Abstract
Background
Plasmodium falciparum and Plasmodium vivax are co-endemic in the Asia-Pacific region. Their capacity to induce and sustain diverse T-cell responses underpins protective immunity. We compared T-cell responses to the largely conserved merozoite surface protein-5 (PfMSP5) during acute and convalescent falciparum and vivax malaria.
Methods
Lymphoproliferation and IFN–γ secretion to PfMSP5 and purified protein derivate were quantified in adults with falciparum (n = 34), and vivax malaria (n = 12) or asymptomatic residents (n = 10) of Papua, Indonesia. Responses were reassessed 7–28 days following treatment.
Results
The frequency of IFN-γ responders to PfMSP5 was similar in acute falciparum (63%) or vivax (67%) malaria. However, significantly more IFN-γ–secreting cells were detectable during vivax compared with falciparum infection. Purified protein derivative responses showed a similarly enhanced pattern. While rapidly lost in vivax patients, PfMSP5-specific responses in falciparum malaria remained to day 28. By contrast, frequency and magnitude of lymphoproliferation to PfMSP5 were similar for falciparum and vivax infections.
Conclusion
Cellular PfMSP5-specific responses are most frequent during either acute falciparum or vivax malaria, indicating functional T-cell responses to conserved antigens. Both effector and central memory T-cell functions are increased. Greater IFN-γ responses in acute P. vivax, suggest enhancement of pre-existing effector T-cells during acute vivax infection.
Half the world’s population lives at risk of malaria. Of the malaria-causing parasites, Plasmodium falciparum is associated with the greatest mortality, responsible for approximately 550 million clinical cases and approximately 1 million deaths per year [1]. Although previously thought to be relatively benign, Plasmodium vivax malaria is increasingly recognized as causing major morbidity and has been associated with mortality [2, 3]. Affecting almost 40% of the world’s population, P. vivax is estimated to cause up to 390 million clinical cases each year. Most vivax malaria cases occur in Southeast Asia [3], a region further burdened with 25% of the global falciparum malaria cases [1], as both parasite species are prevalent. In Southeast Asia, reductions in malaria incidence, and particularly falciparum malaria, following intensive vector control efforts and introduction of artemisinin-based combination therapy (ACT) is likely to increase the relative proportion of vivax malaria in the future. Vaccines will be needed to prevent infection with all Plasmodium species, not just P. falciparum, to achieve the goal of malaria elimination in the Asia-Pacific region.
The development and evaluation of vaccines effective against all Plasmodium species requires understanding of cellular immune responses to different Plasmodium species. T-cell responses are essential for the acquisition of long-lasting immunity to malaria, including B-cell help. Reportedly impaired during acute falciparum malaria [4], T-cell responsiveness is not well defined in acute vivax malaria despite the importance of P. vivax.
Merozoite surface protein 5 (MSP5) is expressed during the blood and liver stages of the Plasmodium life cycle [5] and is a potential target antigen for a combined liver- and blood-stage vaccine. P. falciparum MSP5 (PfMSP5) is relatively conserved between P. falciparum isolates [6, 7], and its EGF-like domain shows 72% homology with that of P. vivax MSP5 (PvMSP5) [8]. We previously reported frequent IgG3, IgG1 and IgM antibody recognition of PfMSP5, in P. falciparum and P. vivax infected residents of Papua, Indonesia [9]. Species-specific and PfMSP5 and PvMSP5 cross-reactive antibodies were identified. The extent of cellular responses to MSP5 following natural exposure and during acute malaria is currently unknown.
In the same region of Papua, Indonesia, we assessed the frequency and magnitude of lymphoproliferation and ex vivo IFN-γ secretion to PfMSP5 and purified protein derivative (PPD) in 34 patients with acute uncomplicated falciparum malaria and 12 patients with acute vivax malaria. The data suggest that acute vivax but not falciparum malaria enhances pre-existing effector T cells specific for malarial and nonmalarial antigens.
MATERIAL AND METHODS
Study Participants and Samples
We recruited 56 participants in Timika, a lowland region of Papua, Indonesia, with perennial unstable malaria transmission of both P. falciparum and P. vivax at a ratio of 57:43. The entomological inoculation rate varies between 1 and 4 infectious bites per year [10]. Patients with malaria were enrolled in trials of chloroquine and sulphadoxine-pyrimethamine or artemisinin combination therapy after providing informed written consent [11]. Individuals with fever or a history of fever within 48 hours prior to enrollment, and with no alternative cause of fever identified, and microscope-identified P. falciparum or P. vivax were included in this study. Venous blood was collected at the time of presentation and approximately 7 and 28 days following antimalarial drug treatment. It was not possible to collect follow-up samples from all participants. Although collection of follow-up samples could be achieved in only 35% of patients (16 of 46), reassuringly there was no significant difference in the demographic breakdown between those patients with full and partial observations. Malaria-exposed asymptomatic participants (AC) were resident in the Timika district for at least 2 years, with no fever or symptoms of malaria within the 2 weeks preceding the study [12]. Malaria patients and controls were both resident in malaria-endemic areas within Timika, with comparable malaria exposure and ethnicity (predominantly highland Papuans). Peripheral blood mononuclear cells (PBMC) were separated and cryopreserved for analysis. The median cell viability following cryopreservation was 91% (interquartile range [IQR]: 82%–93%) for controls, 86% (IQR: 74%–90%) for falciparum, and 87% (IQR: 78%–91%) for vivax patients and did not differ significantly between groups (P = .2). The ethics committees of the National Institute of Health Research and Development, Ministry of Health, Jakarta, Indonesia, and Menzies School of Health Research, Darwin, Australia approved this study.
