 and S6, the fast inactivation gate and the carboxy-terminus are shown. (b) Top view from the extracellular face and side view of the Nav channel from the bacterium Arcobacter butzeri (PDB ID code 3RVY). Using this structure as a guide, the inferred position of each vertebrate α subunit domain (I–IV) is shown colour-coded to match figure 2a. The location of the voltage sensors with the S4 helix for domains III and IV, and the likely position of the S5–S6 extracellular regions of domains I and IV, are indicated.
Currently, there are no high-resolution atomic structures of the complete vertebrate Nav channel α subunit. However, the crystal structures of bacterial Nav channels in different conformational states [4–6] (figure 2b) and a chimeric voltage-gated potassium (Kv) channel [7] have been solved. They have provided rich insights into the molecular basis of channel gating. All of the channels with solved structures are homotetramers rather than a single molecule, so they lack the sequence specializations found in the vertebrate Nav channel α subunit, such as the distinct extracellular regions, the intracellular linkers between domains and the unique carboxy-terminus (figure 2a). Nevertheless, the basic domain organization of the vertebrate Nav channel is likely to be similar to the subunit organization of the bacterial Nav channels and the Kv channel [8]. Using these high-resolution structures as guides, we can say that helices S5 and S6 and the pore loop regions that connect them form the ion-selective pore module. Helices S1–S4 of each domain comprise the voltage sensor and lie on the outer rim of the pore module, at each corner of the α subunit (figure 2b). Helix S4 of each voltage sensor is amphipathic, with one face positively charged (figure 2a,b). In response to the electric field changes produced by membrane depolarization, helix S4 moves towards the extracellular face of the membrane, thereby initiating conformational changes that open the pore [4,7]. The voltage sensors of domains I–III show the fastest kinetics and allow ion flow to begin [9]. The domain IV voltage sensor responds more slowly. Its movement frees an intracellular linker called the inactivation gate that connects helix S6 of domain III with helix S1 of domain IV (figure 2a) [10,11]. As a result, the inactivation gate can now move to occlude the pore and drive the channel into an inactivated state (figure 1). Thus, Nav channel activation and inactivation are structurally, mechanistically and functionally linked [8,12].
Most Nav channels isolated from vertebrate cells contain associated β subunits. There are four β subunit genes (Scn1b–Scn4b) encoding proteins β1–β4, respectively [13,14]. Alternative splicing of the Scn1b gene adds further diversity to the β1 protein [15]. As with the α subunits, individual β subunits are expressed with distinct tissue specificities [16]. All β subunits are type 1 intrinsic membrane proteins. The extracellular amino-terminal region contains a single V-type immunoglobulin (Ig) domain and a short ‘neck’. This is connected to a single α-helical transmembrane domain and a carboxy-terminal intracellular region (figure 2a). However, the primary sequences of the β1 and β3 subunits are more closely related to each other than either is to β2 or β4 [17]. The β1 and β3 subunits are non-covalently bound to the α subunit, but the β2 and β4 subunits are covalently attached to the α subunit via an inter-subunit disulfide bond [18,19].
The β subunits can increase peak current density by increasing the amount of channels in the plasma membrane [13]. They also shift the voltage range over which activation and inactivation occur, and they enhance the rates of inactivation and recovery from inactivation [20,21]. They therefore influence many of the key conformational changes that Nav channels undergo during the action potential cycle [13,16]. Although their gating effects can be subtle, they are clearly important. Mice lacking individual β subunits show a range of isoform-specific pathologies such as epilepsy, ataxia and cardiac conduction diseases. Mutations in the β subunits are associated with a number of human inherited diseases, including epilepsy, neuropathies, cardiac conduction diseases and some types of cancer [13,22].
Site-directed mutagenesis has been used to study the function of β subunits. But these experiments were interpreted using structures inferred from homology modelling [23–25], which is inevitably indirect. However, the structures of the human β3 and β4 subunit Ig domains have recently been solved at atomic resolution by X-ray crystallography [26,27], and should now allow for much clearer functional insights. In this review, we will focus on the new structural determinations, and consider their pathophysiological implications. For a more in-depth discussion of additional aspects of β subunit biology, especially the roles of these molecules in development, signal transduction and their potential as pharmacological targets, we recommend other recent reviews [13,16,22,28].
3. The structure of the Nav channel β3 subunit Ig domain and implications for the β1 structure
The X-ray structure of the human β3 Ig domain has been solved to a resolution of 2.5 Å. Surprisingly, it forms a trimer in the asymmetric unit (figure 3a). This is not a crystal packing artefact: super-resolution imaging detected trimeric full-length β3 subunits as a major species in the plasma membrane of HEK293 cells [27]. Moreover, atomic force microscopy (AFM) imaged full-length β3 subunit monomers, dimers and trimers isolated from transfected cells. The trimer is consistent with previous evidence showing that when expressed in cells, the full-length β3 subunits self-associate in cis, and that the Ig domain is necessary for this interaction [25].
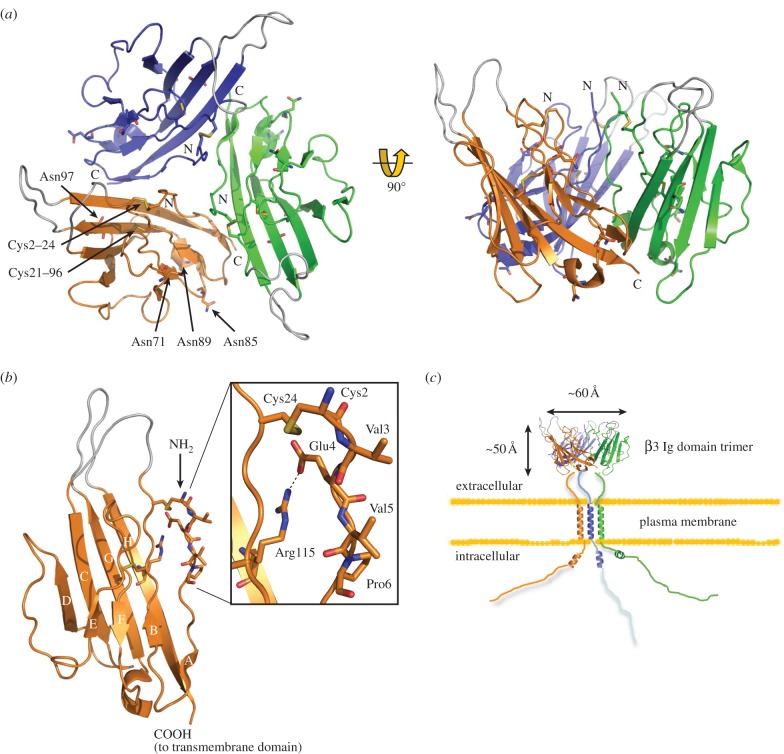
Figure 3.
The atomic resolution structure of the β3 subunit Ig domain (PDB ID code 4L1D). (a) Diagram showing the arrangement of the β3 trimer. Amino (N) and carboxyl (C) termini are labelled. The Cys21–96 disulfide bond and the Cys2–24 disulfide bonds are labelled on the orange protomer. Potential N-linked glycosylation sites Asn71, Asn85, Asn89 and Asn97 are shown as sticks and labelled for the orange protomer. Loops that are not resolved in the electron density maps due to local disorder are shown in grey at their most probable plausible positions. (b) Close-up of the single Nav β3 subunit Ig domain protomer. Residues correspond to loops not visible in the electron density maps due to local disorder are shown in grey. A close-up of the region surrounding the trimer interface is shown in the box. (c) Cartoon of the full-length β3 trimer as it may appear on the plasma membrane, with correct dimensions. Note: in these diagrams, the amino acids are numbered from the first residue of the mature protein (i.e. lacking the endoplasmic reticulum targeting signal) [27].
