Abstract
A regio- and stereoselective cross-coupling reaction is described between internal alkynes and imines that provides selective access to allylic amines and γ-lactams.
Keywords: imines, alkynes, cross coupling, carbometalation, titanium
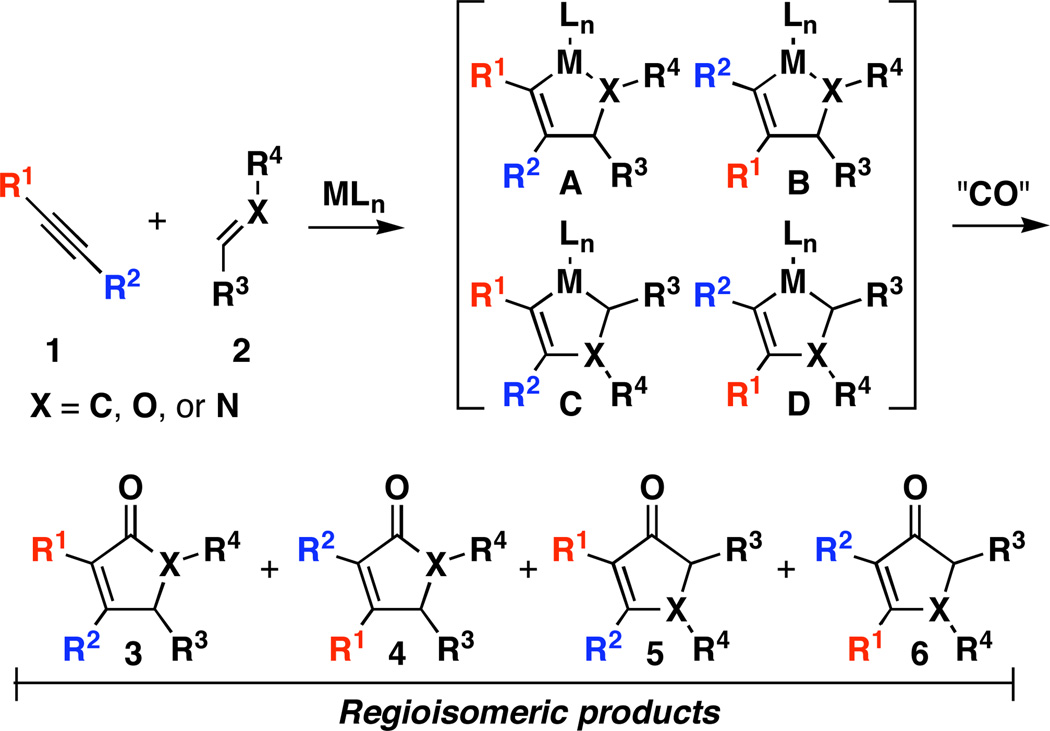
Metal–mediated [2+2+1] annulations are a powerful class of reactions for the synthesis of functionalized five-membered rings.[1] These processes, which proceed by initial formation of a metallacyclopentene followed by insertion of CO, have been described for a variety of functionalized π-systems (alkyne–alkene,[2] alkyne–ketone, alkene–ketone,[3] alkene–aldehyde,[3], [4] and alkyne–imine[5]), and have been useful for the preparation of functionalized carbocyclic and heterocyclic molecules. The vast majority of these annulation reactions are synthetically useful only in intramolecular contexts, whereby geometrical constraints imposed by a tether between the two reacting π-systems dictate site-selectivity in the C–C bond-forming event. The corresponding bimolecular coupling of unsymmetrically substituted π-systems via metal-mediated [2+2+1] annulations has proven much less general due to challenges associated with the control of both reactivity and regioselectivity in the generation of the polysubstituted metallacyclopentene (i.e. Figure 1, 1+2→[A−D]→3−6). Here, we describe a highly regioselective process for bimolecular [2+2+1] annulation that provides a convenient and direct route to tetrasubstituted α,β-unsaturated γ-lactams.