Antigens
We expressed and purified P. falciparum and P. vivax recombinant MSP5 as previously described [9, 13]. We used PPD from Mycobacterium tuberculosis (Statens Serum Institute) as a nonmalarial recall antigen and the mitogen phytohaemagglutinin (PHA; Sigma) as a positive control.
Ex Vivo Enzyme-linked Immunospot Assay
We cultured 0.4 million PBMC per well with or without antigen in 96-well flat-bottom nitrocellulose plates (MAIPS4510, Millipore) pretreated with 70% ethanol, coated with 5 μg/mL of anti-IFN-γ monoclonal antibody (clone 1-D1K, Mabtech) and incubated at 37°C in 5% carbon dioxide (CO2) overnight. We washed and developed and plated using 1 μg/mL biotinylated anti–human IFN-γ mAb (clone 7-B6-1, Mabtech) followed by streptavidin-alkaline phosphatise (AP) (1:1000, Mabtech) and the colorimetric AP Kit (BioRad). We counted spots using an automated enzyme-linked immunospot assay reader (AID) and expressed them as spot-forming units (SFU) per million PBMC. A response was considered positive if the number of spots was significantly different (P ≤ .05) from that of the control well, assuming a Poisson distribution [14].
Proliferation Assay
We plated 100,000 PBMC per well in RPMI-1640 (JRH) with 5% heat-inactivated human AB serum, 2 mM glutamine, 100 μg/mL streptomycin and 100 U/mL penicillin (all Sigma-Aldrich) in triplicate into 96-well round bottom plates (BD). Cells were incubated at 37°C in 5% CO2 for 5 days in the presence of 10 μg/mL of antigen or 2–3 days in the presence of 5 μg/mL mitogen, pulsed with 1 μCi of 3[H]-thymidine (Amersham) and then incubated for a further 16 hours. The stimulation index (SI) was calculated as mean counts per minute (cpm) of stimulated cells divided by mean cpm of unstimulated cells. Responses with SI ≥2 were considered positive.
Flow Cytometry
PBMC were blocked with phosphate-buffered saline containing 10% fetal calf serum, stained with anti–CD3 antibody (Hit3a, Pharmingen), washed and fixed with 1% paraformaldehyde. Data were acquired on a FACSCalibur (BD) and analyzed using FlowJo (TreeStar) software.
Statistical Analysis
We used GraphPad Prism 5 (GraphPad Software) for all statistical analyses. In an a priori analytical plan, we compared responses in the P. falciparum group with P. vivax patients and separately with asymptomatic controls using the Mann–Whitney U test or Fisher exact test. We used the Spearman rank test for correlation analyses. We used the Wilcoxon signed rank test for analysis of longitudinal data.
RESULTS
Study Cohort
We included 34 adult patients with acute uncomplicated P. falciparum malaria (43% female; mean age 26; 60% Highland Papuan; median parasitemia 3068 [IQR 504–8084] parasites/μL), 12 adults with acute P. vivax malaria (54% female; mean age 25; 80% Highland Papuan; median parasitemia 638 [IQR 322–3195] parasites/μL) and 10 asymptomatic malaria-exposed adults (10% female; mean age 27; 100% Highland Papuan; median parasitemia 0 [IQR 0–0] parasites/μL) in this study. There was no significant difference in age, gender, or ethnicity between patients with acute P. falciparum and P. vivax malaria.
Frequent P. falciparum Merozoite Surface Protein 5–specific Proliferation During Acute Malaria
Proliferative responses to PfMSP5 were assessed in 28 patients with falciparum malaria and compared with responses in 10 patients with vivax malaria and 10 exposed asymptomatic persons. PfMSP5-specific proliferation following in vitro stimulation was detected in 13 of 28 patients with acute falciparum (46%) and in 3 of 10 patients with acute vivax malaria (30%; P = .47; Table 1), but only in 1 of 10 exposed asymptomatic individuals (10%; P = .06; Table 1). There was no significant difference in PfMSP5 reactivity between males (11 of 21) and females (5 of 17) either overall or after stratifying by species of infection (P = .35), although it should be noted that this study was not designed or powered to elucidate differences between males and females. All samples proliferated following mitogenic stimulation with PHA, indicating that the cells were viable and capable of division. No significant difference in spontaneous proliferation was observed between falciparum malaria patients and either asymptomatic participants or patients with vivax malaria (Table 2) nor between vivax patients and asymptomatic controls (P = .5). Maximal proliferation in response to PfMSP5 was observed on days 5 and 6 of culture with no significant difference in the stimulation index of responses between the groups (Table 2). The majority (60%–100%) of donors in all groups proliferated following stimulation with the nonmalarial antigen PPD (Table 1). There was no significant difference in the magnitude of PPD proliferative response between falciparum and vivax malaria (Table 2).