The Ig domain is one of the most common protein modules encoded by metazoan genomes, and is found in many immune system proteins and cell-adhesion molecules (CAMs) [29]. It is composed of a two-sheet sandwich of antiparallel β strands which adopt a Greek key topology encasing a disulfide bond-stabilized hydrophobic core. The number and location of the β strands can vary between Ig domains. Loop regions between the β strands often exhibit local disorder when examined in the context of the protein crystal, indicating inherent flexibility [30]. All of these structural features are present in the β3 Ig domain, including a buried disulfide bond Cys21–96 connecting the two faces. However, to promote trimerization, the β3 Ig domain also has a more unusual region of secondary structure. In most Ig domains, the amino-terminal amino acids are held in place by an antiparallel β strand [30]. This feature is absent in β3. Instead, the region is stabilized by a surface disulfide bond (Cys2–24) and a salt bridge between residues Glu4 and Arg115. Consequently, the hydrophobic amino acids Val3, Val5 and Pro6 are forced into a surface-exposed conformation that forms the core of the trimer interface (figure 3b). Replacing residue Cys24 with alanine in a C24A mutant prevents the formation of the Cys2–24 disulfide bond and reduces trimer stability, presumably because it interferes with the correct alignment of the trimer interface [27].
The α-helical transmembrane domain of the β3 subunit contains a conserved glutamic acid residue [23]. Peptides encoding transmembrane α-helices with a membrane-embedded glutamic acid readily form dimers and trimers, stabilized by hydrogen bonds between the protonated glutamic acid side chains [31]. We have therefore suggested that the transmembrane glutamic acid residue acts to further stabilize a full-length β3 trimer [27]. A cartoon of the proposed structure for the full-length β3 trimer is shown in figure 3c.
Residues between β3 and β1 that are identical in all species can be identified using evolutionary trace analysis [32]. In figure 4a,b, these amino acids are mapped onto the trimeric β3 Ig domain structure. Most of the trimer interface, including the Cys2 and Cys24 residues, is fully conserved between β3 and β1 [25], as are the surrounding amino acids that help to align and stabilize the trimer interface. This includes an unusual inward-pointing arginine residue (Arg100), whose mutation in β1 is associated with febrile epilepsy [27,34,35]. The β3 Ig domain can interact with β1 when the two are co-expressed in the same cell [25], suggesting that the trimer interface is functionally conserved between β3 and β1. Furthermore, the α-helical transmembrane domain of β1 includes the unusual membrane-buried glutamic acid residue noted above, and it occurs in the same location on the transmembrane domain as with β3 [23,27]. Hence, many of the key features implicated in β3 trimer stability are present in β1. All these factors taken together suggest that the β1 subunit may also assemble into a trimer in a manner broadly similar to β3.
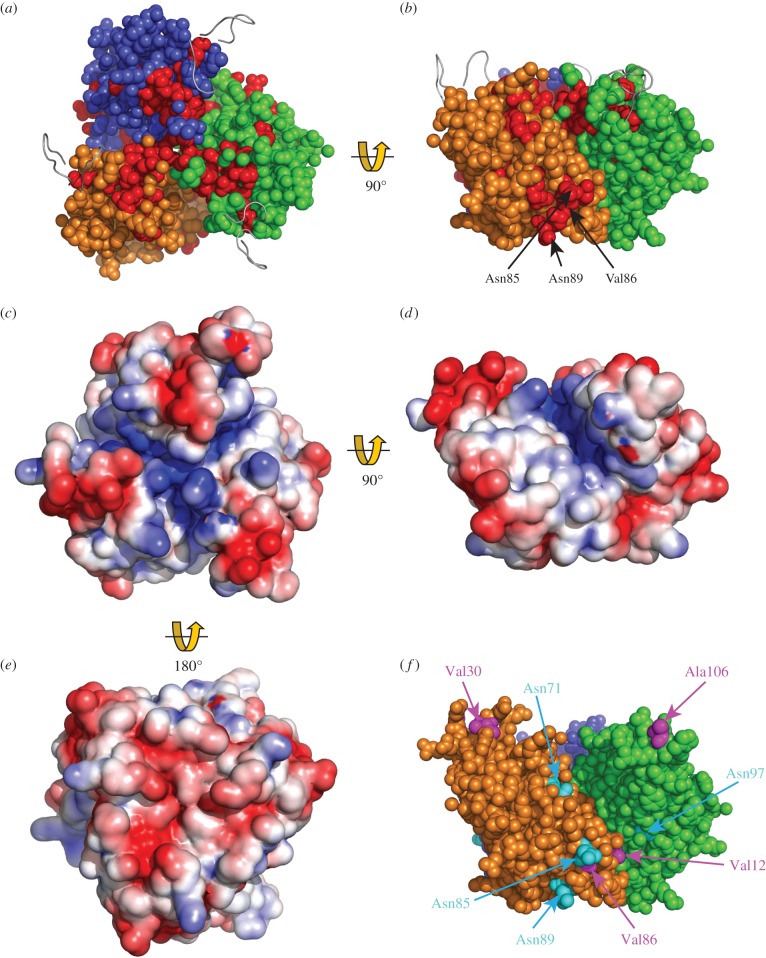
Figure 4.
Structural features of the β3 trimer. (a,b) Evolutionary trace analysis [25] showing residues in red that are identical between β3 and β1 in all species for which sequences are available, and mapped onto the β3 trimer in (a) top and (b) side view. The location of putative N-linked glycosylation sites Asn85 and Asn89, and the cardiopathology mutation V86 within a distinct patch of conserved residues (as mentioned in the text), are indicated for the orange protomer. Disordered loops are shown as grey ribbon. (c–e) Electrostatic surface potential (ESP) of β3 trimers in (c) top, (d) side and (e) bottom view. ESP was calculated for the trimer surface using the Adaptive Poisson-Boltzmann Solver (APBS) plug-in tool in PyMol [33]. (f) The side face of the trimer showing the location of N-linked glycosylation sites (cyan) and the location of separate cardiopathology mutations (purple) mentioned in the text.
4. Insights into Nav channel assembly and α subunit gating from the β3 Ig domain structure
A trimeric β3 structure implies that it may promote the oligomerization of up to three α subunits. Consistent with this, AFM imaging detected heterogeneous complexes containing the cardiac-specific α subunit Nav 1.5 cross-linked by β3 dimers and trimers. The frequency of these α subunit oligomers was reduced to the background level when the Nav 1.5 α subunit was co-expressed with the trimer-disrupting C24A β3 subunit mutant described above [27]. The C24A β3 mutant also attenuated the normal β3-induced gating shift in the voltage sensitivity of inactivation for Nav 1.5 α subunits [20]. Hence the oligomeric status of Nav 1.5 controlled by β3 can affect the electrophysiological properties of the channel.
The β3 subunit is expressed in the heart [36], and it plays an important role in cardiac physiology, because the major pathology associated with the Scn3b−/− mouse is spontaneous cardiac arrhythmia [37,38]. The Scn1b−/−mouse also displays cardiac conduction abnormalities [39]. Evidence that the β1 subunit, like its homologue β3, can cross-link Nav 1.5 α subunits is provided by an interesting study by Mercier et al. [40]. Here an Nav 1.5 α subunit mutant associated with the cardiac conduction disease Brugada syndrome (BrS) was retained in the endoplasmic reticulum of cardiomyocytes, leading to reduced peak current. The mutation was also associated with reduced surface expression of the wild-type α subunit, but only if mutant and wild-type α subunits were co-expressed together with β1. Other 1.5 α subunit mutations linked to BrS show similar dominant negative behaviour when co-expressed with the wild-type α subunit in cardiomyocytes [41–44]. Dominant negative phenotypes can often be explained in molecular terms when mutant and wild-type subunits are jointly assembled into a multi-subunit complex, but then every complex that contains any mutant subunit is functionally inhibited. As a result, heterozygous individuals expressing one mutant and one wild-type copy of a protein display a much greater than 50% reduction of the functional protein activity [45]. We suggest that, in vivo, individual Nav 1.5 α subunits do not behave as independent molecules, but as oligomeric complexes in which multiple α subunits co-assemble, and which the β3 and β1 subunits may help stabilize. This may help explain why inherited Nav channel cardiopathologies often behave as autosomal dominant phenotypes in pedigree analysis studies [46].
