Abstract
Background
Vertebrate otic and epibranchial placodes develop in close proximity in response to localized Fgf signaling. Although less is known about epibranchial induction, the process of otic induction in highly conserved, with important roles for Fgf3 and Fgf8 reported in all species examined. Fgf10 is also critical for otic induction in mouse, but the only zebrafish ortholog examined to date, fgf10a, is not expressed early enough to play such a role. A second zebrafish ortholog, fgf10b, has not been previously examined.
Results
We find that zebrafish fgf10b is expressed at tailbud stage in paraxial cephalic mesoderm beneath prospective epibranchial tissue, lateral to the developing otic placode. Knockdown of fgf10b does not affect initial otic induction but impairs subsequent accumulation of otic cells. Formation of epibranchial placodes and ganglia are also moderately impaired. Combinatorial disruption of fgf10b and fgf3 exacerbates the deficiency of otic cells and eliminates epibranchial induction entirely. Disruption of fgf10b and fgf24 also strongly reduces, but does not eliminate, epibranchial induction.
Conclusions
fgf10b participates in a late phase of otic induction and, in combination with fgf3, is especially critical for epibranchial induction.
Keywords: cranial placodes, otic vesicle, epibranchial ganglia, pax2a, sox3, phox2a
INTRODUCTION
Much has been learned in recent years about developmental regulation of vertebrate cranial placodes, transient ectodermal thickenings that produce key parts of the paired sensory structures in the head. The best characterized cranial placode is the otic placode, the precursor of the inner ear. In a process that is highly conserved amongst vertebrates, the otic placode is induced near the end of gastrulation by locally expressed Fgf3 and Fgf8 (Mansour et al., 1993; Phillips et al., 2001; Léger and Brand, 2002; Maroon et al., 2002; Liu et al., 2003; Alvarez et al. 2003; Wright and Mansour, 2003; Ladher et al., 2005; Martin and Groves, 2006; Freter et al., 2008; Park and Saint-Jeannet, 2008; Padanad et al., 2012). Fgf3 is expressed in the hindbrain adjacent to the otic region, and both Fgf3 and Fgf8 are expressed in subjacent mesoderm or endoderm (Mahmood et al., 1995; Lombardo et al., 1998; Phillips et al., 2001; Alvarez et al., 2003; Wright et al., 2003; Ladher et al., 2005; Nechiporuk et al., 2007; Nikaido et al., 2007). In addition, in mouse Fgf10 is expressed in subjacent mesoderm and plays a critical role in otic induction (Alvarez et al., 2003; Wright and Mansour, 2003), with a similar role played by Fgf19 in chick (Ladher et al., 2000; Freter et al., 2008). Whether Fgf10 (or other Fgfs) contribute to otic induction in zebrafish has not been reported. In all species examined otic induction still occurs when any single Fgf gene is impaired, whereas impairing any two Fgf genes leads to a profound deficiency or complete loss of otic tissue. Thus redundancy provided by multiple Fgfs from multiple tissues renders the process of otic induction highly robust and resistant to perturbation.
In a developmentally related process, epibranchial placodes form in close proximity to the otic placode and produce sensory neurons of the facial, glossopharyngeal and vagal ganglia. Although epibranchial placodes are induced at a slightly later stage, they require the same Fgfs that induce the otic placode (Nechiporuk et al., 2007; Nikaido et al., 2007; Sun et al., 2007). Moreover, otic and epibranchial placodes share expression of some early markers such as pax8 and sox3 (Nikaido et al., 2007; Sun et al., 2007; Padanad and Riley, 2011). Otic expression of pax8 and sox3 occurs first, and several hours later epibranchial expression appears as an arc wrapping laterally around the otic placode. The developmental basis for the delay in epibranchial induction is not fully understood, but in zebrafish it partly reflects an additional requirement for expression of Fgf24 in the nascent otic placode (Padanad and Riley, 2011). In general, induction of epibranchial placodes appears more sensitive to loss of any one Fgf gene than does the otic placode. For example, disruption of fgf24 has no discernable effect on otic development but causes a deficiency in all epibranchial ganglia except for the anterior-most facial ganglion (Padanad and Riley, 2011). Similarly, loss of fgf3 alone causes only slight reduction of the otic placode but results in substantial loss of glossopharyngeal and anterior vagal ganglia (Nechiporuk et al., 2005; Nechiporuk et al., 2007). It is possible that the greater dependency of epibranchial placodes on multiple Fgf genes simply reflects their greater distance from local signaling sources.
To more fully characterize the requirements for otic and epibranchial development in zebrafish, we conducted a survey of Fgf gene expression. We report here for the first time expression of fgf10b, which is first detected at tailbud stage in the developing pronephros and cranial mesoderm subjacent to the prospective epibranchial ectoderm. Knockdown of fgf10b reduces the size of all epibranchial ganglia. Simultaneous disruption of fgf10b and fgf3 blocks formation of all epibranchial ganglia. In contrast, knockdown of fg10b has no effect on early stages of otic induction but instead impairs a later “recruitment phase” of otic induction (Bhat et al., 2011). Accordingly, fgf10b-MO enhances otic deficiencies caused by disruption of either fgf3 or fgf8, but does not ablate otic development altogether. Deficiencies in otic and epibranchial development caused by simultaneous knockdown of fgf10b and fgf3 are rescued by low-level activation of heat shock-inducible fgf8 from tailbud to 10-somite stage (10–14 hpf), identifying a brief window during which these Fgfs are normally required. Thus fgf10b plays an auxiliary role in otic induction but is especially important for epibranchial induction during early to mid-somitogenesis stages.