Table 1. Reactivity to Merozoite Surface Protein 5 and Nonmalarial Antigen.
| AC | Pf | Pv | P value# | P value* | ||
|---|---|---|---|---|---|---|
| MSP5 | Proliferation | 1/10 (10%) | 13/28 (46%) | 3/10 (30%) | .06 | .47 |
| IFN-γ | 2/7 (28%) | 12/19 (63%) | 8/12 (67%) | .19 | 1.0 | |
| PPD | Proliferation | 6/10 (60%) | 17/20 (85%) | 8/8 (100%) | .18 | .54 |
| IFN-γ | 3/7 (43%) | 4/9 (44%) | 7/8 (88%) | 1.0 | .13 |
NOTE. MSP5, merozoite surface protein 5; AC, asymptomatic malaria-exposed; Pf, acute P. falciparum malaria; P v, acute P. vivax malaria.
Fisher exact test AC vs Pf;
Fisher exact test Pf vs Pv.
Table 2. Cellular Immune Responses to Merozoite Surface Protein 5 and Purified Protein Derivative.
| AC | Pf | Pv | P value# | P value* | ||
|---|---|---|---|---|---|---|
| Proliferationa | n | 10 | 28 | 10 | ||
| Media [cpm] | 394 [129–2371] | 381 [189–828] | 685 [375–1535] | .87 | .08 | |
| MSP5 [cpm] | 488 [144–1616] | 691 [230–1134] | 1292 [653–2857] | |||
| MSP5 [SI] | 1 [0.8–1.5] | 1.9 [0.8–4.9] | 1.3 [1–7.7] | .14 | .92 | |
| PPD [cpm] | 5274 [219–11,485] | 6770 [1427–19,062] | 22,124 [7128–49,498] | |||
| PPD [SI] | 5 [1–28] | 13 [5–25] | 16 [10–68] | .18 | .36 | |
| IFN-γb | n | 7 | 19 | 12 | ||
| Media | 0 [0–10] | 5 [0–20] | 9 [3–39] | .09 | .4 | |
| MSP5 | 0 [0–45] | 20 [10–38] | 49 [26–75] | .16 | .01 | |
| PPD | 38 [0–200] | 10 [3–33] | 136 [77–271] | .77 | .04 | |
| T-cell frequencyc | n | 10 | 22 | 12 | ||
| CD3+ | 54 [29–70] | 57 [44–60] | 57 [54–64] | 0.8 | 0.3 |
NOTE. AC, asymptomatic malaria-exposed; Pf, acute P. falciparum malaria; Pv, acute P. vivax malaria; IFN, interferon; MSP5, merozoite surface protein 5; PPD, purified protein derivative; SI, stimulation index.
Data expressed as median cpm or SI [interquartile range (IQR)]
Data expressed as median SFU/million [IQR]
expressed as % of peripheral blood mononuclear cells [IQR];
Mann-Whitney U test AC vs Pf;
Mann-Whitney U test Pf vs Pv.
P. falciparum Merozoite Surface Protein 5–specific Interferon-γ Secretion During Acute P. falciparum and P. vivax Infection
The presence of PfMSP5-specific effector T-cell responses was assessed by ex vivo IFN-γ ELISpot assay in 19 patients with acute P. falciparum, 12 patients with acute P. vivax malaria, and 7 malaria-exposed asymptomatic participants. IFN-γ responses were detected in most patients with falciparum (63%) or vivax (67%) malaria but in only 28% of asymptomatic individuals (Table 1). Similar to proliferative responses, IFN reactivity did not differ significantly when malaria patients were stratified by gender (P = .1). Interestingly, vivax but not falciparum malaria patients showed significantly higher background IFN-γ responses compared with asymptomatic controls (P = .046). There was no statistically significant difference in background IFN-γ responses between falciparum malaria patients and vivax patients (Table 2). In contrast, the magnitude of PfMSP5-specific IFN-γ responses (corrected for background) was significantly higher in patients with vivax malaria compared with those with falciparum malaria (P = .01; Table 2). This difference was not because of differences in the overall proportion of T cells in peripheral blood because both groups showed similar CD3+ T-cell frequencies (Table 2). Similar to PfMSP5 responses, IFN-γ responses to PPD (corrected for background) were also significantly higher in patients with vivax malaria compared with those in patients with falciparum malaria (P = .04; Table 2).