In many—although by no means all—cell-expression systems, the β3 and β1 subunits shift the half maximal voltage V1/2 for channel activation and inactivation by up to 15 mV in the hyperpolarizing direction (i.e. the voltages corresponding to activation or inactivation of half the channels are displaced to more negative values compared to corresponding values shown by the α subunit alone; the action potential threshold therefore assumes a more negative voltage, leading to an increased likelihood of firing; figure 1). With α subunit partners such as Nav 1.2, 1.3 and 1.5, Ig domains of β3 and β1 are largely responsible for these gating shifts [20,21,23,24,47]. To understand how they might influence gating, we need to consider the structure and location of the β subunit binding site(s) on the α subunits. Unfortunately, the detailed answer to this question is uncertain; even the α : β stoichiometry is not clear. Although the conventional view assumes that individual β subunits bind α subunits at a single site [16], there is no over-riding reason to think that this is correct in all cases. In fact, AFM images show that β3 can bind to Nav 1.5 α subunits at up to four locations around the α subunit [27], probably corresponding to sites on each of the four α subunit domains. On the other hand, the pseudo-symmetry of the vertebrate α subunit domains means that there is no requirement for each binding site to be thermodynamically or structurally equivalent. These experiments were also conducted with a high level of β3 expression, and without the presence of other β subunits, so not all of the potential sites may be occupied by β3 in neurons or myocytes. Nevertheless, they establish the fact that at least under some conditions, a given type of β subunit can bind to the α subunit at multiple locations.
There is currently no molecular information concerning the β3 Ig domain binding site(s) on the α subunit. However, given the structural similarity between the β1 and β3 subunit Ig domains (figure 4a,b), it is at least plausible that these two closely related β subunits may bind similar or overlapping sites. The intracellular regions of both β1 and β3 do indeed bind to similar sites on the Nav 1.5 α subunit carboxy-terminus [48]. This would indicate that both β1 and β3 can interact with the α subunit close to domain IV (figure 2a). Interestingly, the local anaesthetic lignocaine binds to the S6 helix of domain IV [49], and both β3 and β1 attenuate lignocaine binding to Nav 1.3 [50]. A binding site for the β1 Ig domain has been localized to the domain IV extracellular S5–S6 region of the brain-type α subunit Nav 1.2 [51]. In a separate study, two β1-binding sites on the muscle Nav 1.4 α subunit were mapped to the extracellular regions connecting the S5–S6 helices of domain I and domain IV (figure 2a) [52]. If the bacterial Nav channel structures accurately reflect the topology of the vertebrate Nav channel, then these two β1 binding sites probably lie about 30–40 Å from each other (figure 2b). Since the three-dimensional structure of these α subunit extracellular regions is currently unknown, it is not yet possible to say whether they extend far enough to form a joint binding site. Assuming a top-view clockwise arrangement of the α subunit domains [3], the S5–S6 extracellular region of domain I lies closest to the voltage sensor of domain IV, while the S5–S6 extracellular region of domain IV abuts the voltage sensor of domain III. If β1 is like β3 and forms a trimer, then viewed from above, its shape will be an approximate equilateral triangle of side length about 60 Å (figure 3c). Based on the dimensions of the bacterial channel, adjacent voltage sensors will be about 60–70 Å apart. Hence, the Ig domains of β1 (and β3) at this site could lie close to the α subunit voltage sensors of both domains IV and III. How then could they influence voltage gating sensitivity?
A hyperpolarizing shift could occur if a negatively charged protein is brought sufficiently close to a voltage sensor so that it generates screening surface charge [53–56]. The calculated isoelectric points of the β1 and the β3 Ig domains are 4.95 and 5.28, respectively, so they will both carry a net negative charge at pH 7.4. Interestingly, the electrostatic surface potential of the β3 trimer suggests a dipole-like quality. Positive charges are concentrated on the top surface and the clefts between protomers, although the loop regions at each trimer vertex are negatively charged (figure 4c,d). By contrast, the trimer underside presents negative charges concentrated within a centrally located, shallow concave face (figure 4e). The full significance of this curious feature will remain unclear until the atomic resolution structure(s) of the β3-binding sites on the α subunit are determined. But it is consistent with a role in presenting negative charge to the α subunit voltage sensors.
An important consideration is that, in vivo, the β subunit Ig domains are heterogeneously glycosylated [25,57]. Asparagine (N)-linked glycosylation sites are defined by a conserved Asn-X-(Ser/Thr) motif, in which X can be any amino acid except proline [58] and mature N-linked sugar residues often contain sialic acid moieties on their terminal branches [59]. When tested in CHO cells with Nav 1.2, 1.5 and 1.7, the hyperpolarizing gating shifts induced by β1 were abolished, both by mutagenic removal of the N-linked sites and by using a cell-line lacking sialyl transferase [60]. This indicates an important role for sialylation of the β1 Ig domain N-linked glycosylation sites in modulating the gating voltage shifts. The same post-translational modifications will undoubtedly occur on the β3 Ig domain. There are four potential N-linked glycosylation sites per β3 Ig domain protomer, and all of them point outwards from the surface-exposed faces of the trimer [27] (figure 4f). The potential N-linked glycosylation sites Asn85 and Asn89 are particularly interesting. They are fully conserved between β3 and β1 in all known species (figure 4b), as are the immediately surrounding residues Val86, Thre87 and Gly92 [25,27]. Furthermore, these two N-linked glycosylation sites lie close to two amino acids that are separately mutated in different inherited cardiopathologies: V86I [61] and V12M [62] (figure 4f). Residue V12 normally forms a hydrophobic contact with Leu116 of an adjacent protomer that helps maintain the correct trimer organization [27]. The V12M mutation probably destabilizes this interaction. Two further mutations in the β3 Ig domain that are associated with inherited cardiopathologies have been described. These are A106V [63] and V30G [64], and occur on adjacent Ig domain loops (figure 4f). In the crystal structure, the conformation of these two regions is unresolved due to local disorder (figure 3a,b) [27]. In other Ig domains, the equivalent loop regions can act as binding sites for interacting proteins. For example, these regions of antibody Fab fragment Ig domains constitute the antigen-binding site [30]. In β3, their flexible nature, outwardly pointing locations and associations with cardiopathologies make them plausible candidates for an α subunit binding site—perhaps interacting with the S5–S6 extracellular regions of domains I and IV (and see also §5).
We suggest that the hyperpolarizing shifts in voltage gating shown by the β3 and β1 subunits are caused by an electrostatic mechanism in which the Ig domain is held in place via binding to extracellular regions of the α subunit. This would position the Ig domains so as to present negative charges from sialic acid residues (and perhaps the proteins themselves), close enough to one or more voltage sensors to affect gating behaviour, as previously proposed [55,56,60]. It should be noted, however, that in some other expression systems the same β subunits can produce depolarizing shifts in the V1/2 values for activation and inactivation [65–67]. Furthermore, the magnitude of the shifts can vary between α subunit partners. For example, it has been reported that β3 subunits have little or no effects on the gating voltage sensitivity of Nav 1.8 and Nav 1.6 [22,68]. It is hard to see how such differences can arise, other than by variations in post-translational modification and/or cell-specific and isoform-specific differences in the precise molecular organization of the subunits within the channel complex. An additional factor may be the wider molecular environment of the channel. The Nav channels do not exist in isolation on the plasma membrane, but as part of local protein clusters that include other ion channels and CAMs [69]. The influence of this clustering on β subunit-induced gating shifts remains to be characterized.