RESULTS
Expression of fgf10b
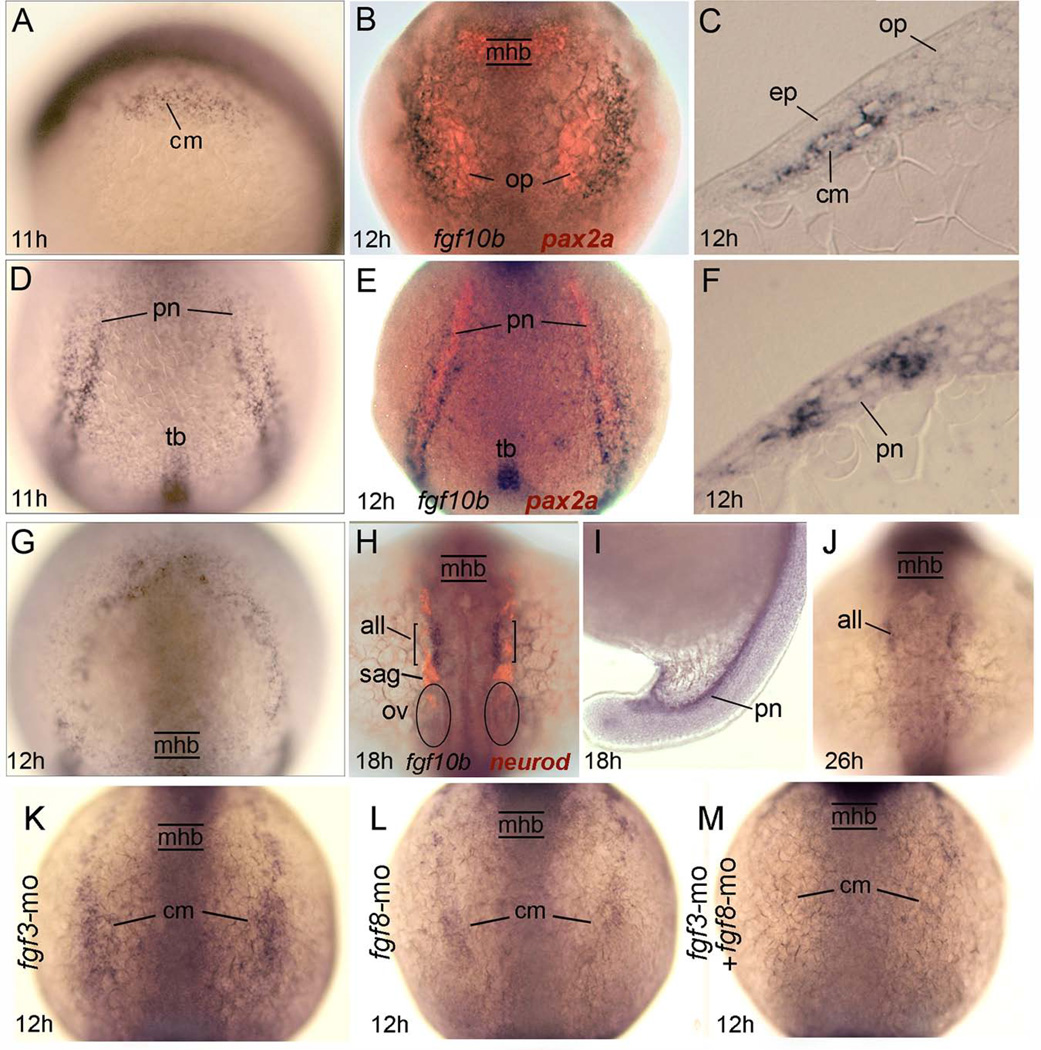
Based on conservation of function with other vertebrates, we hypothesized that an ortholog of Fgf10 is likely to contribute to otic placode induction in zebrafish. A previous study reported that the earliest cranial expression of fgf10a is detected at 14 hpf (10 somite stage) in the posterior lateral line (Nechiporuk and Raible, 2008), making it a poor candidate for an early otic inducer. In contrast, we found that a second ortholog, fgf10b, is expressed in a pattern suggesting it could play a role in otic induction. Expression of fgf10b is first detected at 10.7 hpf (tailbud stage) in bilateral longitudinal stripes adjacent to the hindbrain and a posterior set of stripes wrapping around the tailbud, as well as a small domain within the tailbud itself (Fig. 1A, D). The level of expression in these domains upregulates by 12 hpf (Fig. 1B, E). Co-labeling for pax2a and analysis of sections showed that the domain of maximal expression in the head marks paraxial cephalic mesendoderm just lateral to the developing otic placode (Fig. 1B, C). This domain predominantly marks mesodermal cells rather than endoderm, as expression was unaltered in embryos lacking endoderm due to knock down for sox32 (casanova) (data not shown). The posterior domain of fgf10b occurs in parallel stripes of mesoderm flanking the pronephric domain of pax2a (Fig. 1E, F). Also appearing at 12 hpf is an additional domain of diffuse expression wrapping around the anterior neural plate (Fig. 1G). By 18 hpf, expression is limited to bilateral stripes in the head corresponding to the anterior lateral line and the developing pronephros, domains that persist through 26 hpf (Fig. 1H–J). We detected no expression at later stages (not shown).
Fig. 1.
Expression and regulation of fgf10b. A–G: Early expression of fgf10b marks mesoderm near the otic/epibranchial domain (A–C), flaking the pronephros (D–F) and surrounding the anterior neural plate (G). Specimens in (B) and (E) also show pax2a expression (red) in the otic placode and pronephros, while (C) and (F) show fgf10b expression in cross sections. H–J: Late expression in the anterior lateral line and pronephros. The specimen in (H) also shows neurod expression (red) to reveal neurogenesis in various placodal derivatives. K–M: Expression of fgf10b in cranial mesoderm appears normal in fgf3 morphants (K) but is reduced in fgf8 morphants (L) and nearly abolished in fgf3-fgf8 double morphants (M). Abbreviations: all, anterior lateral line; cm, cranial mesoderm; ep, epibranchial placode; mhb, midbrain-hindbrain border. op, otic placode; ov, otic vesicle; pn, pronephros; sag, statoacoustic ganglion; tb, tailbud. Wholemount specimens are shown with dorsal views (anterior to the top) except for (A, I), which are lateral views with dorsal to the right. Cross sections are shown with dorsal to the top.
Because expression of Fgf10 in mouse subotic mesenchyme requires Fgf3 and Fgf8 (Ladher et al., 2005), we tested whether there is a similar requirement for zebrafish fgf10b. Expression of fgf10b appeared normal in fgf3 morphants but was markedly reduced in the posterior limit of the cranial domain in fgf8 morphants and was almost undetectable in fgf3-fgf8 double morphants (Fig. 1K–M). Thus expression of fgf10b adjacent to prospective otic/epibranchial tissue requires prior expression of fgf3 and fgf8, similar to the regulation seen in mouse.
Testing morpholinos for fgf10b
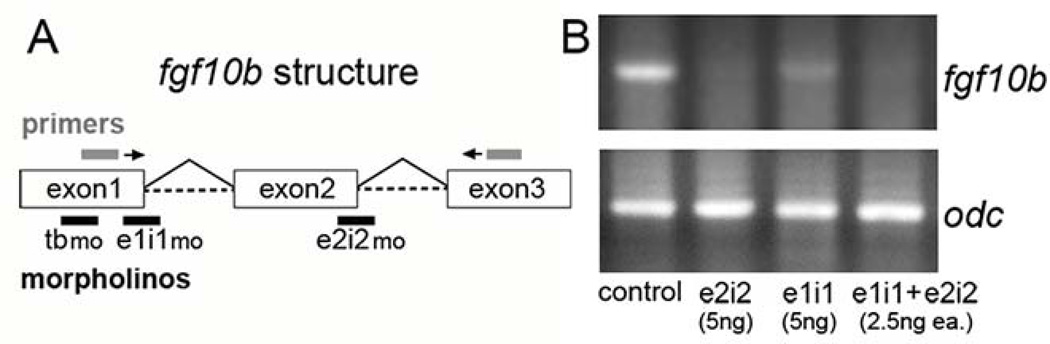
We next tested three different morpholino oligomers (MOs) to knockdown fgf10b function, one to block translation and two to block splicing at different exon-intron junctions (Fig. 2A). Injection of 5 ng of individual MOs caused similar phenotypes, although the translation blocker caused moderate non-specific cell death in the brain, a phenotype often associated with off-target effects (not shown). The effects of splice-blocking MOs appeared more specific and showed little or no brain necrosis. To further minimize the risk of potential off-target effects all data reported herein were generated by co-injecting 2.5 ng of each splice-blocker. This combination effectively and specifically reduced accumulation of mature fgf10b mRNA (Fig. 2B) and caused a highly reproducible phenotype with little or no cell death in the brain (Fig. 3A, D).
Figure 2.
Efficacy of fgf10b knockdown. A: Diagram showing the exon/intron structure of the fgf10b locus. Above the diagram are primer-binding sites for PCR, and below are target sites for translation-blocking MO (tbmo) and splice-blocking MOs (e1i1mo) and (e2i2mo). B: RT-PCR amplification of fgf10b mRNA at 12 hpf in control embryos or embryos injected with the indicated mounts of splice-blocking MO. Expression of ornithine decarboxylase (odc) at 12 hpf serves as a control.
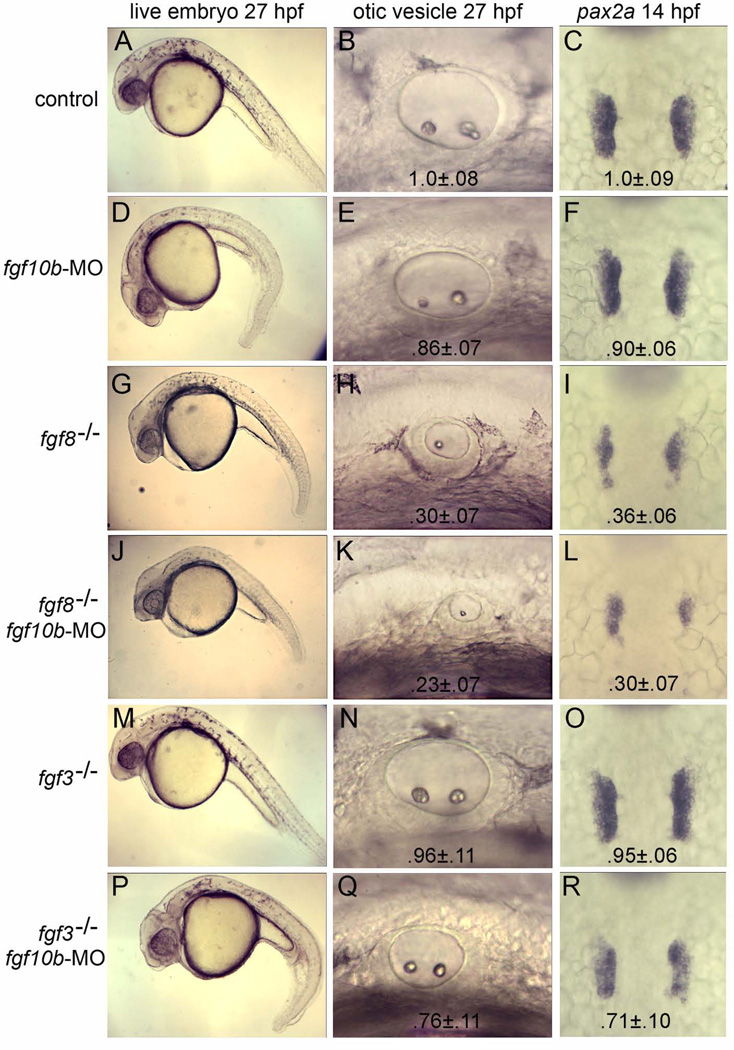
Figure 3.