P. falciparum Merozoite Surface Protein 5 Responses are Short-lived in P. vivax but Not in P. falciparum Malaria
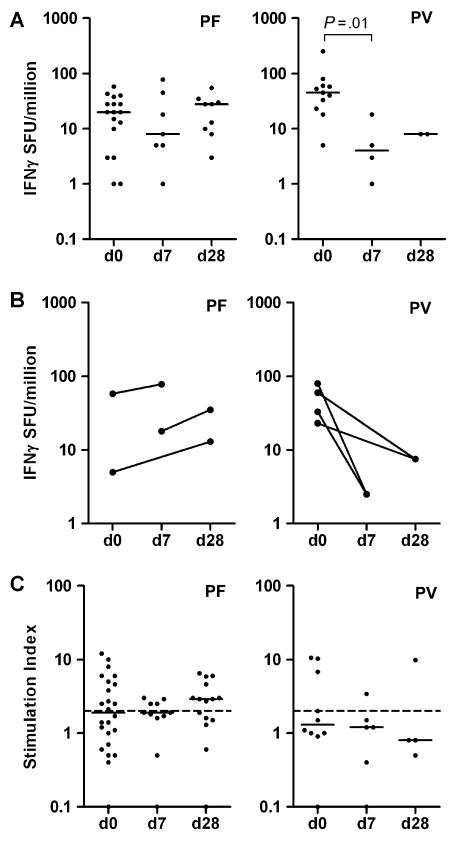
Whereas on the day of admission, PfMSP5-specific IFN-γ responses were higher in patients with vivax malaria, these responses were short-lived and dropped significantly within 7 days post treatment (Figure 1A, right panel). In contrast, the magnitude of PfMSP5-specific IFN-γ responses in patients with falciparum malaria remained unchanged up to at least 28 days post treatment (Figure 1A, left panel). This observation was further supported by longitudinal samples from individual patients with falciparum (n = 3) and vivax (n = 4) malaria showing that PfMSP5-specific IFN-γ responses were short lived in vivax malaria (Figure 1B, right panel) but were maintained during convalescence following falciparum malaria treatment (Figure 1B, left panel).
Figure 1.
Longitudinal responses to merozoite surface protein 5. (A) Longitudinal interferon-γ (IFN-γ) responses to merozoite surface protein 5 (MSP5) in samples from patients with acute P. falciparum malaria (Pf, left panel) and acute P. vivax malaria (Pv, right panel). (B) Longitudinal IFN-γ response to MSP5 in paired samples from 3 patients with acute P. falciparummalaria (Pf, left panel) and 4 patients with acute P. vivax malaria (Pv, right panel). All graphs show number of spot forming units (SFU) per million PBMC with background SFU subtracted. Horizontal lines depict the median. (C) Longitudinal proliferation response to PfMSP5 in cross-sectional samples from patients with acute P. falciparum malaria (Pf, left panel) and acute P. vivax malaria (Pv, right panel). Positive response defined as stimulation index (SI) > 2. Horizontal lines depict the median. Dashed lines represent cut-off value for positive response (SI = 2).
Similar to IFN-γ responses, PfMSP5-specific proliferation was still observed in 60% of individuals 28 days following treatment of falciparum malaria (Figure 1C, left panel) compared with only 1/4 following vivax malaria (Figure 1C, right panel). We observed no correlation between proliferative responses and IFN-γ responses in patients with falciparum (rs = .19; P = .5) or vivax malaria (rs = .32; P = .4). Similarly, there was no significant relationship between proliferative or IFN-γ responses and parasitemia in either P. falciparum or P. vivax infection. We observed no correlation between T-cell responses and previously reported antibody responses [9] (data not shown).
The Majority of P. falciparum Merozoite Surface Protein 5 Responders also Recognize P. vivax Merozoite Surface Protein 5
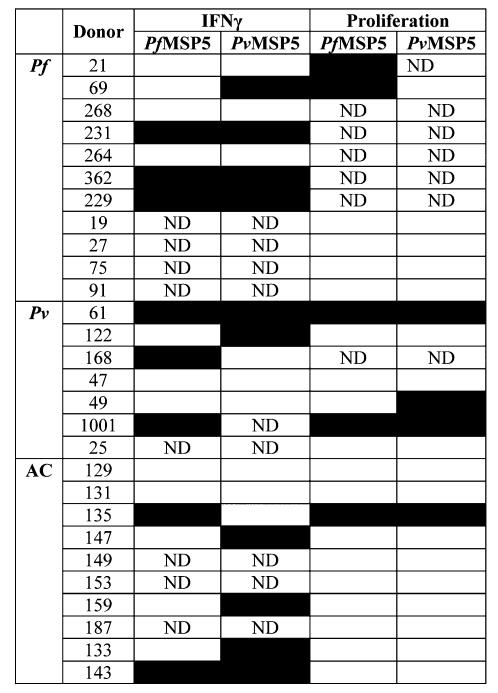
The high effector T-cell reactivity to PfMSP5 in patients with vivax malaria led us to further test a subgroup of 11 individuals with acute P. falciparum, 7 with acute P. vivax malaria and the 10 asymptomatic individuals for concordant reactivity to recombinant P. vivax MSP5 (PvMSP5). Figure 2 shows the reactivity pattern of IFN-γ and proliferative responses for each individual. Reactivity to PvMSP5 was observed in all groups. Overall, during acute malaria, 71% of PfMSP5 responders also responded to PvMSP5.
Figure 2.