The β3 and β1 subunits can also enhance the rate of inactivation and recovery from inactivation, and increase the fraction of channels that gate in a fast-acting mode [23,70,71]. Within the α subunit, the carboxy-terminal domain binds to the inactivation gate, and this interaction stabilizes fast inactivation [72]. The α subunit carboxy-terminal domain also binds to the intracellular region of β3 and β1. An epilepsy-associated mutation in the carboxy-terminal domain of Nav 1.1 disrupts this interaction and attenuates the normal β1-induced enhancement of inactivation [48]. This suggests that these β subunits may facilitate fast inactivation by binding to, and optimally aligning the complex between, the α subunit carboxy-terminal domain and the inactivation gate. NMR studies on the intracellular region β3 intracellular region indicate that it is largely disordered, but with a short juxtamembrane sequence that can adopt a negatively charged amphipathic α-helix [65] (figure 3c). The intracellular regions are long enough to form a complex with the α subunit carboxy-terminus and the inactivation gate. But the formation of α subunit oligomers via β3- and β1-induced cross-linking could greatly facilitate and stabilize these interactions. The Nav channel oligomer would then behave as an integrated allosteric protein [73]. Consistent with this proposal, it has been shown that mutational disruption of the β1 region equivalent to the β3 trimer interface reduced the fraction of Nav 1.2 that acted in the fast-gating mode [74].
5. The β4 Ig domain structure and functional implications
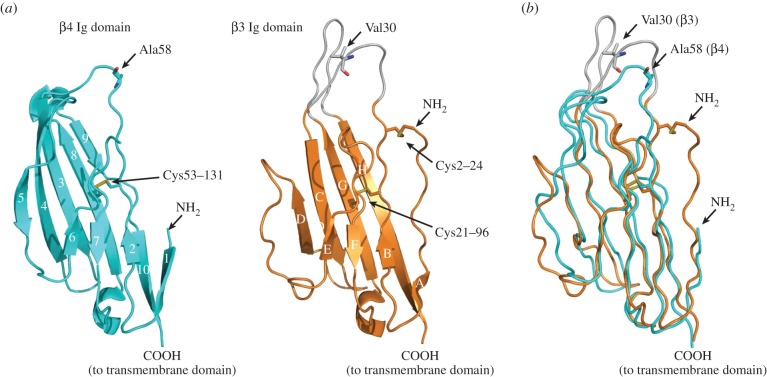
The structure of the human β4 Ig domain has been solved at 1.7 Å resolution [26]. Overall, the Ig domains of β4 and a β3 protomer are remarkably similar, despite their low level of sequence similarity (figure 5a,b). However, there are also some striking differences. The most obvious is that the β4 Ig domain is a monomer in the crystal asymmetric unit. There is currently no evidence that β4 forms cis homodimers or homotrimers in vivo. Unusually for an Ig domain, the first seven amino-terminal residues (which in β3 comprise the trimer interface) are disordered and unresolved in the β4 Ig domain crystal structure. There are also significant sequence differences in this region between β4 and β3. In particular, the C2–24 disulfide bond is not present in β4, because the equivalent cysteine residue at position 2 is not present. The Cys24-equivalent residue is present in β4 as Cys58, but to avoid potential complications caused by the presence of a free cysteine, this residue was mutated to alanine in the protein used for crystallization. The Cys58 residue forms a disulfide bond with the α subunit in vivo [18], and so must be located within an α subunit binding site of the β4 subunit. Residue 58 is surrounded by side chains of bulky hydrophobic amino acids—particularly Phe59, which is exposed and outward-pointing in the crystal [26], but which is probably buried into a suitable hydrophobic pocket on the α subunit when correctly assembled in vivo. Interestingly, residue 58 is located on one of the β4 Ig domain surface loops, although the region appears notably less flexible than the corresponding loop in the β3 protomer (figure 5a) [26]. When the β4 and β3 structures are overlaid (figure 5b), the region of β4 surrounding residue 58 directly corresponds to the loop region of β3 that contains the V30G cardiopathology mutation noted above (figure 4f). This provides some additional evidence that the loop regions of the β subunit Ig domains may be α subunit binding sites (see §4).
Figure 5.
The atomic resolution structure of the β4 subunit Ig domain. (a) The β4 Ig domain (PDB ID code 4MZ2) and comparison with similar orientation with the β3 Ig domain. (b) Supposition of the β4 Ig domain (cyan) and a single β3 Ig domain protomer (orange), showing the location of the Ala58 residue (corresponding to the free Cys58 residue of β4) and the Val30 residue of β3 mentioned in the text.
In common with most Ig domains, the β4 structure contains a buried disulfide bond (C53–131) (figure 5a) [30]. The conventional view has been that the disulfide bond stabilizes Ig domain folding by covalently connecting the two β sheet faces. Surprisingly, however, a C131W mutation still folded and was efficiently exported to the plasma membrane through the secretory pathway. Rather than completely disrupting folding, X-ray crystallographic analysis revealed that the C131W mutation had a more subtle and interesting effect. Breaking the internal disulfide bond led to a local remodelling of the β sheets surrounding the Cys58-containing loop region [26]. This work has important implications for other β subunits. In particular, an equivalent mutation (C121W) in β1 causes generalized epilepsy with febrile seizures plus (GEFS+). Here, the mutant β1 subunit can no longer bind to and modulate the brain-specific Nav 1.2 α subunit [75]. It is therefore probable that in β1, the C121W mutation selectively perturbs an α subunit interaction site on the β1 Ig domain loop region.
The β4 binding site(s) on the α subunit are unclear. However, one clue comes from the ability of β4 to inhibit toxin-binding. For example, co-expression of β4 with the Nav 1.2 α subunit reduced the affinity of the tarantula toxin ProTx-II for the channel, and β4 also reduced sodium influx in Nav 1.2 when tested with the scorpion toxin TsVII [26]. The ProTx-II toxin binds to the α subunit voltage sensors of domains I, II and IV, and TsVII binds predominantly to the α subunit voltage sensor of domain II [10]. On this basis, it has been suggested that β4 may influence the domain II voltage sensor [26]. This presumed β4 binding site on the α subunit is different from the one known β1 binding site noted above, and Nav 1.1 channels can be isolated containing both non-covalently associated β1 and covalently bound β4 subunits [76]. On the other hand, under conditions of high expression, β4 can partially (but not completely) displace β1 from Nav 1.1 [76]. Hence, a more subtle possibility is that the β4 and β1 subunits may bind at more than one site on this α subunit, some of which are overlapping. This is reminiscent of the multiple β3 binding sites on Nav 1.5 detected by AFM and noted earlier. If so, then there are some interesting implications. In neurons, expression of the α and β subunit isoforms can change significantly, both during development [77] and in some pathologies such as diabetic neuropathy [78] and neuropathic pain [79]. If β3 and β1 can induce α subunit cross-linking, while monomeric β4 cannot, then varying expression of different structural classes of β subunits may differentially modulate the oligomeric state of the channels, and this will contribute to both structural and functional heterogeneity within the Nav channels on the plasma membrane.
A unique aspect of β4 behaviour is its ability to promote ‘resurgent current’. This phenomenon occurs when channels are opened by membrane depolarization but are rapidly blocked by an endogenous protein that inhibits current flow, yet also prevents binding of the fast inactivation gate to the pore. Following membrane repolarization, the blocker is removed, leading to a brief resurgent current flow before the channels inactivate via the conventional pathway [80]. The blocking factor has been localized to sequences within the β4 intracellular region [81]. As with β3, the secondary structure of the β4 intracellular region is predominantly disordered, but with a short segment adjacent to the membrane that displays amphipathic α-helical potential—although in the case of β4, the charges along one face of the helix are positive. It is these charges that are critical for the resurgent current effect [82]. Disordered regions of proteins can sample many different conformations simultaneously, and can provide high-specificity binding even with low intrinsic affinity [83]. They may be advantageous in situations encountered by the intracellular regions of both β3 and β4, with their requirement for different binding interactions occurring at specific parts in the channel gating cycle.