Figure 1.
Regioselection in bimolecular [2+2+1] annulation reactions.
Nitrogen-containing heterocycles are ubiquitous structural motifs in natural products and small molecules of biomedical relevance. Many methods for the convergent assembly of such architectures target C–C or C–N bond-formation, by nucleophilic addition to C=N based π-systems, condensation or metal-mediated cross-coupling.[6] An alternative, and potentially more powerful, pathway to functionalized heterocycles is through multicomponent coupling reactions between all-carbon-based π-systems, imines, and CO2 via [2+2+1] annulation reactions.[5] To date, these annulation processes have been of limited utility in organic synthesis due to the poor levels of regioselection commonly observed in the initial cross coupling reaction between the internal alkyne and imine (alkyne + imine → azametallacyclopentene).[7] A general means to control site- and stereoselective carbon–carbon bond-formation in these bimolecular coupling reactions would render such processes versatile for the synthesis of highly functionalized nitrogen-containing acyclic and heterocyclic targets. Our recent success in developing selective cross-coupling reactions of unactivated and differentially functionalized π-systems (alkyne–alkyne[8] and alkyne–alkene[9]) via harnessing the unique reactivity of Group IV metal alkoxides led us to question whether we could define such a process for alkyne–imine cross coupling via directed carbometalation of an internal alkyne with an azametallacyclopropane.
Our initial results describing regioselective cross coupling reactions between internal alkynes and imines are described in Table 1. In short, preformation of an azametallacyclopropane (imine, Ti(Oi-Pr)4, and c-C5H9MgCl, −78 to −40 °C) is followed by addition of an internal alkyne bearing a homopropargylic alkoxide, and warming to 0 °C. Protonation of the presumed bicyclic azametallacyclopentene then delivers an unsaturated 1,5-amino alcohol. As illustrated in entry 1, coupling of imine 7 with the homopropargylic alkoxide 8 provided the unsaturated 1,5-amino alcohol 9. Importantly, no evidence was found for the production of a minor regio- or olefin isomer. This single result represents the first highly regioselective cross-coupling reaction between an unsymmetrically substituted internal alkyne and an imine that proceeds without the requirement of electronic or steric differentiation of the internal alkyne. Although metal-mediated coupling reactions between alkynes and imines have been described, these coupling reactions proceed in a regioselective manner with only a small subset of alkynes: terminal, TMS-substituted, or conjugated alkynes.[5], [7]