Reactivity pattern to P. falciparum merozoite surface protein 5 and P. vivax merozoite surface protein 5. Eleven patients with falciparum malaria (Pf),7 with vivax malaria (Pv) and 10 asymptomatic individuals (AC) were tested for IFN-gamma and proliferation responses to PfMSP5 and PvMSP5. Positive responses are shown as black squares. ND, not determined.
DISCUSSION
We report frequent lymphoproliferation and IFN-γ secretion to PfMSP5 and PPD in adults with acute P. falciparum or P. vivax malaria in Papua, Indonesia. IFN-γ-secretion, but not proliferative responses, was significantly greater during acute vivax compared with falciparum malaria but was rapidly lost following anti-malarial treatment. Cellular responses to PfMSP5 were detectable in P. falciparum malaria patients until at least day 28 post treatment. The higher magnitude of IFN-γ responses to PfMSP5 and PPD during acute vivax malaria suggests both specific and non-specific effector T-cell activation by P. vivax, which may contribute to the stronger host inflammatory responses per infected erythrocyte known to occur for vivax malaria [15, 16].
Proliferative capacity is a fundamental prerequisite for the maintenance of long-term protective immunity. The low proliferative reactivity in residents without clinical malaria suggests that long-lasting PfMSP5-specific memory responses did not develop in these donors despite at least 2 years of P. falciparum exposure and high frequencies of antibodies to PfMSP5 [9], albeit in a region of unstable rather than hyperendemic transmission [10]. Similarly low responsiveness has been reported to other malarial antigens [17–20], whereas studies from hyperendemic areas, especially those with seasonal transmission, show higher proliferation reactivity to malarial antigens [21–23] in individuals with life-long exposure. Together these studies highlight the broad diversity of T-cell responsiveness amongst different geographic areas with different malaria endemicity.
The low frequency of PfMSP5-specifc IFN-γ responses detected in asymptomatic persons was anticipated because ex vivo ELISpot assays detect circulating effector T cells, and overnight culture is too short to expand cells. In acute malaria, however, such expansion occurs in vivo as evidenced by the presence of proliferating Ki-67 positive T cells in patients with acute falciparum or vivax malaria [24]. PfMSP5-specific IFN-γ responses detected by ex vivo ELISpot in asymptomatic participants likely reflect expansion of circulating PfMSP5-specific effector T cells due to a recent P. falciparum infection [25]. IFN-γ-secreting effector memory T cells directly contribute to antibody independent protective immunity to malaria blood-stages as evidenced by rodent [26, 27] and human experimental malaria challenge data [28]. IFN-γ responses similar to the PfMSP5-specific reactivity reported here have also been observed to other malarial antigens [14, 20, 29].
Frequent detection of IFN-γ and proliferative responses to PfMSP5 during both acute falciparum and vivax malaria suggests that PfMSP5 responses are boosted by the current Plasmodium infection. Interestingly, significantly more PfMSP5- and PPD-specific IFN-γ–secreting cells were detected in vivax malaria patients compared with falciparum malaria patients. PfMSP5-specific responses following acute P. vivax infection were only short-lasting and declined by day 7 post infection. Thus, observed responses may represent MSP5-specific cross-reactive memory T cells directly activated by the current infection or indirectly activated as bystanders by the inflammatory P. vivax infection [15, 30]. We previously reported cross-reactive IgG responses in a minority of responders [9]; hence cross-reactive T-cell epitopes may also exist. However, greater IFN-γ responses to the nonmalarial antigen PPD in vivax malaria compared with falciparum malaria and the high background IFN-γ responses suggest either bystander activation and enhanced T-cell mobilization or less immune suppression in vivax malaria. Regardless of the mechanism by which reactivity to P. falciparum antigen is started during P. vivax infection, such reactivity is important because cellular immune responses to blood-stage antigens are shown herein to have potential to be highly interdependent of the other parasite.
The T-cell frequency was similar among falciparum and vivax malaria patients and asymptomatic controls, suggesting that the fewer IFN-γ–secreting effector T cells seen in falciparum malaria were not attributable to a proportional loss of T cells from the periphery. P. falciparum antigen specific T cells may transiently relocate from circulation [31, 32], particularly because IFN-γ responses increased following falciparum malaria treatment, potentially reflecting the return of sequestered T cells into the periphery [33]. However, such relocation would not account for the fewer PPD-specific T cells detected in falciparum compared with vivax malaria patients. The increase in IFN-γ secreting cells during convalescence of falciparum malaria is in line with a previously reported increase of CD4 and CD8 T cells capable of IFN-γ production (following phorbol myristate acetate–ionomycin stimulation) during early convalescence [34]. We show here that these contain antigen-specific circulating effector T cells, both to P. falciparum malarial antigen and PPD, which are detectable in the periphery until at least day 28 of convalescence.