The β4 subunit is closest in sequence identity to β2. The β2 subunit also contains a free cysteine at the equivalent position to Cys58, and likewise forms a disulfide bond with the α subunit in vivo [19]. However, it is not known if β2 and β4 share overlapping binding sites. For Nav 1.5, the β2 subunit induces hyperpolarizing gating shifts that are sialic-acid-dependent [84]. On the other hand, the β2 subunit induces sialic-acid-independent depolarizing gating shifts for Nav 1.2 [84]. There are currently no structural explanations for these strikingly disparate effects. Again, they emphasize the complex isoform-specific nature of the binding interactions between Nav α and β subunits. Mice lacking β2 expression have a relatively mild phenotype, although they are more prone to seizures. Interestingly, these mice display a significantly reduced Nav channel density in some classes of neurons, especially in the hippocampus [85]. It is therefore possible that β2 has a particularly important role in stabilizing Nav channels in the plasma membrane [86].
6. The Nav β subunits as cell-adhesion molecules
The Nav β subunits are related to members of the CAMs superfamily [17], and there is growing evidence that β subunits display CAM-like behaviour both in cis and in trans. The β1 subunit Ig domain can interact in cis with other CAMs such as neurofascin-155 and neurofascin-186, contactins and cadherins, and β3 can bind neurofascin-186 [87,88]. The β1 and β2 subunits interact with extracellular matrix proteins such as tenascin-R that are secreted by oligodendrocytes during myelination [89]. These multiple interactions all help localize Nav channels to discrete clusters at the nodes of Ranvier in myelinated neurons, and probably increase the total amount of Nav channels in the membrane.
The Nav β subunits also act as trans-binding CAMs independently of α subunits. This has been conveniently studied by expressing them in the Drosophila S2 cell-line and monitoring cell adhesion visually. Here, the β1 and β2 subunits promoted cell adhesion [90], while the β3 subunit did not [91]. On the other hand, β3-mediated cell adhesion was detected when assayed in more physiologically mammalian cell-lines, both by immunoprecipitation [25] and by immunofluorescence (figure 6). The trans cell adhesion of β3-subunits required an intact Cys2–24 disulfide bond [25]. Hence, the cis-formed β3 trimer is probably needed to form the trans-binding cell–cell contacts. This is similar to the behaviour shown by the CAM myelin P0 which stabilizes the myelin sheath around peripheral neurons [94]. The myelin P0 molecules assemble as tetramers in cis, and then the tetramers associate in trans to form tight intercellular contacts [94]. It may be significant that myelin P0 is a distant paralogue of the Nav β subunits, and its Ig domain has previously been used to model both the β1 and β3 Ig domains [23,25,74,95].
Figure 6.
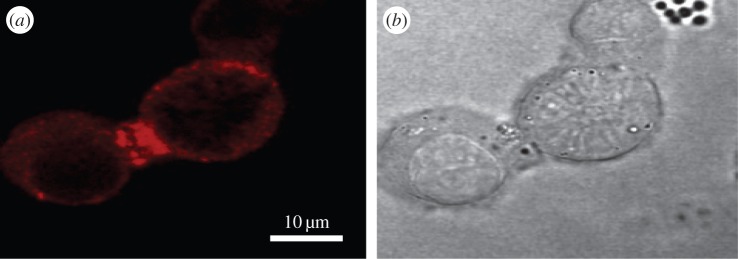
The rat neuronal-like cell-line PC12 expresses the β3 subunit endogenously [23]. (a) β3 accumulation at the contact site between two PC12 cells, as detected by immunofluorescence microscopy, and (b) DIC image of the same cells. Cells were stained with an affinity-purified rabbit polyclonal antibody raised against a β3-specific peptide corresponding to the intracellular region of the molecule. The antibody has been described previously. It detects β3 in both Western blotting and immunohistochemistry experiments, and its binding is inhibited by prior incubation with the immunizing peptide [92]. The PC12 cells were fixed, permeablized and stained as described previously [93].
The demonstration that at least some β subunits have both cis and trans CAM activity complicates the interpretation of knockout mouse phenotypes. For example, rapid conduction in the heart requires the cardiomyocytes to be electrically coupled to each other via their apposed intercalated discs [46,96]. The β subunits are located on the intercalated discs of ventricular cardiomyocytes [38,97,98], and their absence will probably have multiple effects: the Nav channels will display altered gating behaviour and their location at the intercalated discs may be destabilized, but cell adhesion between cardiomyocytes could also be compromised, potentially leading to loss of signal cohesion during the intercellular propagation of the cardiac action potential. We should be open to the possibility that some of the pathologies shown by β subunit knockout mice (and by the human pathologies associated with β subunit mutations) reflect abnormalities in cell adhesion rather than direct electrophysiological effects on the Nav channels themselves.
7. Summary
An earlier review described Nav channel β subunits as ‘anything but auxiliary’ [99]. We agree with this sentiment, and we emphasize the integration of the β subunits with other Nav channel components. As a case in point, we note the current interest in the pharmacological potential of animal toxins that target Nav channels [100]. In some examples, the binding affinity of these toxins for Nav channels is actually increased by the presence of specific β subunits [101,102]. This raises interesting questions about the nature of the toxin-binding sites. The important point is that these toxins evolved to act against Nav channels in their normal physiological context, which includes the β subunits. We suggest that screening assays for such toxins and drugs should include, wherever possible, the relevant β subunit(s) for the Nav channels in question.
These are interesting times for Nav channel β subunit research. The new results from X-ray crystallography and molecular imaging provide the first detailed look at the molecules, and will encourage both the generation of detailed functional hypotheses and their experimental testing.
Acknowledgements
We thank Manoj Patel, Bojana Popovic and Trevor Wilkinson for their helpful comments on the manuscript.
Funding statement
S.N. is supported by a Cambridge Nehru Trust scholarship. D.Y.C. is funded by the Crystallographic X-ray Facility, Department of Biochemistry, University of Cambridge. C.L.-H.H. is funded by the Medical Research Council (UK) grant MR/M001288/1 and the MacVeigh Benefaction.