Table 1.
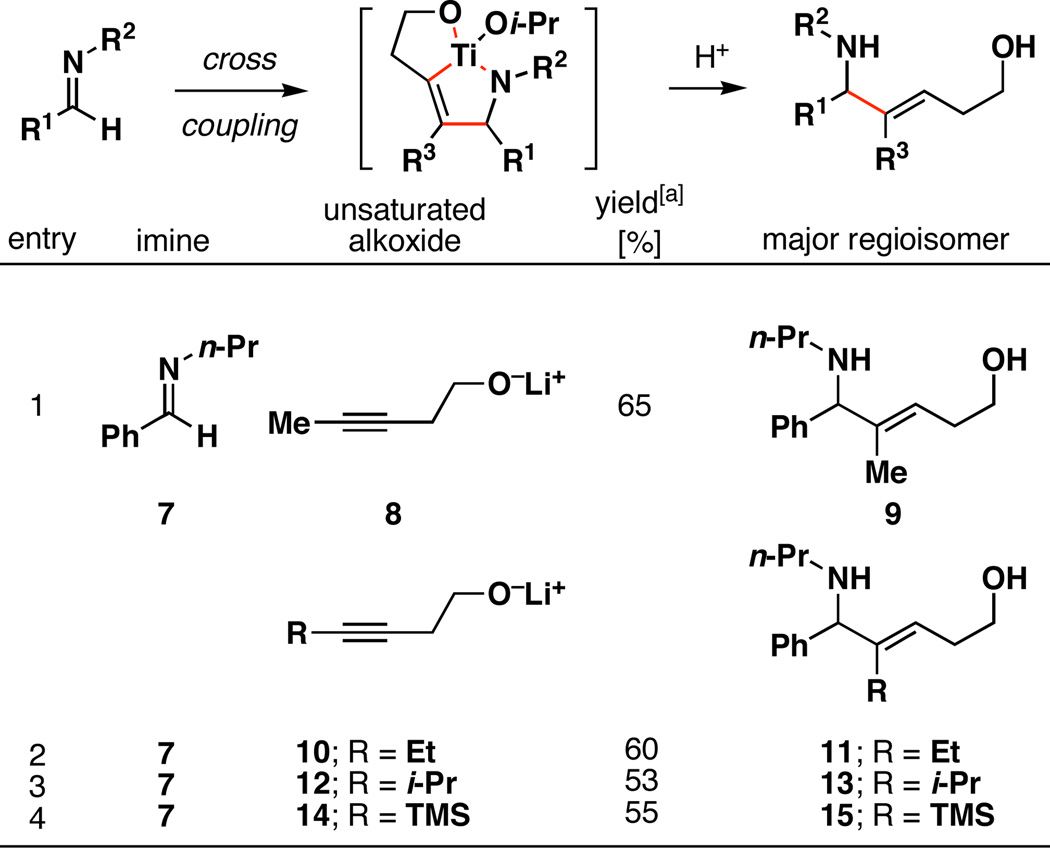 |
Reaction conditions: Ti(Oi-Pr)4, c-C5H9MgCl, Et2O, −78 to −40 °C, then add unsaturated alkoxide (−40 to 0 °C), quench with sat. NH4Cl(aq).
As observed in our alkyne–alkyne and alkene–alkyne cross coupling reactions,[8], [9] the current coupling process is relatively insensitive to non-bonded steric interactions imposed by substitution at the alkyne. For example, as depicted in entries 2–4, ethyl-, isopropyl-, and trimethylsilyl-substituted alkynes are all effective cross-coupling partners, and furnish the unsaturated 1,5-amino alcohols 11, 13, and 15 as single regioisomeric products – in all cases C–C bond-formation occurs distal to the homoallylic alkoxide, independent of steric considerations. Interestingly, entry 4 demonstrates a complete reversal in regioselection with respect to known cross-coupling reactions of TMS-substituted alkynes and imines (C–C bond-formation typically occurs β to the TMS-substitutent), hence demonstrating that the directing effect of the tethered alkoxide completely overrides the directing effect of the trimethylsilyl-substituent.[10]
With this site-selective alkyne–imine cross–coupling reaction in hand, we focused our attention on developing an intermolecular aza Pauson-Khand-like annulation reaction for the synthesis of tetrasubstituted γ-lactams (Table 2).[5c] As illustrated in entry 1, coupling of imine 16 and the methyl substituted internal alkyne 8, followed by exposure to CO2 (20 psi) and heating to 90 °C, furnishes the tetrasubstituted γ-lactam 17 as a single regioisomer in 66% yield. This annulation process is similarly effective with alkyne substrates possessing more sterically demanding substituents at the terminus of the alkyne (entries 2–4), and provides access to the ethyl-, isopropyl-, and TMS-substituted unsaturated γ-lactams 18–20 as single regioisomers. Substituted aromatic imines are also effective coupling partners in this reaction; o-Me, m-Br and p-Br-substituted aromatic imines 21, 23 and 25 provide the functionalized lactams 22, 24 and 26 in 55–63% yield (entries 5–7).[11] Interestingly, coupling of the ortho-substituted aromatic imine 21 with alkoxide 8 proceeded in a diastereoselective manner, producing the corresponding atropisomeric lactam 21 in a 4:1 ratio.
Table 2.
 |
Reaction conditions: Ti(Oi-Pr)4, c-C5H9MgCl, PhMe, −78 to −30 °C, then add unsaturated alkoxide (−30 to 0 °C), then CO2 (20 psi) 90 °C, 48 h.