fgf10b cooperates with fgf3 and fgf8 in otic induction. A–R: Live specimens at 27 hpf viewed at low magnification to show general morphology and high magnification to show otic vesicles; and dorsal views of in situ hybridizations to visualize pax2a in the otic placodes at 14 hpf. Genetic manipulations are indicated down the left side of the figure. Mean surface area (± standard deviation, n≥8) of otic placodes and vesicles, normalized to wild-type control embryos, are indicated for each genotype/knockdown.
Role in otic placode induction
Knockdown of fgf10b resulted in development of a ventrally curved embryonic axis and, additionally, the circumference of the otic vesicle was reduced by 10–15% at 27 hpf (p<.05) (Fig. 3D, E). Examination of early otic markers showed that knockdown of fgf10b had no affect on the pattern of pax8 expression at 11 hpf (not shown). By 14 hpf, however, the otic domain of pax2a was reduced in area by about 10% in fgf10b morphants (p<.05) (Fig. 3F). Thus, fgf10b is not required for initial induction of pax8 but is instead required for subsequent expansion of the otic domain. This is consistent with the slightly later onset of fgf10b expression relative to early otic expression of pax8.
We hypothesized that redundancy from other inductive Fgfs partially compensates for loss of fgf10b. In support, loss of fgf8 caused a 65–70% reduction in the size of the otic placode and vesicle (Fig. 3H, I) and there was a slight additional decrease (though not statistically significant) following knockdown of fgf10b in fgf8−/− mutants (Fig. 3K, L). Knockdown of fgf10b also enhanced the short axis defect seen in fgf8−/− mutants (Fig. 3G, J). Because the severity of the fgf8−/− mutant phenotype complicates analysis of fgf10b function, we also examined the interaction between fgf10b and fgf3. Loss of fgf3 alone caused only 5% reduction in the size of the otic placode and vesicle (Fig. 3N, O), whereas disruption of both fgf3 and fgf10b reduced the size of the otic placode and vesicle by 25–30% (Fig. 3Q, R). This is significantly more severe than the phenotype seen in fgf10b morphants (pax2a domain, p<.01). These findings are consistent with a model in which fgf3 and fgf8 are required for initial otic induction whereas fgf10b is required only to increase the size of the otic placode after 11 hpf (see Discussion).
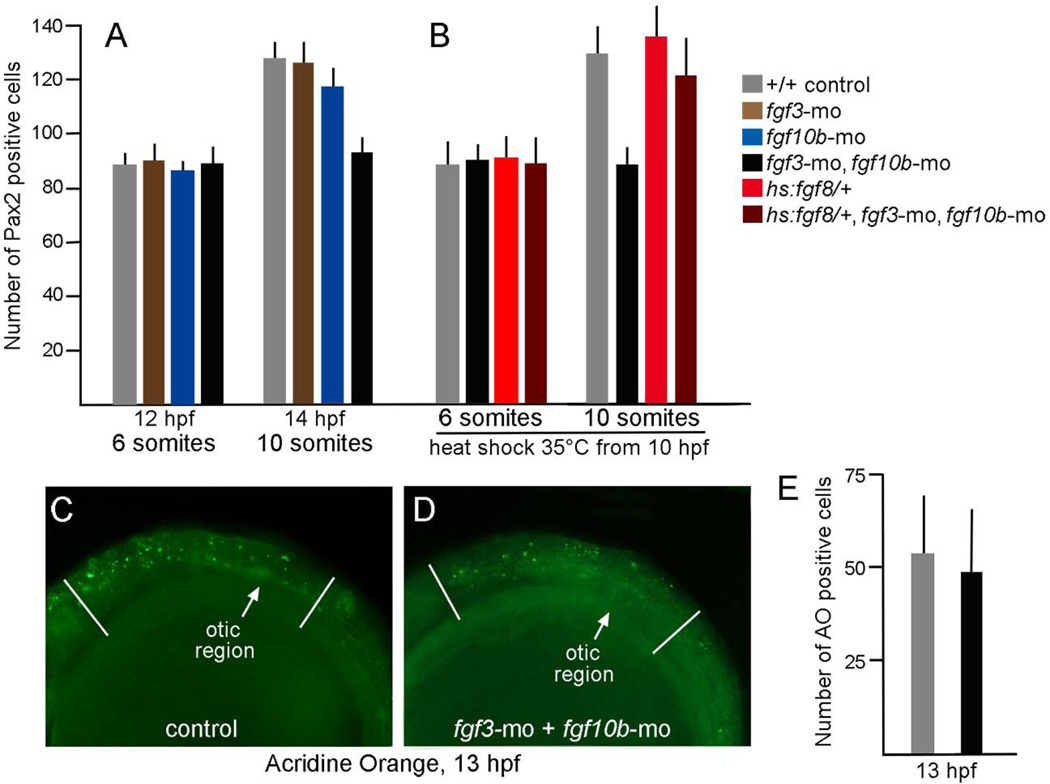
The deficiency in otic tissue caused by knockdown of fgf10b could arise from insufficient induction of otic cells or failure to maintain otic fate. To distinguish between these possibilities, we determined the number of pax2a-expressing cells at stages after fgf10b is normally expressed. Staining of otic cells with Pax2 polyclonal antibody is first detected at around 12 hpf, indicating a lag of roughly 1 hour from the onset of pax2a transcription. At 12 hpf the number of Pax2-positive cells appeared normal in embryos knocked down for fgf10b and/or fgf3 (Fig. 4A). We previously documented that the number of Pax2-positive cells shows a 50% increase between 12 hpf and 14 hpf (10 somites) through a recruitment process that does not require cell division (Riley et al. 2010; Bhat et al., 2011). The same fold-increase is seen in fgf3 morphants, while fgf10b morphants show a 35% increase by 14 hpf (Fig. 4A). In contrast, there is no detectable change in the number of Pax2-positive cells in fgf3-fgf10b double morphants by 14 hpf (ig. 4A). This failure in otic expansion does not reflect attrition from elevated cell death, as the number of acridine orange-staining cells is normal in fgf3-fgf10b double morphants during this period (Fig. 4C–E). We next tested whether the deficiency in otic expansion seen in fgf3-fgf10b double morphants could be rescued by weakly elevating Fgf signaling after the initial phase of otic induction. For this we used a heat shock-inducible transgene, hs:fgf8, activated at a low level by mild heat shock at 35°C. Under these conditions it takes about 1 hour to detect a rise in Fgf signaling (not shown). Unlike standard heat shock conditions at 39°C (Padanad et al., 2012), incubating hs:fgf8/+ embryos at 35°C beginning at 10 hpf did not significantly enhance otic development (Fig. 4B). Nevertheless, weak activation of hs:fgf8 at 35°C was sufficient to rescue the otic deficiency in fgf3-fgf10b double morphants (Fig. 4B). Together these data indicate that fgf10b, acting together with fgf3, is required for a late secondary phase of otic induction.
Figure 4.