During acute P. falciparum malaria, impairment of T-cell responsiveness is manifest by reduced IFN-γ responses [35], lymphoproliferation [4, 36], delayed type hypersensitivity reaction to recall antigens [37] and virus-specific T-cell responses [38, 39]. However, the magnitude of lymphoproliferation or IFN-γ response to PfMSP5 or PPD was no higher in falciparum malaria patients than in asymptomatic exposed individuals, with stimulation indices in both groups being low. We previously observed a transient increase in regulatory T cells [40] in adults with acute falciparum malaria from the same area, which may contribute to impaired T-cell responsiveness during acute disease. T-cell responses during acute vivax malaria are not well described. Patients with acute clinical P. vivax malaria have been reported to show reduced delayed type hypersensitivity reaction to recall antigens [37] and low lymphoproliferative responsiveness to P. vivax schizont lysate and recombinant P. vivax proteins has been observed in convalescent vivax malaria patients from malaria-endemic Sri Lanka [41]. We found no immediate evidence of immune suppression in patients with acute P. vivax malaria, indeed responses were greater during acute malaria compared with convalescence and background IFN-γ responses were greater than those of controls. The lack of suppression of IFN-γ responses during acute P. vivax malaria suggests elementary differences in immune regulation between infections with these 2 Plasmodium species that are yet to be explained. A recent comparative study reports that T cells from adult falciparum patients [42, 43] but not vivax patients [43] show elevated expression of the T-cell inhibitory antigen CTLA-4. Such inhibition may occur independent of regulatory T cells because, like falciparum malaria [40], vivax malaria is accompanied by a relative increase in CD4+CD25+Foxp3+ regulatory T cells [44, 45]. Further comparative studies are required for a better understanding of immune regulatory differences in falciparum and vivax malaria.
The lack of correlation of lymphoproliferation with antibody responses or IFN-γ responses is in accordance with previous reports [46–48] as is the lack of correlation between lymphoproliferation and parasitemia [49, 50], highlighting our still limited understanding of the complex network of immune responses in malaria infection.
This study presents the first data on cellular responsiveness to PfMSP5 and demonstrates that unstable Plasmodium exposure generates PfMSP5-specifc cellular and antibody responses [9] that are boosted by a current Plasmodium infection. The contribution of MSP5-specific cellular and humoral responses to protection against clinical disease remains to be determined. Greater T-cell reactivity during acute vivax malaria suggests that acute vivax but not falciparum malaria enhances or mobilises pre-existing effector T cells specific for malarial and nonmalarial antigens. Greater understanding of interspecies differences in cellular responses is needed for the development of vaccines against all species causing malaria.
Acknowledgments
We thank Ferryanto Chalfein, Buhari, Prayoga, Rosmini, Elvi Yoshi, for technical and logistical assistance; Hadjar Siswantoro, Alison Ratcliff and Mitra Masyarakat Hospital staff for clinical support; Mauritz Okeseray, Erna Tresnaningsih, Jeanne Rini Poespoprodjo, and Paulus Sugiarto for support; the staff of PT Freeport Indonesia Public Health & Malaria Control Department, International SOS and Lembarga Pengembangan Masyarakat Amungme Kamoro for support and technical assistance in the community-based studies. Cross checking of slides was performed by Ferryanto Chalfein (Timika) and Budi Prasetyorini (NIHRD, Jakarta).
Funding This work was supported by the National Health and Medical Research Council of Australia (NHMRC ICRG ID 283321; Program Grants 496600, 290208 and 323229, and Fellowships to G.M., N.A., and M.P.); the Wellcome Trust (ICRG GR071614MA and Career Development Award to R.P.). The Timika Research Facility is supported by AusAID.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: XIIth International Congress of Parasitology (ICOPA), Melbourne, Australia, 15–20 August 2010.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: Severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–5. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: Neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 4.Hviid L, Theander TG, Abu-Zeid YA, et al. Loss of cellular immune reactivity during acute Plasmodium falciparum malaria. FEMS Microbiol Immunol. 1991;3:219–27. doi: 10.1111/j.1574-6968.1991.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 5.Bodescot M, Silvie O, Siau A, et al. Transcription status of vaccine candidate genes of Plasmodium falciparum during the hepatic phase of its life cycle. Parasitol Res. 2004;92:449–52. doi: 10.1007/s00436-003-1061-9. [DOI] [PubMed] [Google Scholar]
- 6.Polson HE, Conway DJ, Fandeur T, Mercereau-Puijalon O, Longacre S. Gene polymorphism of Plasmodium falciparum merozoite surface proteins 4 and 5. Mol Biochem Parasitol. 2005;142:110–5. doi: 10.1016/j.molbiopara.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Black CG, Wang L, Hibbs AR, Coppel RL. Lack of sequence diversity in the gene encoding merozoite surface protein 5 of Plasmodium falciparum. Mol Biochem Parasitol. 1999;103:243–50. doi: 10.1016/s0166-6851(99)00134-6. [DOI] [PubMed] [Google Scholar]