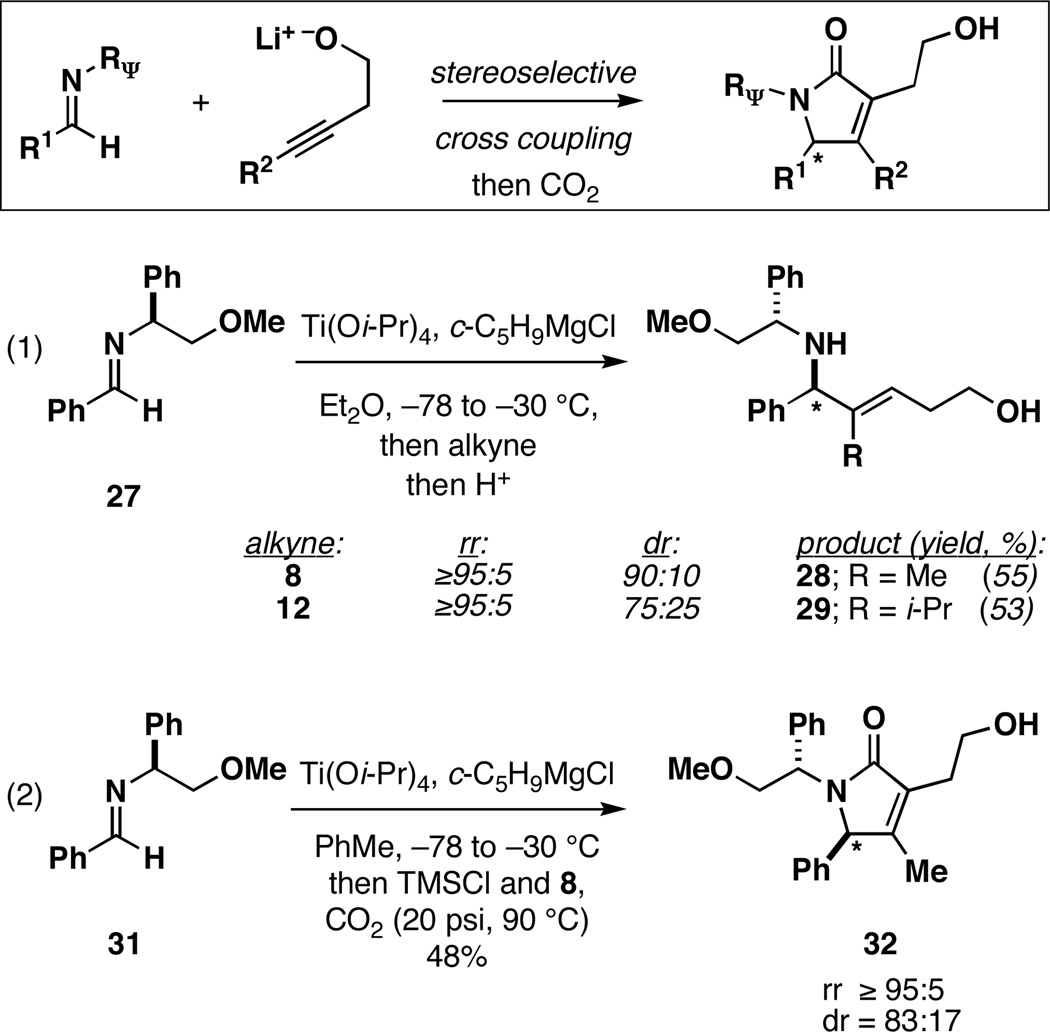
This [2+2+1] cross-coupling reaction can also be performed in a stereoselective manner. As depicted in Figure 2, use of a chiral imine 27[12] in the cross coupling reaction with alkyne 8 or 12 provides the unsaturated 1,5-amino alcohol products with ≥95:5 regioselection in all cases. Interestingly, diastereoselection in these reactions appears to be a function of the size of the terminal substituent of the alkyne (dr = 90:10 when R = Me, dr = 75:25 when R = i-Pr).