Otic deficiencies in fgf3-fgf10b double morphants and rescue by timed misexpression of fgf8. A–B: The number of Pax2-expressing cells in the otic vesicle at 12 hpf and 14 hpf in embryos with various genotypes and gene knockdown, as indicated in the color key. Embryos in (B) were incubated at 35°C from 10 hpf until fixation at the 8- or 10-somite stage. Note that incubation at 35°C accelerates development such that these stages occur slightly earlier than normal. C–D: Comparison of the number of apoptotic cells stained with acridine orange (AO) in the head between the eye and first somite (marked by white lines), as seen from a lateral view in control embryos (C) or fgf3-fgf10b double morphants (D). Data in (E) show means and standard deviations of eight specimens each.
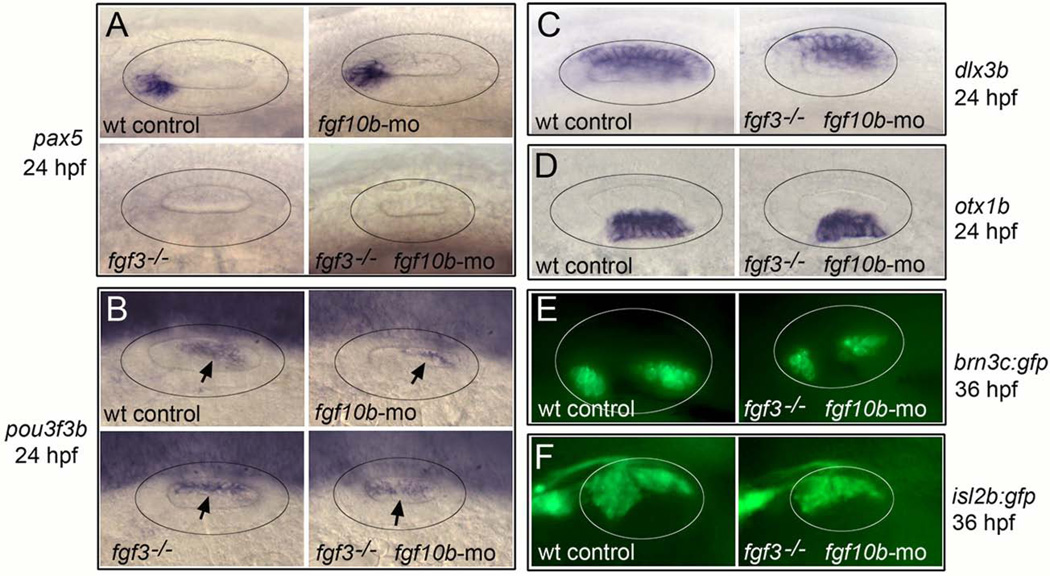
To test whether the early requirement for fgf10b affects subsequent otic patterning, we examined expression of regionally expressed markers within the developing otic vesicle. fgf10b morphants showed normal expression of all markers tested, including anterior marker pax5, posterior marker pou3f3b (zp23), dorsal marker dlx3b and ventrolateral marker otx1b (Fig. 5A, B). In fgf3-fgf10b-deficient embryos, dlx3b and otx1b were expressed normally, whereas pax5 expression was lost and the domain of pou3f3b expanded anteriorly (Fig. 5A–D). Altered expression of pax5 and pou3f3b was also seen in fgf3−/− mutants (Fig. 5A, B), reflecting the role of fgf3 in regulating antero-posterior patterning in the otic vesicle (Kwak et al., 2002; Kwak et al., 2006; Hammond and Whitfield, 2011). Despite the changes in patterning in fgf3-fgf10b-deficient embryos, sensory epithelia and neurons of the statoacoustic ganglion (SAG) appeared to form relatively normally, albeit in slightly smaller domains corresponding to the reduced size of the otic vesicle (Fig. 5E, F). Thus, knockdown of fgf10b does not cause obvious changes in otic patterning despite the early deficiency in otic placode development. Moreover, altered patterning seen in fgf3-fgf10b-deficient morphants is attributable solely to loss of fgf3.
Figure 5.
Effects of fgf10b knockdown on otic vesicle patterning. A–F: Lateral view (anterior to the left) of the otic vesicle (circled) at 24 hpf (A–D) and 36 hpf (E–F) labeled for the indicated genes. Arrows in (B) indicate the otic domain of pou3f3b.
Role in epibranchial placode induction
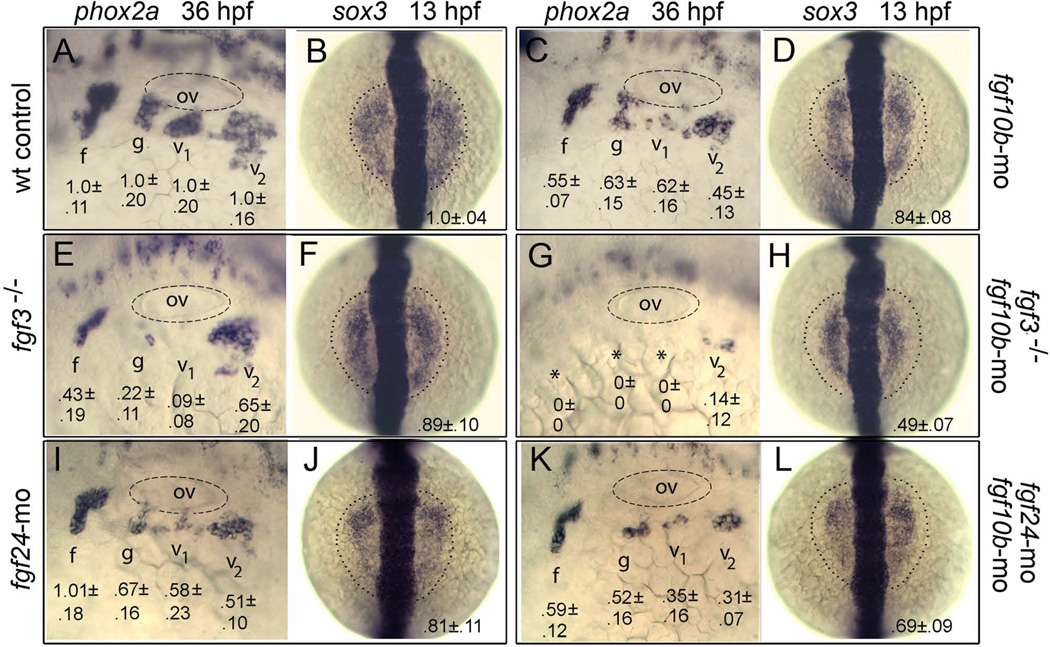
Epibranchial placodes, which produce sensory neurons of epibranchial ganglia, are produced in close proximity to the otic placode and are co-regulated in part by mesodermal expression of fgf3 (Nechiporuk et al., 2007). We therefore examined whether fgf10b cooperates with fgf3 in epibranchial regulation. Knockdown of fgf10b alone caused reduction in all epibranchial ganglia, as marked by reduced domains of phox2a at 36 hpf (Fig. 6A, C). As previously reported (Nechiporuk et al., 2007), disruption of fgf3 resulted in loss of glossopharyngeal and vagal I ganglia and reduction in the facial ganglion (Fig. 6E). Embryos deficient for both fgf3 and fgf10b produced little or no epibranchial ganglia (Fig. 6G). To determine the developmental basis for loss of epibranchial ganglia, embryos were examined for expression of sox3, the earliest known marker of epibranchial placode formation (Nikaido et al., 2007; Sun et al., 2007). In control embryos expression of sox3 is initially co-induced with pax8 in the otic placode by 9.5–10 hpf. Between 11–12 hpf, sox3 expression expands outward to mark adjacent epibranchial tissue and simultaneously downregulates in otic tissue. Knockdown of fgf10b did not affect otic expression of sox3 at 11 hpf, but the epibranchial domain of sox3 appeared slightly reduced at 13 hpf (p<.05) (Fig. 6B, D). A slight but insignificant reduction in the epibranchial domain of sox3 was also seen in fgf3 morphants or mutants (Fig. 6F). In contrast, the epibranchial domain of sox3 was reduced by half in fgf3-fgf10b double morphants (Fig. 6H), a highly significant reduction relative to fgf10b morphants (p<.01).