References
- 1.Waxman SG. 2012. Sodium channels, the electrogenisome and the electrogenistat: lessons and questions from the clinic. J. Physiol. 590, 2601–2612 (doi:10.1113/jphysiol.2012.228460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantegazza M, Catterall WA. 2012. Voltage-gated Na+ channels: structure, function, and pathophysiology. In Jasper's basic mechanisms of the epilepsies (eds Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV.). Bethesda, MD. [PubMed] [Google Scholar]
- 3.Dudley SC, Jr, Chang N, Hall J, Lipkind G, Fozzard HA, French RJ. 2000. µ-conotoxin GIIIA interactions with the voltage-gated Na+ channel predict a clockwise arrangement of the domains. J. Gen. Physiol. 116, 679–690 (doi:10.1085/jgp.116.5.679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payandeh J, Scheuer T, Zheng N, Catterall WA. 2011. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (doi:10.1038/nature10238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, et al. 2012. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486, 130–134 (doi:10.1038/nature11054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCusker EC, Bagneris C, Naylor CE, Cole AR, D'Avanzo N, Nichols CG, Wallace BA. 2012. Structure of a bacterial voltage-gated sodium channel pore reveals mechanisms of opening and closing. Nat. Commun. 3, 1102 (doi:10.1038/ncomms2077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long SB, Tao X, Campbell EB, MacKinnon R. 2007. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (doi:10.1038/nature06265) [DOI] [PubMed] [Google Scholar]
- 8.Catterall WA. 2012. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J. Physiol. 590, 2577–2589 (doi:10.1113/jphysiol.2011.224204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanda B, Bezanilla F. 2002. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J. Gen. Physiol. 120, 629–645 (doi:10.1085/jgp.20028679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosmans F, Martin-Eauclaire MF, Swartz KJ. 2008. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature 456, 202–208 (doi:10.1038/nature07473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capes DL, Goldschen-Ohm MP, Arcisio-Miranda M, Bezanilla F, Chanda B. 2013. Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. J. Gen. Physiol. 142, 101–112 (doi:10.1085/jgp.201310998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas E, et al. 2012. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 140, 587–594 (doi:10.1085/jgp.201210873) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brackenbury WJ, Isom LL. 2011. Na+ channel β subunits: overachievers of the ion channel family. Front. Pharmacol. 2, 53 (doi:10.3389/fphar.2011.00053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusdin FS, Clare JJ, Jackson AP. 2008. Trafficking and cellular distribution of voltage-gated sodium channels. Traffic 9, 17–26 (doi:10.1111/j.1600-0854.2007.00673.x) [DOI] [PubMed] [Google Scholar]
- 15.Qin N, D'Andrea MR, Lubin ML, Shafaee N, Codd EE, Correa AM. 2003. Molecular cloning and functional expression of the human sodium channel β1B subunit, a novel splicing variant of the β1 subunit. Eur. J. Biochem. 270, 4762–4770 (doi:10.1046/j.1432-1033.2003.03878.x) [DOI] [PubMed] [Google Scholar]
- 16.Patino GA, Isom LL. 2010. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease. Neurosci. Lett. 486, 53–59 (doi:10.1016/j.neulet.2010.06.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopra SS, Watanabe H, Zhong TP, Roden DM. 2007. Molecular cloning and analysis of zebrafish voltage-gated sodium channel beta subunit genes: implications for the evolution of electrical signaling in vertebrates. BMC Evol. Biol. 7, 113 (doi:10.1186/1471-2148-7-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buffington SA, Rasband MN. 2013. Na+ channel-dependent recruitment of Navβ4 to axon initial segments and nodes of Ranvier. J. Neurosci. 33, 6191–6202 (doi:10.1523/JNEUROSCI.4051-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Calhoun JD, Zhang Y, Lopez-Santiago L, Zhou N, Davis TH, Salzer JL, Isom LL. 2012. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel α and β2 subunits. J. Biol. Chem. 287, 39 061–39 069 (doi:10.1074/jbc.M112.397646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu EJ, Ko SH, Lenkowski PW, Pance A, Patel MK, Jackson AP. 2005. Distinct domains of the sodium channel β3-subunit modulate channel-gating kinetics and subcellular location. Biochem. J. 392, 519–526 (doi:10.1042/BJ20050518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C, Cannon SC. 1995. Modulation of Na+ channel inactivation by the β1 subunit: a deletion analysis. Pflugers Arch. 431, 186–195 (doi:10.1007/BF00410190) [DOI] [PubMed] [Google Scholar]
- 22.Chahine M, O'Leary ME. 2011. Regulatory role of voltage-gated Na+ channel β subunits in sensory neurons. Front. Pharmacol. 2, 70 (doi:10.3389/fphar.2011.00070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan K, et al. 2000. β 3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl Acad. Sci. USA 97, 2308–2313 (doi:10.1073/pnas.030362197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick KA, Srinivasan J, White K, Scheuer T, Catterall WA. 1999. The extracellular domain of the β1 subunit is both necessary and sufficient for β1-like modulation of sodium channel gating. J. Biol. Chem. 274, 32 638–32 646 (doi:10.1074/jbc.274.46.32638) [DOI] [PubMed] [Google Scholar]
- 25.Yereddi NR, Cusdin FS, Namadurai S, Packman LC, Monie TP, Slavny P, Clare JJ, Powell AJ, Jackson AP. 2013. The immunoglobulin domain of the sodium channel β3 subunit contains a surface-localized disulfide bond that is required for homophilic binding. Faseb J. 27, 568–580 (doi:10.1096/fj.12-209445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilchrist J, Das S, Van Petegem F, Bosmans F. 2013. Crystallographic insights into sodium-channel modulation by the β4 subunit. Proc. Natl Acad. Sci. USA 110, E5016–E5024 (doi:10.1073/pnas.1314557110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namadurai S, Balasuriya D, Rajappa R, Wiemhofer M, Stott K, Klingauf J, Edwardson JM, Chirgadze DY, Jackson AP. 2014. Crystal structure and molecular imaging of the Nav channel β3 subunit indicates a trimeric assembly. J. Biol. Chem. 289, 10 797–10 811 (doi:10.1074/jbc.M113.527994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calhoun JD, Isom LL. 2014. The role of non-pore-forming beta subunits in physiology and pathophysiology of voltage-gated sodium channels. Handb. Exp. Pharmacol. 221, 51–89 (doi:10.1007/978-3-642-41588-3_4) [DOI] [PubMed] [Google Scholar]
- 29.Barclay AN. 1999. Ig-like domains: evolution from simple interaction molecules to sophisticated antigen recognition. Proc. Natl Acad. Sci. USA 96, 14 672–14 674 (doi:10.1073/pnas.96.26.14672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bork P, Holm L, Sander C. 1994. The immunoglobulin fold: structural classification, sequence patterns and common core. J. Mol. Biol. 242, 309–320 (doi:10.1006/jmbi.1994.1582) [DOI] [PubMed] [Google Scholar]
- 31.Gratkowski H, Lear JD, DeGrado WF. 2001. Polar side chains drive the association of model transmembrane peptides. Proc. Natl Acad. Sci. USA 98, 880–885 (doi:10.1073/pnas.98.3.880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lichtarge O, Bourne HR, Cohen FE. 1996. An evolutionary trace method defines binding surfaces common to protein families. J. Mol. Biol. 257, 342–358 (doi:10.1006/jmbi.1996.0167) [DOI] [PubMed] [Google Scholar]
- 33.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. 2001. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10 037–10 041 (doi:10.1073/pnas.181342398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fendri-Kriaa N, Kammoun F, Salem IH, Kifagi C, Mkaouar-Rebai E, Hsairi I, Rebai A, Triki C, Fakhfakh F. 2011. New mutation c.374C > T and a putative disease-associated haplotype within SCN1B gene in Tunisian families with febrile seizures. Eur. J. Neurol. 18, 695–702 (doi:10.1111/j.1468-1331.2010.03216.x) [DOI] [PubMed] [Google Scholar]