Figure 2.
Stereoselective synthesis of ene-1,5-amino alcohols and tetrasubstituted α,β-unsaturated γ-lactams.
This diastereoselective cross coupling can be extended to [2+2+1] annulation processes. As depicted in equation 2, coupling of imine 31, alkyne 8 and CO2 proceeds in a regio- and stereoselective manner to provide the γ-lactam 31 in 48% yield (rr ≥ 95:5; dr = 83:17).[13]
In conclusion, we have developed a highly regioselective cross coupling reaction between internal alkynes and imines that provides convergent access to ene-1,5-aminoalcohols or tetrasubstituted α,β-unsaturated γ-lactams.[14] Regiochemical control in these bimolecular coupling reactions results from alkoxide-directed carbometalation between a preformed azametallacyclopropene and an internal alkyne. Selectivity in these processes is independent of the differential size of substituents about the internal alkyne, and is completely dictated by the presence of a neighboring alkoxide. Finally, we have demonstrated the potential to employ this coupling reaction in a stereoselective manner whereby absolute stereochemical control is derived from a chiral imine. Further studies focused on controlling related intermolecular [2+2+1] processes are in progress.
Experimental Section
General Procedure (entry 1, Table 2): To a Schlenk tube containing a solution of imine 16 (0.292 g, 1.50 mmol) and Ti(Oi-Pr)4 (0.383 g, 1.35 mmol) in toluene (5 mL) at −78 °C was added c-C5H9MgCl (1.8 M in diethyl ether, 2.70 mmol) in a drop wise manner with a gas-tight syringe. The yellow solution was slowly warmed to −30 °C over one hour and the brown solution was stirred at −30 °C for a further two hours. Next, a solution of lithium alkoxide 8, generated from the deprotonation of the corresponding alcohol (0.028 g, 0.338 mmol) with n-BuLi (2.5 M in hexanes, 0.371 mmol), in toluene (900 µl) at −78 °C warming to 0 °C over twenty minutes, was added in a drop wise manner to the brown solution of imine 16 at −30 °C. The reaction was allowed to warm to 0 °C over one hour and stirred at 0 °C for four hours. The reaction was then cooled to −30 °C, evacuated, and backfilled with 20 psig CO2 (evacuation and backfilling repeated three times) and heated to 90 °C for 48 hours. Next, the reaction was removed from the oil bath, the CO2 was released, and the reaction was quenched with one mL of H2O. The resulting biphasic mixture was rapidly stirred until the precipitate became white in color. The solution was then further diluted with 0.5 M HCl (40 mL) and extracted with ether. The combined organic phases were washed with saturated NaHCO3, and brine, dried over MgSO4, and concentrated in vacuo. The crude material was purified by column chromatography on silica gel (50% → 66% EtOAc/Hexanes) to yield γ-lactam 17 as an off white solid (68 mg, 66%). For characterization of all new compounds, see the accompanying supporting information.
Footnotes
We gratefully acknowledge financial support of this work by the American Cancer Society, the American Chemical Society (PRF-G), the Arnold and Mabel Beckman Foundation, Boehringer Ingelheim, Eli Lilly & Co., and Yale University.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For reviews, see: Ojima I, Tzamarioudaki M, Li Z, Donovan RJ. Chem. Rev. 1996;96:635–662. doi: 10.1021/cr950065y. Schore NE. Chem. Rev. 1988;88:1081–1119. Gibson SE, Mainolfi N. Angew. Chem. Int. Ed. 2005;44:3022–3037. doi: 10.1002/anie.200462235.
- 2.For reviews, see: Pauson PL. Tetrahedron. 1985;41:5855–5860. Gibson SE, Stevanazzi A. Angew. Chem. Int. Ed. 2003;42:1800–1810. doi: 10.1002/anie.200200547.