Figure 6.
fgf10b cooperates with fgf3 and fgf24 in epibranchial induction. A–L: Lateral views (anterior to the left) of phox2a expression in epibranchial ganglia at 36 hpf; and dorsal views (anterior to the top) of sox3 expression at 13 hpf (normal boundaries of the control are outlined). Genetic manipulations are indicated along the sides. Positions of the otic vesicle (ov) and facial (f), glossopharyngeal (g) and vagal ganglia (v1 and v2) are indicated. Mean surface areas (± standard deviation, n≥8), normalized to wild-type control embryos, are indicated for each structure.
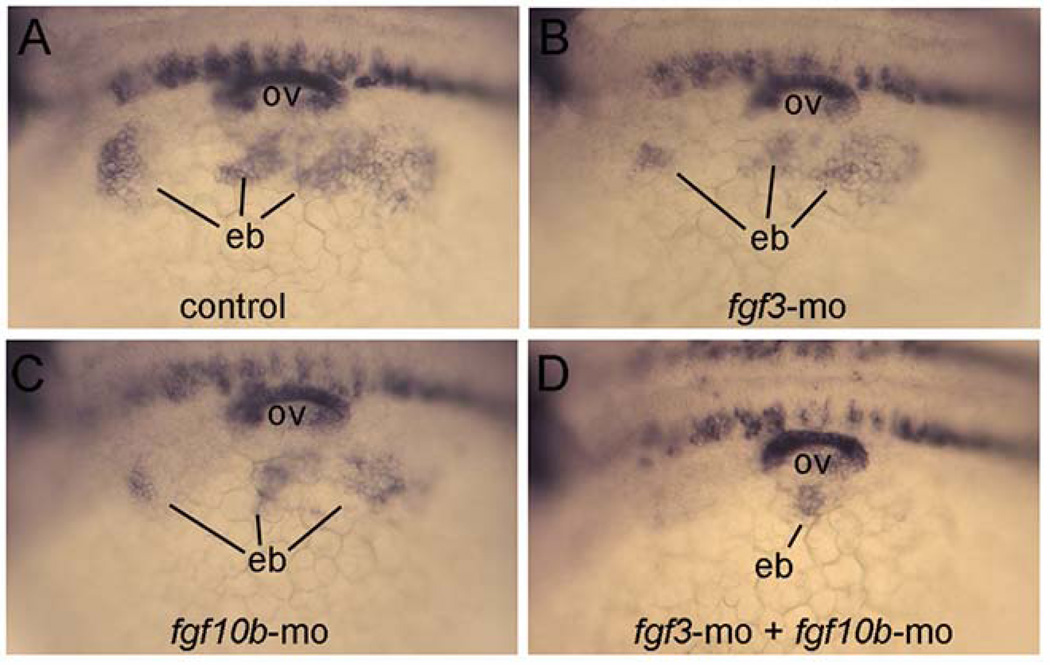
To confirm the early deficiency in epibranchial placode formation, we examined expression of pax2a at 24 hpf when epibranchial placode thickening first becomes evident. As expected, moderate deficiencies were seen in the epibranchial domain of pax2a in fgf3 morphants and fgf10b morphants, and this domain was almost completely ablated in fgf3-fgf10b double morphants (Fig. 7). Thus, fgf10b and fgf3 are together required for proper induction of epibranchial placodes and subsequent differentiation of epibranchial ganglia.
Figure 7.
Loss of epibranchial expression of pax2a in fgf3-fgf10b deficient embryos. A–D: Lateral views (anterior to the left) showing expression of pax2a in the otic vesicle (ov) and nascent epibranchial placode (eb) at 24 hpf. Compared to a control embryo (A), knockdown of fgf3 (B) or fgf10b (C) causes moderate deficiencies in epibranchial placodes, whereas knockdown of both fgf3 and fgf10b nearly abolishes the epibranchial domain (D).
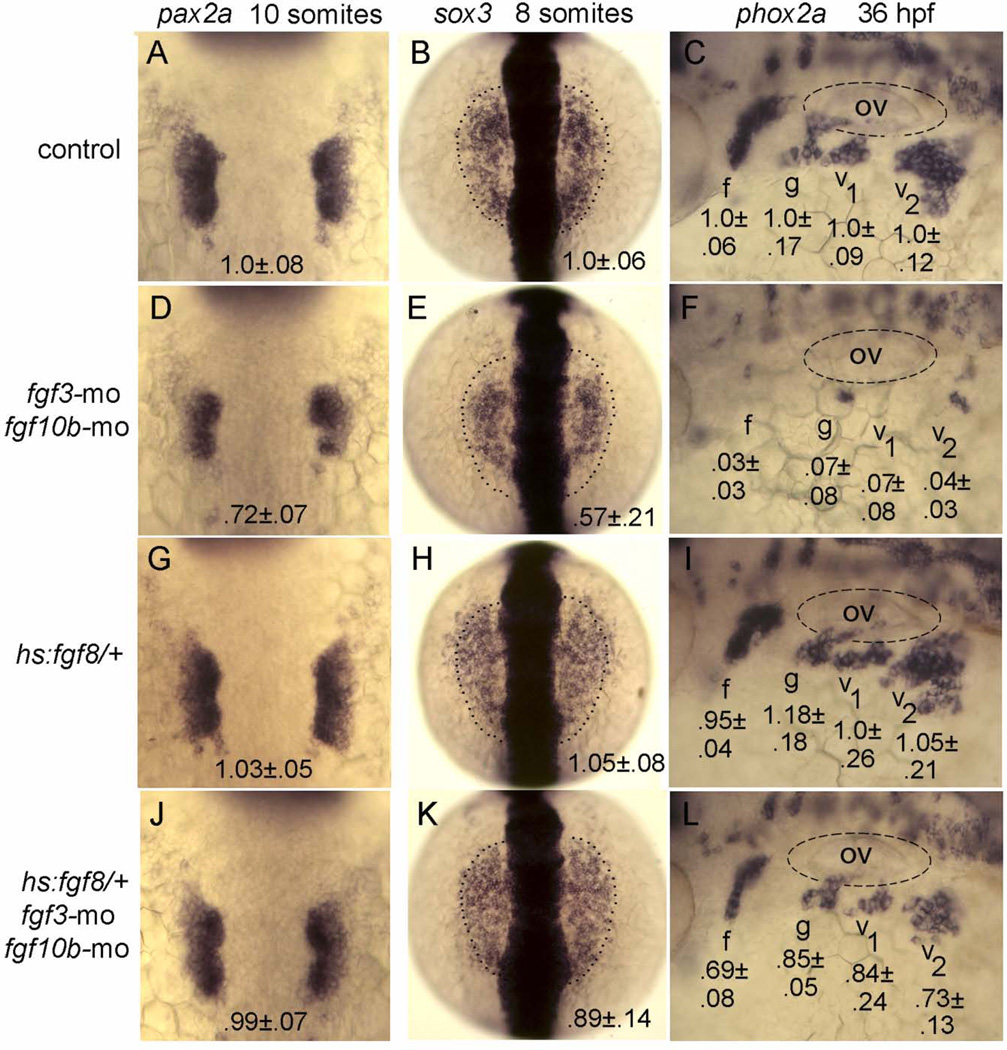
To examine the temporal requirements for Fgf in epibranchial placode induction, we tested whether weak early activation of hs:fgf8 could rescue the epibranchial deficiency in fgf3-fgf10b double morphants. Incubation of hs:fgf8/+ transgenic embryos at 35°C from 10 hpf until 8- or 10-somite stage (equivalent to 13 or 14 hpf, respectively) had negligible effects on placodal expression of pax2a or sox3, nor was development of epibranchial ganglia significantly altered at 36 hpf (Fig. 8G–I). Nevertheless, this brief pulse of hs:fgf8 activity was sufficient to fully rescue otic development and provide substantial rescue of epibranchial development in fgf3-fgf10b double morphants (compare Fig. 8D–F, J–L). Similar rescue was obtained by activating hs:fgf8 at 37°C from 12 to 14 hpf (data not shown). Rescue of epibranchial neurogenesis was not complete, however (Fig. 8L), probably reflecting a later requirement for fgf3 expressed in pharyngeal endoderm at 24 hpf (Nechiporuk et al., 2005). Nevertheless rescue by early hs:fgf8 activity suggests that fgf10b and fgf3 are required during early somitogenesis stages for epibranchial placode induction.