- 35.Patino GA, et al. 2009. A functional null mutation of SCN1B in a patient with Dravet syndrome. J. Neurosci. 29, 10 764–10 778 (doi:10.1523/JNEUROSCI.2475-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fahmi AI, et al. 2001. The sodium channel beta-subunit SCN3b modulates the kinetics of SCN5a and is expressed heterogeneously in sheep heart. J. Physiol. 537, 693–700 (doi:10.1113/jphysiol.2001.012691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hakim P, Gurung IS, Pedersen TH, Thresher R, Brice N, Lawrence J, Grace AA, Huang CL. 2008. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog. Biophys. Mol. Biol. 98, 251–266 (doi:10.1016/j.pbiomolbio.2009.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakim P, Brice N, Thresher R, Lawrence J, Zhang Y, Jackson AP, Grace AA, Huang CL. 2010. Scn3b knockout mice exhibit abnormal sino-atrial and cardiac conduction properties. Acta Physiol. 198, 47–59 (doi:10.1111/j.1748-1716.2009.02048.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Santiago LF, et al. 2007. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J. Mol. Cell. Cardiol. 43, 636–647 (doi:10.1016/j.yjmcc.2007.07.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercier A, Clement R, Harnois T, Bourmeyster N, Faivre JF, Findlay I, Chahine M, Bois P, Chatelier A. 2012. The β1-subunit of Nav1.5 cardiac sodium channel is required for a dominant negative effect through α-α interaction. PLoS ONE 7, e48690 (doi:10.1371/journal.pone.0048690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshi M, Du XX, Shinlapawittayatorn K, Liu H, Chai S, Wan X, Ficker E, Deschenes I. 2014. Brugada syndrome disease phenotype explained in apparently benign sodium channel mutations. Circ. Cardiovasc. Genet. 7, 123–131 (doi:10.1161/CIRCGENETICS.113.000292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poelzing S, et al. 2006. SCN5A polymorphism restores trafficking of a Brugada syndrome mutation on a separate gene. Circulation 114, 368–376 (doi:10.1161/CIRCULATIONAHA.105.601294) [DOI] [PubMed] [Google Scholar]
- 43.Keller DI, et al. 2005. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc. Res. 67, 510–519 (doi:10.1016/j.cardiores.2005.03.024) [DOI] [PubMed] [Google Scholar]
- 44.Clatot J, et al. 2012. Dominant-negative effect of SCN5A N-terminal mutations through the interaction of Nav1.5 α-subunits. Cardiovasc. Res. 96, 53–63 (doi:10.1093/cvr/cvs211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veitia RA. 2007. Exploring the molecular etiology of dominant-negative mutations. Plant Cell 19, 3843–3851 (doi:10.1105/tpc.107.055053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei M, Huang CL, Zhang Y. 2008. Genetic Na+ channelopathies and sinus node dysfunction. Progr. Biophys. Mol. Biol. 98, 171–178 (doi:10.1016/j.pbiomolbio.2008.10.003) [DOI] [PubMed] [Google Scholar]
- 47.Meadows LS, Chen YH, Powell AJ, Clare JJ, Ragsdale DS. 2002. Functional modulation of human brain Nav1.3 sodium channels, expressed in mammalian cells, by auxiliary β1, β2 and β3 subunits. Neuroscience 114, 745–753 (doi:10.1016/S0306-4522(02)00242-7) [DOI] [PubMed] [Google Scholar]
- 48.Spampanato J, et al. 2004. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J. Neurosci. 24, 10 022–10 034 (doi:10.1523/JNEUROSCI.2034-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. 1994. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265, 1724–1728 (doi:10.1126/science.8085162) [DOI] [PubMed] [Google Scholar]
- 50.Lenkowski PW, Shah BS, Dinn AE, Lee K, Patel MK. 2003. Lidocaine block of neonatal Nav1.3 is differentially modulated by co-expression of β1 and beta3 subunits. Eur. J. Pharmacol. 467, 23–30 (doi:10.1016/S0014-2999(03)01595-4) [DOI] [PubMed] [Google Scholar]
- 51.Qu Y, Rogers JC, Chen SF, McCormick KA, Scheuer T, Catterall WA. 1999. Functional roles of the extracellular segments of the sodium channel alpha subunit in voltage-dependent gating and modulation by β1 subunits. J. Biol. Chem. 274, 32 647–32 654 (doi:10.1074/jbc.274.46.32647) [DOI] [PubMed] [Google Scholar]
- 52.Makita N, Bennett PB, George AL., Jr 1996. Molecular determinants of beta 1 subunit-induced gating modulation in voltage-dependent Na+ channels. J. Neurosci. 16, 7117–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta AR, Huang CL, Skepper JN, Fraser JA. 2008. Extracellular charge adsorption influences intracellular electrochemical homeostasis in amphibian skeletal muscle. Biophys. J. 94, 4549–4560 (doi:10.1529/biophysj.107.128587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandler WK, Hodgkin AL, Meves H. 1965. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J. Physiol. 180, 821–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrera L, Moran O. 2006. Beta1-subunit modulates the Nav1.4 sodium channel by changing the surface charge. Exp. Brain Res. 172, 139–150 (doi:10.1007/s00221-005-0323-4) [DOI] [PubMed] [Google Scholar]
- 56.Ednie AR, Bennett ES. 2012. Modulation of voltage-gated ion channels by sialylation. Compr. Physiol. 2, 1269–1301 (doi:10.1002/cphy.c110044) [DOI] [PubMed] [Google Scholar]
- 57.Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. 1992. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science 256, 839–842 (doi:10.1126/science.1375395) [DOI] [PubMed] [Google Scholar]
- 58.Bause E. 1983. Structural requirements of N-glycosylation of proteins: studies with proline peptides as conformational probes. Biochem. J. 209, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hart GW. 1992. Glycosylation. Curr. Opin. Cell Biol. 4, 1017–1023 (doi:10.1016/0955-0674(92)90134-X) [DOI] [PubMed] [Google Scholar]
- 60.Johnson D, Montpetit ML, Stocker PJ, Bennett ES. 2004. The sialic acid component of the β1 subunit modulates voltage-gated sodium channel function. J. Biol. Chem. 279, 44 303–44 310 (doi:10.1074/jbc.M408900200) [DOI] [PubMed] [Google Scholar]
- 61.Ishikawa T, et al. 2013. Novel SCN3B mutation associated with brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ. J. 77, 959–967 (doi:10.1253/circj.CJ-12-0995) [DOI] [PubMed] [Google Scholar]
- 62.Tan BH, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, Medeiros-Domingo A, Makielski JC, Ackerman MJ. 2010. Sudden infant death syndrome-associated mutations in the sodium channel β subunits. Heart Rhythm 7, 771–778 (doi:10.1016/j.hrthm.2010.01.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang P, et al. 2010. Functional dominant-negative mutation of sodium channel subunit gene SCN3B associated with atrial fibrillation in a Chinese GeneID population. Biochem. Biophys. Res. Commun. 398, 98–104 (doi:10.1016/j.bbrc.2010.06.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valdivia CR, Medeiros-Domingo A, Ye B, Shen WK, Algiers TJ, Ackerman MJ, Makielski JC. 2010. Loss-of-function mutation of the SCN3B-encoded sodium channel β3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc. Res. 86, 392–400 (doi:10.1093/cvr/cvp417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cusdin FS, Nietlispach D, Maman J, Dale TJ, Powell AJ, Clare JJ, Jackson AP. 2010. The sodium channel β3-subunit induces multiphasic gating in NaV1.3 and affects fast inactivation via distinct intracellular regions. J. Biol. Chem. 285, 33 404–33 412 (doi:10.1074/jbc.M110.114058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qu Y, Curtis R, Lawson D, Gilbride K, Ge P, DiStefano PS, Silos-Santiago I, Catterall WA, Scheuer T. 2001. Differential modulation of sodium channel gating and persistent sodium currents by the β1, β2, and β3 subunits. Mol. Cell. Neurosci. 18, 570–580 (doi:10.1006/mcne.2001.1039) [DOI] [PubMed] [Google Scholar]