- 3.Kablaoui NM, Hicks FA, Buchwald SL. J. Am. Chem. Soc. 1996;118:5818–5819. [Google Scholar]
- 4.a) Crowe WE, Vu AT. J. Am. Chem. Soc. 1996;118:1557–1558. [Google Scholar]; b) Mandal SK, Amin R, Crowe WE. J. Am. Chem. Soc. 2001;123:6457–6458. doi: 10.1021/ja005568m. [DOI] [PubMed] [Google Scholar]
- 5. Buchwald SL, Wannamaker MW, Watson BT. J. Am. Chem. Soc. 1989;111:776–777. Gao Y, Shirai M, Sato F. Tetrahedron Lett. 1996;37:7787–7790. For the conversion of an azatitanacyclopentene to a γ-lactam via exposure to carbon dioxide, see: Gao Y, Shirai M, Sato F. Tetrahedron Lett. 1997;38:6849–6852.
- 6.For a recent review of transition-metal-catalyzed reactions in heterocycle synthesis, see: Nakamura I, Yamamoto Y. Chem. Rev. 2004;104:2127–2198. doi: 10.1021/cr020095i.
- 7.Control of regioselection in bimolecular metal-mediated coupling reactions between internal alkynes and imines has been possible in only a small subset of reactions, whereby the origin of selectivity is derived by steric or electronic effects. For zirconium-mediated coupling, see: Buchwald SL, Watson BT, Wannamaker MW, Dewan JC. J. Am. Chem. Soc. 1989;111:4486–4494. Grossman RB, Davis WM, Buchwald SL. J. Am. Chem. Soc. 1991;113:2321–2322. For titanium-mediated coupling, see: Gao Y, Harada K, Sato F. Tetrahedron Lett. 1995;36:5913–5916. Gao Y, Yoshida Y, Sato F. Synlett. 1997:1353–1354. For nickel-mediated coupling, see: Patel SJ, Jamison TF. Angew. Chem. Int. Ed. 2003;42:1364–1367. doi: 10.1002/anie.200390349. Patel SJ, Jamison TF. Angew. Chem. Int. Ed. 2004;43:3941–3944. doi: 10.1002/anie.200460044. For rhodium-mediated coupling, see: Kong J-R, Cho C-W, Krische MJ. J. Am. Chem. Soc. 2005;127:11269–11276. doi: 10.1021/ja051104i.
- 8.Ryan J, Micalizio GC. J. Am. Chem. Soc. 2006;128:2764–2765. doi: 10.1021/ja057352w. [DOI] [PubMed] [Google Scholar]
- 9.Reichard HA, Micalizio GC. Angew. Chem. Int. Ed. 2006 accepted. [Google Scholar]
- 10. Gao Y, Harada K, Sato F. Tetrahedron Lett. 1995;36:5913–5916. See also: ref. 9. For an example of a unique nickel-catalyzed reductive coupling with TMS-C≡C-Ph and n-PrCHO that provides similar regioselection, see: Miller KM, Huang W-S, Jamison TF. J. Am. Chem. Soc. 2003;125:3442–3443. doi: 10.1021/ja034366y.
- 11.An aromatic heterocycle was tolerated on the imine, yet yields in the coupling reaction with 2-furylbenzylimine were uniformly lower (23%) than those between homopropargylic alkoxides and imines 7, 16, 21, 23 and 25, see supporting information for details.
- 12.Fukuhara K, Okamoto S, Sato F. Org. Lett. 2003;5:2145–2148. doi: 10.1021/ol034599u. [DOI] [PubMed] [Google Scholar]
- 13.In this process, the addition of TMSCl prior to heating in the presence of carbon dioxide minimized scrambling of the γ-stereocenter.
- 14.For an alternative coupling reaction of imines fumarates for the synthesis of α,β-unsaturated γ-lactams, see: Barluenga J, Palacios F, Fustero S, Gotor V. Synthesis. 1981:200–201.