Abstract
This study presents a cross-sectional investigation of functional networks in temporal lobe epilepsy (TLE) as they evolve over years of disease. Networks of interest were identified based on a priori hypotheses: the network of seizure propagation ipsilateral to the seizure focus, the same regions contralateral to seizure focus, the cross hemisphere network of the same regions, and a cingulate midline network. Resting functional magnetic resonance imaging data were acquired for 20 min in 12 unilateral TLE patients, and 12 age- and gender-matched healthy controls. Functional changes within and between the four networks as they evolve over years of disease were quantified by standard measures of static functional connectivity and novel measures of dynamic functional connectivity. The results suggest an initial disruption of cross-hemispheric networks and an increase in static functional connectivity in the ipsilateral temporal network accompanying the onset of TLE seizures. As seizures progress over years, the static functional connectivity across the ipsilateral network diminishes, while dynamic functional connectivity measures show the functional independence of this ipsilateral network from the network of midline regions of the cingulate declines. This implies a gradual breakdown of the seizure onset and early propagation network involving the ipsilateral hippocampus and temporal lobe as it becomes more synchronous with the network of regions responsible for secondary generalization of the seizures, a process that may facilitate the spread of seizures across the brain. Ultimately, the significance of this evolution may be realized in relating it to symptoms and treatment outcomes of TLE.
Key words: : brain, functional connectivity, functional magnetic resonance imaging, network, seizure propagation, temporal lobe epilepsy
Introduction
Temporal lobe epilepsy (TLE) is a common and relatively homogeneous form of epilepsy in adults. While the success rate for surgical therapy for medically intractable seizures has been reported to be ∼50% or higher (Goellner et al., 2013; Wiebe et al., 2001), this remains a grossly underutilized treatment (Wiebe et al., 2001), resulting in a large number of patients experiencing seizures over years or decades. These characteristics make TLE a powerful model for examining the effect of seizures and their propagation in the brain over years. Furthermore, the elucidation of these effects may potentially improve the clinical outcomes of these patients.
Functional connectivity mapping (Rogers et al., 2007) measured by correlations in functional magnetic resonance imaging (fMRI) time series have been used extensively to examine the strength of functional relationships in TLE in humans. Studies have investigated the functional connectivity between the presumed seizure focus in the hippocampus and the contralateral hippocampus (Morgan et al., 2011), between local hippocampal regions (Bettus et al., 2009; Pereira et al., 2010), and across the brain (Bettus et al., 2010; Frings et al., 2009; Haneef et al., 2014; Liao et al., 2010; Pittau et al., 2012). These studies generally report decreases within the ipsilateral temporal lobe and between the hemispheres, and some increases in functional connectivity in the contralateral temporal lobe. Other studies in TLE have focused on specific networks previously identified in healthy controls (Vlooswijk et al., 2010; Zhang et al., 2009a, 2009b), finding mostly decreases in connectivity associated with cognitive and behavioral deficits in the patients. Taken together, there is clear support for the ability of functional connectivity mapping to detect functional brain changes in TLE, but the specific effects of seizure propagation on the brain over the years have yet to be elucidated.
In past computations of functional connectivity using fMRI, the relationship between the regions or signals was assumed to be stationary over the duration of the acquisition. This, however, may not be true in many cases for reasons such as physiological noise, attention or scanner drift [see Hutchison et al. (2013) for review]. One emerging method for quantifying the dynamic nature of functional connectivity over the course of the imaging acquisition is the windowing approach (Chang and Glover, 2010; Kiviniemi et al., 2011). In this approach, the acquisition time series is parsed into windows of a fixed length over which individual measures of functional connectivity can be calculated. This approach yields a time series of functional connectivity. The covariance between the time series in two different paths may be used to quantify the dynamic functional relationship between them (Allen et al., 2014).