Figure 8.
Rescue of epibranchial development in fgf3-fgf10b deficient embryos by timed misexpression of fgf8. A–L: Dorsal views (anterior to the top) of pax2a expression at the 10-somite stage and sox3 expression at the 8-somite stage (normal boundaries of the control are outlined), and lateral views (anterior to the left) of phox2a expression at 36 hpf in embryos incubated at 35°C from 10 hpf until the 8-somite stage (for sox3) or the 10-somite stage (for pax2a and phox2a). Genetic manipulations are indicated along the left side. Positions of the otic vesicle (ov) and facial (f), glossopharyngeal (g) and vagal ganglia (v1 and v2) are indicated. Mean surface areas (± standard deviation, n≥8), normalized to wild-type control embryos, are indicated for each structure
Because epibranchial placode development also partly depends on expression of fgf24 in the otic placode (Padanad and Riley, 2011), we examined genetic interaction between fgf24 and fgf10b. As previously reported (Padanad and Riley, 2011), knockdown of fgf24 caused a deficiency of glossopharyngeal and vagal ganglia at 36 hpf, whereas the facial ganglion developed normally (Fig. 6I). Note, the deficiency seen in posterior epibranchial ganglia is less severe than what we previously reported at 30 hpf (Padanad and Riley, 2011), indicating that fgf24 morphants show partial recovery by 36 hpf. Knockdown of both fgf10b and fgf24 led to a more pronounced deficiency of glossopharyngeal and vagal ganglia at 36 hpf than was seen in single morphants (compare Fig. 6C, I, K). The deficiencies in epibranchial ganglia seen at 36 hpf reflected corresponding deficiencies in the area of sox3 expression seen at 13 hpf (Fig. 6J, L). Specifically, knockdown of fgf24 caused a decrease of nearly a 20% in the sox3 domain (p<.01), and fgf10b-fgf24 double morphants showed a decrease of about 30% (p<.01). Thus, fgf10b and fgf24 work together to induce a normal amount of epibranchial tissue.
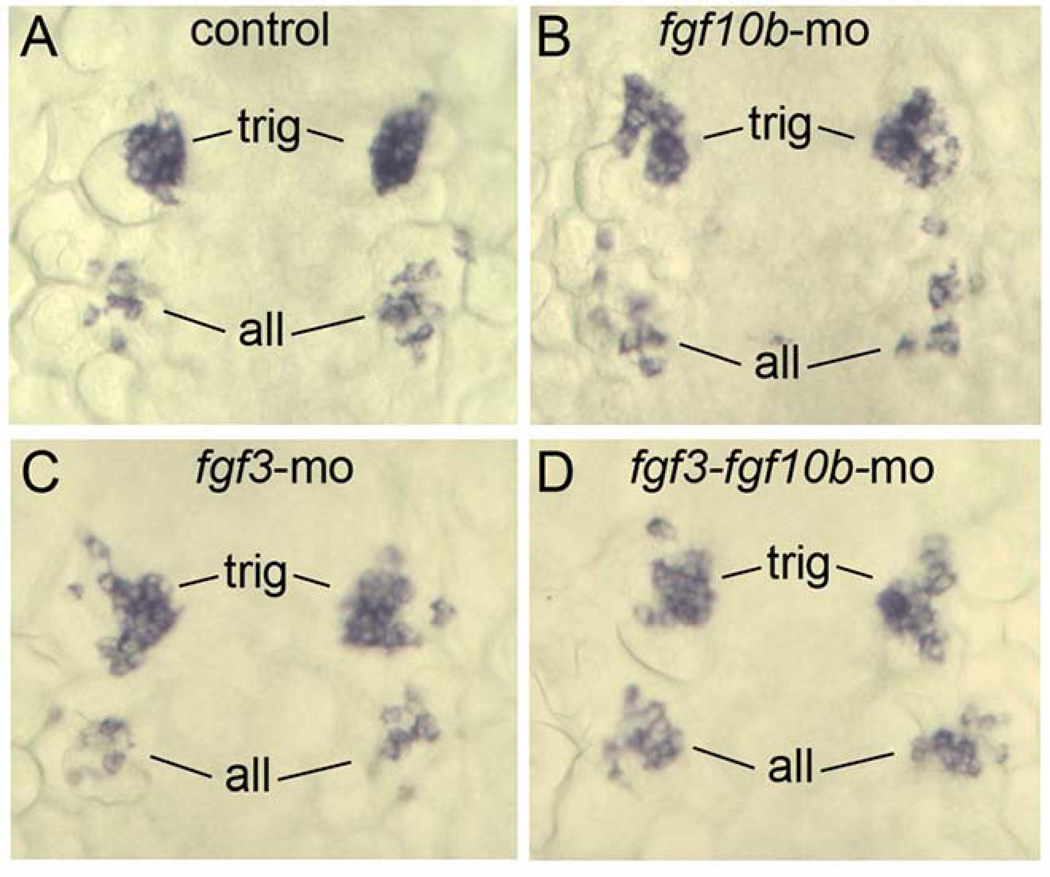
No role in more anterior placodes
Because fgf10b expression extends into more anterior mesoderm, we examined whether more anterior placodes were affected by knockdown of fgf10b. Expression of neurod marks early stages of development of the anterior lateral line and trigeminal placodes at 14 hpf, and these domains appeared normal in fgf10b morphants (Fig. 9A, B). Similarly, neurod expression was normal in fgf3 and fgf3-fgf10b double morphants (Fig. 9C, D). We also noted that nasal pit and the lens of the eye form with normal morphology in embryos disrupted for fgf3 and/or fgf10b (not shown). Thus, only the otic and epibranchial placodes require fgf10b and fgf3 for proper development.
Figure 9.
fgf10b is not required for development of other cranial placodes. A–D: Dorsal views (anterior to the top) showing expression of neurod in nascent trigeminal (trig) and anterior lateral line (all) placodes at 14 hpf in a control embryo (A), fgf10b morphant (B), fgf3 morphant (C) and fgf3-fgf10b double morphant.
DISCUSSION
We have shown that fgf10b is expressed in cranial paraxial mesoderm by tailbud stage and contributes to induction of both otic and epibranchial placodes (Fig. 10). As detailed below, our findings complement and extend earlier studies and shed light on evolutionary conservation of the underlying mechanisms. We also document expression of fgf10b in mesoderm flanking the developing pronephros but did not investigate the function of fgf10b in this domain.
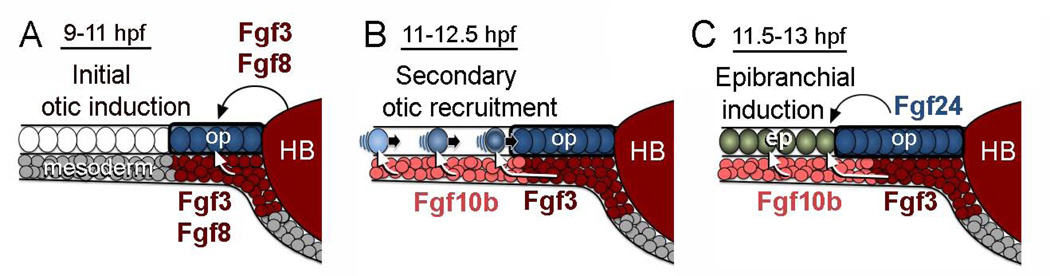
Figure 10.
A network of Fgf ligands regulates otic-epibranchial induction. A: Initial otic induction is mediated by Fgf3 and Fgf8 from the hindbrain (HB) and sub-otic mesoderm. B: During secondary otic recruitment, cells converging from more lateral regions initially encounter mesodermal Fgf10b followed by Fgf3. Newly recruited cells continue to join the otic placode through 12.5 hpf (Bhat et al., 2010). C: In a final phase, epibranchial fates are induced by a combination of mesodermal Fgf10b and Fgf3 and otic Fgf24. Cells in the facial placode are induced first, followed by cells in more posterior epibranchial tissue (Nechiporuk et al., 2007).