- 8.Black CG, Barnwell JW, Huber CS, Galinski MR, Coppel RL. The Plasmodium vivax homologues of merozoite surface proteins 4 and 5 from Plasmodium falciparum are expressed at different locations in the merozoite. Mol Biochem Parasitol. 2002;120:215–24. doi: 10.1016/s0166-6851(01)00458-3. [DOI] [PubMed] [Google Scholar]
- 9.Woodberry T, Minigo G, Piera KA, et al. Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: Species-specific and cross-reactive responses. J Infect Dis. 2008;198:134–42. doi: 10.1086/588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karyana M, Burdarm L, Yeung S, et al. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar J. 2008;7:148. doi: 10.1186/1475-2875-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: An open-label randomised comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randall LM, Kenangalem E, Lampah DA, et al. Age-related susceptibility to severe malaria associated with galectin-2 in highland Papuans. J Infect Dis. 2010;202:117–24. doi: 10.1086/653125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall VM, Tieqiao W, Coppel RL. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol Biochem Parasitol. 1998;94:13–25. doi: 10.1016/s0166-6851(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan KL, Plebanski M, Akinwunmi P, et al. Broadly distributed T cell reactivity, with no immunodominant loci, to the pre-erythrocytic antigen thrombospondin-related adhesive protein of Plasmodium falciparum in West Africans. Eur J Immunol. 1999;29:1943–54. doi: 10.1002/(SICI)1521-4141(199906)29:06<1943::AID-IMMU1943>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Hemmer CJ, Holst FG, Kern P, Chiwakata CB, Dietrich M, Reisinger EC. Stronger host response per parasitized erythrocyte in Plasmodium vivax or ovale than in Plasmodium falciparum malaria. Trop Med Int Health. 2006;11:817–23. doi: 10.1111/j.1365-3156.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- 16.Yeo TW, Lampah DA, Tjitra E, et al. Greater endothelial activation, Weibel–Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: A prospective study in Papua, Indonesia. J Infect Dis. 2010;202:109–12. doi: 10.1086/653211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: Evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–31. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.al-Yaman F, Genton B, Taraika J, Anders R, Alpers MP. Cellular immunity to merozoite surface protein 2 (FC27 and 3D7) in Papua New Guinean children. Temporal variation and relation to clinical and parasitological status. Parasite Immunol. 1997;19:207–14. doi: 10.1046/j.1365-3024.1997.d01-198.x. [DOI] [PubMed] [Google Scholar]
- 19.Braga EM, Carvalho LH, Fontes CJ, Krettli AU. Low cellular response in vitro among subjects with long-term exposure to malaria transmission in Brazilian endemic areas. Am J Trop Med Hyg. 2002;66:299–303. doi: 10.4269/ajtmh.2002.66.299. [DOI] [PubMed] [Google Scholar]
- 20.Reece WH, Plebanski M, Akinwunmi P, et al. Naturally exposed populations differ in their T1 and T2 responses to the circumsporozoite protein of Plasmodium falciparum. Infect Immun. 2002;70:1468–74. doi: 10.1128/IAI.70.3.1468-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley EM, Morris-Jones S, Taylor-Robinson AW, Holder AA. Lymphoproliferative responses to a merozoite surface antigen of Plasmodium falciparum: Preliminary evidence for seasonal activation of CD8+/HLA-DQ–restricted suppressor cells. Clin Exp Immunol. 1993;94:64–7. doi: 10.1111/j.1365-2249.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck HP, Felger I, Genton B, et al. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect Immun. 1995;63:596–600. doi: 10.1128/iai.63.2.596-600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connelly M, King CL, Bucci K, et al. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect Immun. 1997;65:5082–7. doi: 10.1128/iai.65.12.5082-5087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worku S, Troye-Blomberg M, Christensson B, Björkman A, Fehniger T. Activation of T cells in the blood of patients with acute malaria: Proliferative activity as indicated by Ki-67 expression. Scand J Immunol. 2001;53:296–301. doi: 10.1046/j.1365-3083.2001.00861.x. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan KL, Mwangi T, Plebanski M, et al. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: Longevity and risk of Plasmodium falciparum infection. Am J Trop Med Hyg. 2003;68:421–30. [PubMed] [Google Scholar]
- 26.Pouniotis DS, Proudfoot O, Bogdanoska V, Apostolopoulos V, Fifis T, Plebanski M. Dendritic cells induce immunity and long-lasting protection against blood-stage malaria despite an in vitro parasite-induced maturation defect. Infect Immun. 2004;72:5331–9. doi: 10.1128/IAI.72.9.5331-5339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makobongo MO, Riding G, Xu H, et al. The purine salvage enzyme hypoxanthine guanine xanthine phosphoribosyl transferase is a major target antigen for cell-mediated immunity to malaria. Proc Natl Acad Sci USA. 2003;100:2628–33. doi: 10.1073/pnas.0337629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pombo DJ, Lawrence G, Hirunpetcharat C, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360:610–7. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 29.Lee EA, Palmer DR, Flanagan KL, et al. Induction of T helper type 1 and 2 responses to 19-kilodalton merozoite surface protein 1 in vaccinated healthy volunteers and adults naturally exposed to malaria. Infect Immun. 2002;70:1417–21. doi: 10.1128/IAI.70.3.1417-1421.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–7. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Hviid L, Theander TG, Abdulhadi NH, Abu-Zeid YA, Bayoumi RA, Jensen JB. Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparum malaria. Eur J Immunol. 1991;21:1249–53. doi: 10.1002/eji.1830210523. [DOI] [PubMed] [Google Scholar]