- 67.Cummins TR, Aglieco F, Renganathan M, Herzog RI, Dib-Hajj SD, Waxman SG. 2001. Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 21, 5952–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao J, O'Leary ME, Chahine M. 2011. Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary β-subunits. J. Neurophysiol. 106, 608–619 (doi:10.1152/jn.00107.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leterrier C, Brachet A, Dargent B, Vacher H. 2011. Determinants of voltage-gated sodium channel clustering in neurons. Semin. Cell Dev. Biol. 22, 171–177 (doi:10.1016/j.semcdb.2010.09.014) [DOI] [PubMed] [Google Scholar]
- 70.Patton DE, Isom LL, Catterall WA, Goldin AL. 1994. The adult rat brain β1 subunit modifies activation and inactivation gating of multiple sodium channel alpha subunits. J. Biol. Chem. 269, 17 649–17 655. [PubMed] [Google Scholar]
- 71.Bennett PB, Jr, Makita N, George AL., Jr 1993. A molecular basis for gating mode transitions in human skeletal muscle Na+ channels. FEBS Lett. 326, 21–24 (doi:10.1016/0014-5793(93)81752-L) [DOI] [PubMed] [Google Scholar]
- 72.Motoike HK, Liu H, Glaaser IW, Yang AS, Tateyama M, Kass RS. 2004. The Na+ channel inactivation gate is a molecular complex: a novel role of the COOH-terminal domain. J. Gen. Physiol. 123, 155–165 (doi:10.1085/jgp.200308929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Changeux JP. 2012. Allostery and the Monod–Wyman–Changeux model after 50 years. Annu. Rev. Biophys. 41, 103–133 (doi:10.1146/annurev-biophys-050511-102222) [DOI] [PubMed] [Google Scholar]
- 74.McCormick KA, Isom LL, Ragsdale D, Smith D, Scheuer T, Catterall WA. 1998. Molecular determinants of Na+ channel function in the extracellular domain of the beta1 subunit. J. Biol. Chem. 273, 3954–3962 (doi:10.1074/jbc.273.7.3954) [DOI] [PubMed] [Google Scholar]
- 75.Wallace RH, et al. 1998. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nat. Genet. 19, 366–370 (doi:10.1038/1252) [DOI] [PubMed] [Google Scholar]
- 76.Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. 2009. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J. Neurosci. 29, 2027–2042 (doi:10.1523/JNEUROSCI.4531-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah BS, Gonzalez MI, Bramwell S, Pinnock RD, Lee K, Dixon AK. 2001. β3, a novel auxiliary subunit for the voltage gated sodium channel is upregulated in sensory neurones following streptozocin induced diabetic neuropathy in rat. Neurosci. Lett. 309, 1–4 (doi:10.1016/S0304-3940(01)01976-0) [DOI] [PubMed] [Google Scholar]
- 78.Craner MJ, Klein JP, Renganathan M, Black JA, Waxman SG. 2002. Changes of sodium channel expression in experimental painful diabetic neuropathy. Ann. Neurol. 52, 786–792 (doi:10.1002/ana.10364) [DOI] [PubMed] [Google Scholar]
- 79.Shah BS, Stevens EB, Gonzalez MI, Bramwell S, Pinnock RD, Lee K, Dixon AK. 2000. β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. Eur. J. Neurosci. 12, 3985–3990 (doi:10.1046/j.1460-9568.2000.00294.x) [DOI] [PubMed] [Google Scholar]
- 80.Bean BP. 2005. The molecular machinery of resurgent sodium current revealed. Neuron 45, 185–187 (doi:10.1016/j.neuron.2005.01.006) [DOI] [PubMed] [Google Scholar]
- 81.Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. 2005. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron 45, 233–244 (doi:10.1016/j.neuron.2004.12.035) [DOI] [PubMed] [Google Scholar]
- 82.Lewis AH, Raman IM. 2011. Cross-species conservation of open-channel block by Na channel beta4 peptides reveals structural features required for resurgent Na current. J. Neurosci. 31, 11 527–11 536 (doi:10.1523/JNEUROSCI.1428-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Z, Huang Y. 2014. Advantages of proteins being disordered. Protein Sci. 23, 539–550 (doi:10.1002/pro.2443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson D, Bennett ES. 2006. Isoform-specific effects of the beta2 subunit on voltage-gated sodium channel gating. J. Biol. Chem. 281, 25 875–25 881 (doi:10.1074/jbc.M605060200) [DOI] [PubMed] [Google Scholar]
- 85.Chen C, et al. 2002. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proc. Natl Acad. Sci. USA 99, 17 072–17 077 (doi:10.1073/pnas.212638099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt JW, Catterall WA. 1986. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell 46, 437–444 (doi:10.1016/0092-8674(86)90664-1) [DOI] [PubMed] [Google Scholar]
- 87.Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. 2001. Sodium channel β1 and β3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J. Cell Biol. 154, 427–434 (doi:10.1083/jcb.200102086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kazarinova-Noyes K, et al. 2001. Contactin associates with Na+ channels and increases their functional expression. J. Neurosci. 21, 7517–7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao ZC, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL. 1999. Tenascin-R is a functional modulator of sodium channel beta subunits. J. Biol. Chem. 274, 26 511–26 517 (doi:10.1074/jbc.274.37.26511) [DOI] [PubMed] [Google Scholar]
- 90.Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. 2000. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell–cell contact. J. Biol. Chem. 275, 11 383–11 388 (doi:10.1074/jbc.275.15.11383) [DOI] [PubMed] [Google Scholar]
- 91.McEwen DP, Chen C, Meadows LS, Lopez-Santiago L, Isom LL. 2009. The voltage-gated Na+ channel beta3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neurosci. Lett. 462, 272–275 (doi:10.1016/j.neulet.2009.07.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Gassen KL, de Wit M, van Kempen M, van der Hel WS, van Rijen PC, Jackson AP, Lindhout D, de Graan PN. 2009. Hippocampal Naβ3 expression in patients with temporal lobe epilepsy. Epilepsia 50, 957–962 (doi:10.1111/j.1528-1167.2008.02015.x) [DOI] [PubMed] [Google Scholar]
- 93.Pance A, Morgan K, Guest PC, Bowers K, Dean GE, Cutler DF, Jackson AP. 1999. A PC12 variant lacking regulated secretory organelles: aberrant protein targeting and evidence for a factor inhibiting neuroendocrine gene expression. J. Neurochem. 73, 21–30 (doi:10.1046/j.1471-4159.1999.0730021.x) [DOI] [PubMed] [Google Scholar]
- 94.Spiryda LB. 1998. Myelin protein zero and membrane adhesion. J. Neurosci. Res. 54, 137–146 (doi:10.1002/(SICI)1097-4547(19981015)54:2<137::AID-JNR2>3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- 95.Islas AA, Sanchez-Solano A, Scior T, Millan-PerezPena L, Salinas-Stefanon EM. 2013. Identification of Navbeta1 residues involved in the modulation of the sodium channel Nav1.4. PLoS ONE 8, e81995 (doi:10.1371/journal.pone.0081995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meadows LS, Isom LL. 2005. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc. Res. 67, 448–458 (doi:10.1016/j.cardiores.2005.04.003) [DOI] [PubMed] [Google Scholar]
- 97.Malhotra JD, Thyagarajan V, Chen C, Isom LL. 2004. Tyrosine-phosphorylated and nonphosphorylated sodium channel β1 subunits are differentially localized in cardiac myocytes. J. Biol. Chem. 279, 40 748–40 754 (doi:10.1074/jbc.M407243200) [DOI] [PubMed] [Google Scholar]
- 98.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. 2004. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 109, 1421–1427 (doi:10.1161/01.CIR.0000121421.61896.24) [DOI] [PubMed] [Google Scholar]
- 99.Isom LL. 2001. Sodium channel beta subunits: anything but auxiliary. Neuroscientist 7, 42–54 (doi:10.1177/107385840100700108) [DOI] [PubMed] [Google Scholar]
- 100.Billen B, Bosmans F, Tytgat J. 2008. Animal peptides targeting voltage-activated sodium channels. Curr. Pharm. Des. 14, 2492–2502 (doi:10.2174/138161208785777423) [DOI] [PubMed] [Google Scholar]
- 101.Zhang MM, Wilson MJ, Azam L, Gajewiak J, Rivier JE, Bulaj G, Olivera BM, Yoshikami D. 2013. Co-expression of NaVbeta subunits alters the kinetics of inhibition of voltage-gated sodium channels by pore-blocking µ-conotoxins. Br. J. Pharmacol. 168, 1597–1610 (doi:10.1111/bph.12051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson MJ, Zhang MM, Azam L, Olivera BM, Bulaj G, Yoshikami D. 2011. Navbeta subunits modulate the inhibition of Nav1.8 by the analgesic gating modifier muO-conotoxin MrVIB. J. Pharmacol. Exp. Ther. 338, 687–693 (doi:10.1124/jpet.110.178343) [DOI] [PMC free article] [PubMed] [Google Scholar]