Otic induction
The principal otic inducers in zebrafish are Fgf3 and Fgf8 secreted from the hindbrain and subotic mesendoderm (Fig. 10A). Accordingly, disruption of both fgf3 and fgf8 abolishes otic induction entirely (Phillips et al., 2001; Léger and Brand, 2002; Maroon et al., 2002; Liu et al., 2003). In contrast, fgf10b is expressed after the initial phase of otic induction such that knockdown of fgf10b results in only 10–15% reduction in the size of the otic placode and vesicle (Fig. 3). This change is consistent with a role for fgf10b in “secondary otic recruitment” (Fig. 10B). We previously documented that the otic placode continues to expand after 11 hpf through a mechanism that does not involve cell division (Riley et al., 2010). Instead, during the later phase of recruitment cells converge by directed migration from surrounding regions into the growing otic placode, a process that requires integrin-α5 (itga5) (Bhat et al., 2011). In itga5 mutants, cells meander haphazardly such that fewer cells move into range of inductive signals in time to join the otic placode. Time-lapse imaging of lineage-labeled cells shows that secondary otic recruitment occurs during a window from 11–12.5 hpf (Bhat et al., 2011). This is consistent with the timing of fgf10b expression. Moreover, the domain of fgf10b expression is optimally positioned to impact late-migrating cells just prior to entering the otic domain (Fig. 1A, 10B). We do not know whether disruption of fgf10b affects the pattern of cell migration, but it clearly impedes later expansion of the otic domain. Mesodermal Fgf3 also appears to contribute to secondary otic recruitment, as fgf3-fgf10b double morphants show a significantly more pronounced otic deficiency than single morphants, and this deficiency is rescued by a weak pulse of hs:fgf8 activity beginning after initial otic induction (Figs. 3, 4).
Comparison between mouse and zebrafish suggests a broadly conserved role for Fgf10 in otic development. In a pattern similar to zebrafish fgf10b, mouse Fgf10 is first expressed in cranial mesoderm beneath the prospective otic placode at the zero-somite stage and requires earlier expression of Fgf8 (Ladher et al. 2005). The mesodermal domain of Fgf10 is clearly important for otic induction in mouse (Alvarez at el., 2003; Wright and Mansour, 2003). Subsequently, mouse Fgf10 is expressed in the developing otic vesicle where it regulates neurogenesis (Pauley et al., 2003). In zebrafish, comparable expression in the otic vesicle is observed for fgf10a but not fgf10b (Feng and Xu, 2010). Thus the two fgf10 paralogs in zebrafish appear to have undergone subfunctionalization to regulate early vs. late functions in otic development. In chick Fgf10 is first detected in the otic placode and vesicle to regulate otic neurogenesis (Alsina et al., 2004), but no earlier mesodermal domain has been detected. Instead, mesodermal expression of Fgf19 contributes to otic induction in chick (Ladher et al., 2000), a function not shared by Fgf19 orthologs in zebrafish or mouse (Wright et al., 2004; Miyake et al., 2005).
Epibranchial induction
The role of Fgf in epibranchial induction has been studied most extensively in zebrafish. Early markers of epibranchial induction include sox3, pax8 and pax2a, all of which require Fgf (Nikaido et al., 2007; Sun et al., 2007; Padanad et al., 2011; McCarroll et al., 2012). Expression of sox3 is particularly dynamic and expands from a central otic domain into adjacent epibranchial tissue between 11 and 12 hpf. Time lapse imaging of cell migration shows that cells continue to enter the epibranchial domain of pax2a expression until just after 13 hpf (Bhat et al., 2011; Fig. 10C). Timed disruption of Fgf using the pharmacological inhibitor SU5402 reveals that epibranchial induction follows an anterior-to-posterior progression: Induction of the facial placode requires Fgf by around11.5 hpf whereas induction of glossopharyngeal and vagal placodes require Fgf at slightly later stages (Nechiporuk et al., 2007). The above studies suggest a general timeframe for epibranchial induction that is consistent with the requirement for Fgf3 and Fgf10b. Nearly all epibranchial development is abolished in fgf3-fgf10b deficient embryos, and this deficiency is substantially rescued by a low pulse of hs:fgf8 activity at 35°C from 10–14 hpf (Figs. 3 and 8) or a moderate pulse at 37°C from 12–14 hpf (data not shown). In addition, Fgf24 secreted from the nascent otic placode during the same timeframe is required for normal development of gossopharyngeal and vagal placodes (Padanad et al., 2011; Fig. 6; Fig. 10C). Interestingly, otic fgf24 is expressed relatively normally in fgf3-fgf10b deficient embryos yet is not sufficient to support epibranchial induction. Thus epibranchial induction is critically dependent on the spatial distribution and overall level of Fgf signaling during early somitogenesis stages.
In addition to its early inductive role, fgf3 is also expressed at later stages in pharyngeal endoderm and helps initiate neurogenesis within developing epibranchial tissue (Nechiporuk et al., 2005). Blocking endoderm formation does not block induction of sox3 in nascent epibranchial placodes but profoundly affects subsequent neurogenesis. Thus, loss of fgf3 affects both epibranchial induction and later neurogenesis, compounding its interaction with fgf10b. Pharyngeal Bmp is also required for epibranchial neurogenesis (Begbie et al., 1999; Holzschuh et al., 2005), although the relationship between pharyngeal Fgf3 and Bmp is not known.
Studies in other vertebrates on the role of Fgf in epibranchial induction are still limited. The earliest potential marker for epibranchial development in chick and mouse is Foxi2, which is expressed in ectoderm immediately surrounding the nascent otic placode and likely encompasses epibranchial precursors (Oyama and Groves, 2004; Freter et al., 2008). Expression of chick Foxi2 requires Fgf and increases in response to exogenous Fgf2 (Freter et al., 2008; Yang et al., 2013), but there have been no knockdown studies to indicate which endogenous Fgf genes are involved. The situation in mouse is less clear, as the epibranchial domain of Foxi2 expands at the expense of otic markers in Fgf3-Fgf10 double mutants (Urness et al., 2010). Clearly additional studies are needed to clarify these issues and to evaluate the degree to which mechanisms have been conserved in various vertebrate species.
EXPERIMENTAL PROCEDURES
Fish Strains and Staging
Wild-type zebrafish were obtained from that AB line (Eugene, OR). Embryos were developed under standard conditions at 28.5°C and staged according to standard morphological criteria (Kimmel et al., 1995). To ensure precise staging of young embryos used for gene expression studies, embryos were co-stained for myoD to count somites. Mutant alleles fgf3t26212 and fgf8x15 and PCR-based genotyping protocols have been previously described (Herzog et al., 2004; Kwon and Riley, 2009). Transgenic lines TG(brn3c:gap43-GFP) (Xiao et a., 2005) and TG(-17.6isl2b:GFP)zc7 (Pitman et al., 2008) were used to visualize sensory hair cells and mature neurons of the statoacoustic ganglion, respectively. Misexpression of fgf8 was achieved by briefly heat shocking heterozygous transgenic embryos carrying Tg(hsp70:fgf8)x17 (Millimaki et al., 2010) at 35°C.
Morpholinos and Phenotypic Analysis
Morpholino oligomers (MOs) were obtained from Gene Tools, Inc. Sequences and testing of MOs to knockdown fgf3, fgf8, fgf24, p53 and casanova (sox32) have been previously described (Draper et al., 2001; Sakaguchi et al., 2001; Fischer et al., 2003; Robu et al., 2007; Kwon and Riley, 2009). To knockdown fgf10b, the following MO sequences were used: tb-MO to block translation TCCATCTCCTCATGGTACTCCTCAT; e1i1-MO to target the exon1-intron1 splice junction AATCGTGACTCACTGTACGGGTCGT; e2i2-MO to target the exon2-intron2 splice junction GACACTCACCACTCCAAACAGCTCC. For most target sequences, embryos were injected with 5ng per MO at the one-cell stage. All three fgf10b-MOs produced similar phenotypes. For most experiments involving knockdown of fgf10b, embryos were co-injected with 2.5ng each of e1i1-MO and e2i2-MO to minimize off-target effects. To further guard against nonspecific cell death caused from off-target effects, embryos were also co-injected with p53-MO (Robu et al., 2007), which did not detectably alter the phenotypes reported herein. Interactions with fgf3 were conducted initially with fgf3-MO and confirmed in fgf3 mutants. Phenotypes described herein were assessed with at least 20 specimens per time point and probe. Quantification of otic vesicles size (area presented in lateral views) and gene expression areas was performed using Photoshop. Areas presented in figures were normalized relative to wild-type control embryos and indicate the mean ± standard deviation of 8–10 specimens. Where indicated, statistical significance was evaluated using students’ t-test or, where appropriate, ANOVA and Tukey post-hoc HSD tests.