- 32.Elhassan IM, Hviid L, Satti G, et al. Evidence of endothelial inflammation, T cell activation, and T cell reallocation in un-complicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 1994;51:372–9. doi: 10.4269/ajtmh.1994.51.372. [DOI] [PubMed] [Google Scholar]
- 33.Hviid L, Kurtzhals JA, Goka BQ, Oliver-Commey JO, Nkrumah FK, Theander TG. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65:4090–3. doi: 10.1128/iai.65.10.4090-4093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkler S, Willheim M, Baier K, et al. Reciprocal regulation of Th1-and Th2-cytokine–producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect Immun. 1998;66:6040–4. doi: 10.1128/iai.66.12.6040-6044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bejon P, Mwacharo J, Kai O, et al. The induction and persistence of T cell IFN-γ responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol. 2007;179:4193–201. doi: 10.4049/jimmunol.179.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho M, Webster HK, Green B, Looareesuwan S, Kongchareon S, White NJ. Defective production of and response to IL-2 in acute human falciparum malaria. J Immunol. 1988;141:2755–9. [PubMed] [Google Scholar]
- 37.Walsh DS, Looareesuwan S, Vaniganonta S, Viravan C, Webster HK. Cutaneous delayed-type hypersensitivity responsiveness in patients during and after Plasmodium falciparum and Plasmodium vivax-infections. Clin Immunol Immunopathol. 1995;77:89–94. doi: 10.1016/0090-1229(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 38.Njie R, Bell AI, Jia H, et al. The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis. 2009;199:31–8. doi: 10.1086/594373. [DOI] [PubMed] [Google Scholar]
- 39.Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus–specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195:799–808. doi: 10.1086/511984. [DOI] [PubMed] [Google Scholar]
- 40.Minigo G, Woodberry T, Piera KA, et al. Parasite-dependent expansion of TNF receptor II–positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goonewardene R, Carter R, Gamage CP, et al. Human T cell proliferative responses to Plasmodium vivax antigens: Evidence of immunosuppression following prolonged exposure to endemic malaria. Eur J Immunol. 1990;20:1387–91. doi: 10.1002/eji.1830200626. [DOI] [PubMed] [Google Scholar]
- 42.Schlotmann T, Waase I, Jülch C, et al. CD4 αβ T lymphocytes express high levels of the T lymphocyte antigen CTLA-4 (CD152) in acute malaria. J Infect Dis. 2000;182:367–70. doi: 10.1086/315690. [DOI] [PubMed] [Google Scholar]
- 43.Gonçalves RM, Salmazi KC, Santos BA, et al. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in un-complicated malaria: Do different parasite species elicit similar host responses? Infect Immun. 2010;78:4763–72. doi: 10.1128/IAI.00578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jangpatarapongsa K, Chootong P, Sattabongkot J, et al. Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol. 2008;38:2697–705. doi: 10.1002/eji.200838186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bueno LL, Morais CG, Araújo FF, et al. Plasmodium vivax: Induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010;5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chougnet C, Deloron P, Savel J. Persistence of cellular and humoral response to synthetic peptides from defined Plasmodium falciparum antigens. Ann Trop Med Parasitol. 1991;85:357–63. doi: 10.1080/00034983.1991.11812574. [DOI] [PubMed] [Google Scholar]
- 47.Flanagan KL, Lee EA, Gravenor MB, et al. Unique T cell effector functions elicited by Plasmodium falciparum epitopes in malaria-exposed Africans tested by three T cell assays. J Immunol. 2001;167:4729–37. doi: 10.4049/jimmunol.167.8.4729. [DOI] [PubMed] [Google Scholar]
- 48.Kabilan L, Sharma VP, Kaur P, Ghosh SK, Yadav RS, Chauhan VS. Cellular and humoral immune responses to well-defined blood stage antigens (major merozoite surface antigen) of Plasmodium falciparum in adults from an Indian zone where malaria is endemic. Infect Immun. 1994;62:685–91. doi: 10.1128/iai.62.2.685-691.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chougnet C, Lepers JP, Astagneau P, Rason MD, Savel J, Deloron P. Lymphoproliferative responses to synthetic peptides from merozoite ring-infected erythrocyte surface antigen and circumsporozoite protein: A longitudinal study during a falciparum malaria episode. Am J Trop Med Hyg. 1991;45:560–6. doi: 10.4269/ajtmh.1991.45.560. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira-Ferreira J, Banic DM, Santos F, Ferreira-da-Cruz MF, Dubois P, Daniel-Ribeiro CT. Cellular and antibody responses to the Plasmodium falciparum heat shock protein Pf72/HSP70 during and after acute malaria in individuals from an endemic area of Brazil. Acta Trop. 1999;73 doi: 10.1016/s0001-706x(99)00008-x. [DOI] [PubMed] [Google Scholar]