PCR
Efficacy of fgf10b splice-blocking MOs was evaluated at 12 hpf by RT-PCR using the following primers: sense 5’-GAT GGA CAG TGA GTA AGA GTG TGT-3’; antisense 5’-TTC TCC TCG ATG CGC TCC TTC A-3’. The fgf10b sequence was amplified through 36 cycles with an annealing temperature of 64°C. Amplification of ornithine decarboxylase (odc) was used as a control.
Gene expression, cell death and Sectioning
Gene expression patterns were visualized by whole-mount in situ hybridization or staining with Pax2 polyclonal antibody (Covance, 1:100 dilution) as previously described (Phillips et al., 2001). For tissue sections, embryos were embedded in Immunobed resin (Polysciences No. 17324) and cut into 7 µm sections. Cell death was visualized by incubating live dechorionated embryos in acridine orange as previously described (Millimaki et al., 2010).
Key Findings.
Fgf10b is required for a late phase of otic induction, accounting for 10–15% of the otic placode.
Fgf10b plus Fgf3 are absolutely required for epibranchial induction.
Fgf10b also cooperates with Fgf24 in epibranchial induction.
Acknowledgments
Grant support: NIDCD R01-DC03806.
REFERENCES
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev. Bio. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bosl MR, Kato S, Maconochie M, Riethmacher D. Requirements for FGF3 and FGF10 during inner ear formation. Development. 2003;130:6329–6338. doi: 10.1242/dev.00881. [DOI] [PubMed] [Google Scholar]
- 3.Begbie J, Brunet JF, Rubenstein JLR, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- 4.Bhat N, Riley BB. Integrin-α5 coordinates assembly of posterior cranial placodes in zebrafish and enhances Fgf-dependent regulation of otic/epibranchial cells. PLoS ONE. 2011;6(12):e27778. doi: 10.1371/journal.pone.0027778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Draper BW, Morcos PA, Kimmel CB. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: A quantifiable method for gene knockdown. Genesis. 2001;30:154–156. doi: 10.1002/gene.1053. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Xu Q. Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development. Dev Biol. 2010;339:507–518. doi: 10.1016/j.ydbio.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Fischer S, Draper BW, Neumann CJ. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- 8.Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–3424. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- 9.Hammond KL, Whitfield TT. Fgf and Hh signaling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle. Development. 2011;138:3977–3987. doi: 10.1242/dev.066639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog W, Sonntag C, von der Hardt S, Roehl HH, Varga ZM, Hammerschmidt M. Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development. 2004;131:3681–3692. doi: 10.1242/dev.01235. [DOI] [PubMed] [Google Scholar]
- 11.Holzschuh Y, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuß A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- 12.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 13.Kwak SJ, Phillips BT, Heck R, Riley BB. An expanded domain of fgf3 expression in the hindbrain of zebrafish valentine mutants results in mis-patterning of the otic vesicle. Development. 2002;129:5279–5287. doi: 10.1242/dev.129.22.5279. [DOI] [PubMed] [Google Scholar]
- 14.Kwak SJ, Vemaraju S, Moorman SJ, Zeddies D, Popper AN, Riley BB. Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev Dyn. 2006;235:3026–3038. doi: 10.1002/dvdy.20961. [DOI] [PubMed] [Google Scholar]
- 15.Kwon HJ, Riley BB. Mesendodermal signals required for otic induction: Bmp-antagonists cooperate with fgf and can facilitate formation of ectopic otic tissue. Dev Dyn. 2009;238:1582–1594. doi: 10.1002/dvdy.21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH. Identification of synergistic signals initiating inner ear development. Science. 2000;290:1965–1968. doi: 10.1126/science.290.5498.1965. [DOI] [PubMed] [Google Scholar]
- 17.Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC. FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 2005;19:603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Léger S, Brand M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 2002;119:91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- 20.Lombardo A, Isaacs HV, Slack JMW. Expression and functions of FGF-3 in Xenopus development. Dev Dyn. 1998;212:75–85. [PubMed] [Google Scholar]
- 21.Mahmood R, Kiefer P, Guthrie S, Dickson C, Mason I. Multiple roles for FGF-3 during cranial neural development in the chicken. Development. 1995;121:1399–1410. doi: 10.1242/dev.121.5.1399. [DOI] [PubMed] [Google Scholar]
- 22.Mansour SL, Goddard JM, Capecchi MR. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- 24.Martin K, Groves AK. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development. 2006;133:877–887. doi: 10.1242/dev.02267. [DOI] [PubMed] [Google Scholar]
- 25.McCarroll MN, Lewis ZR, Culbertson MD, Martin BL, Kimelman D, Nechiporuk AV. Graded levels of Pax2a and Pax8 regulate differentiation during sensory placode formation. Development. 2012;139:2740–2750. doi: 10.1242/dev.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyake A, Nakayama Y, Konishi M, Itoh N. Fgf19 regulated by Hh signaling is required for zebrafish forebrain development. Dev Biol. 2005;288:259–275. doi: 10.1016/j.ydbio.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 28.Nechiporuk A, Raible DW. FGF-dependent mechanosensory organ patterning in zebrafish. Science. 2008;320:1774–1777. doi: 10.1126/science.1156547. [DOI] [PubMed] [Google Scholar]
- 29.Nechiporuk A, Linbo T, Raible DW. Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–3730. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- 30.Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–623. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- 31.Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–571. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- 32.Ohyama T, Groves AK. Expression of mouse Foxi1 class genes in early craniofacial development. Dev Dyn. 2004;231:640–646. doi: 10.1002/dvdy.20160. [DOI] [PubMed] [Google Scholar]
- 33.Padanad MS, Riley BB. Pax2a proteins coordinate sequential induction of otic and epibranchial placodes through differential regulation of foxi1, soxi3, and fgf24. Dev Biol. 2011;351:90–98. doi: 10.1016/j.ydbio.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padanad MS, Bhat N, Guo B, Riley BB. Conditions that influence the response to Fgf during otic placode induction. Dev Biol. 2012;364:1–10. doi: 10.1016/j.ydbio.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park BY, Saint-Jeannet JP. Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Dev Biol. 2008;324:108–121. doi: 10.1016/j.ydbio.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittman AJ, Law MY, Chien CB. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135:2865–2871. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips BT, Bolding K, Riley BB. Zebrafish fgf3 and fgf8 Encode Redundant Functions Required for Otic Placode Induction. Dev Biol. 2001;235:351–365. doi: 10.1006/dbio.2001.0297. [DOI] [PubMed] [Google Scholar]
- 39.Riley BB, Sweet EM, Heck R, Evans A, McFarland KN, Warga RM, Kane DA. Characterization of harpy/Rca1/emi1 mutants: Patterning in the absence of cell division. Dev Dyn. 2010;239:828–843. doi: 10.1002/dvdy.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genetics. 2007;3(%):e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi T, Kuroiwa A, Takeda H. A novel sox gene 226D7, acts downstream of Nodal signaling to specify endoderm precursors in zebrafish. Mech Dev. 2001;107:25–38. doi: 10.1016/s0925-4773(01)00453-1. [DOI] [PubMed] [Google Scholar]
- 42.Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol. 2007;303:675–686. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Urness LD, Paxton CN, Wang X, Schoenwolf GC, Mansour SL. FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8c. Dev Biol. 2010;340:595–604. doi: 10.1016/j.ydbio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright TJ, Mansour SL. Fgf3 and Fgf10 are required for mouse otic placode induction. Development. 2003;130:3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- 45.Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour SL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol. 2004;269:264–275. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Xiao T, Roeser T, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2955–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, O’Neill P, Martin K, Maass JC, Vassilev V, Ladher R, Groves AK. Analysis of FGF-dependent and FGF-independent pathways in otic placode induction. PLoS ONE. 2013;8(1):e55011. doi: 10.1371/journal.pone.0055011. [DOI] [PMC free article] [PubMed] [Google Scholar]