Our motivation for using these dynamic connectivity methods was to more specifically quantify how the seizure propagation or interictal electrical activity may alter functional connectivity at different time scales. First, we considered that frequent random, interictal electroencephalography (EEG) activity in the seizure propagation network, similar to that detected on intracranial electrodes (Tao et al., 2005), may randomly interrupt the functional connection of this network within itself or to others, leading to higher variability in the functional connectivity over the fMRI acquisition. Without the intracranial EEG we cannot verify this mechanism, but this initial investigation will investigate whether such dynamic functional connectivity changes exist. Second, we believed that dynamic changes in network interactions measured by the covariance of the functional connectivity may reflect the differing connectivity patterns or “states” of the system. For example, high negative covariance between two networks could indicate that the system as a whole modulates between the high functional connectivity state of one network and the high state of another network. We modified this idea from the investigation of structured patterns of functional connectivity using k-means clustering described by Allen and associates (2014). The goal of this work was to use both static and dynamic functional connectivity mapping to quantify network changes in the brain over the years in TLE in an a priori defined seizure propagation network across the brain.
There is much evidence that an extensive brain network exists that may be primarily associated with hippocampal seizure propagation in TLE. In addition to the presumed seizure focus in the hippocampus and/or mesial temporal structures, the insula has been implicated in TLE using fMRI (Fahoum et al., 2012; Morgan et al., 2010), positron emission tomography (PET) (Bouilleret et al., 2002; Chassoux et al., 2004), single photon emission tomography (SPECT) (Kim et al., 2008), and EEG (Blauwblomme et al., 2013; Isnard et al., 2000).
The thalamus is believed to play a role in the secondary generalization of seizures in TLE (Blumenfeld et al., 2009; Englot et al., 2008; Norden and Blumenfeld, 2002; Yu and Blumenfeld, 2009), and it has been shown to experience structural atrophy in TLE (Bernasconi et al., 2004; Labate et al., 2008; McMillan et al., 2004). Our recent work suggested that the atrophy in the thalamus is linearly related to functional connectivity changes across several other potential seizure-related regions, including the hippocampus and the mid cingulate gyrus in TLE (Holmes et al., 2013).
The precuneus is a midline region with a potential role in seizure propagation. It is considered a primary component of the Default-Mode Network (Buckner et al., 2008), a set of brain regions more active during internal mental focus rather than externally driven attention, and possibly related to consciousness (Cavanna, 2007). Simultaneous fMRI with EEG has detected functional responses to interictal discharges in the precuneus and other parts of the Default-Mode Network (Fahoum et al., 2012; Kobayashi et al., 2006; Laufs et al., 2007). Using SPECT, hypoperfusion has been seen ictally in TLE in this region (Dupont et al., 2009). Changes in functional connectivity in the precuneus have been found in TLE (Haneef et al., 2014), which may be related to memory ability (Holmes et al., 2011) and gray matter atrophy in the left thalamus (Holmes et al., 2013) in left TLE.
Similar to the precuneus, the mid cingulate gyrus is another midline region of interest in seizure propagation. While not prominently discussed in the literature of TLE, Blumenfeld and coworkers (2009) detected hypoperfusion in SPECT in this region in the secondary generalization of seizures. In a group of 32 TLE patients studied with simultaneous fMRI with EEG, some of the strongest regions of group activation due to interictal spiking occurred in this region and the insula (Fahoum et al., 2012). Similar to the precuneus and thalamus, the mid cingulate gyrus was found to have functional connectivity decreases linearly related to gray matter atrophy in the left thalamus and left hippocampus in TLE (Holmes et al., 2013).
In this study, we examined a system of four brain networks consisting of different combinations of the seizure propagation regions proposed earlier. We quantified functional changes within and between these networks as they evolve over years of the duration of disease using static functional connectivity and novel assessments of dynamic functional connectivity (Allen et al., 2014; Chang and Glover, 2010; Hutchison et al., 2013). We believe that this network approach will illustrate more specifically how seizures affect the brain over years in TLE. We hypothesize that the regions in the network ipsilateral to the seizure focus will have the most significant changes between TLE subjects and healthy controls, and that the relationship between this network and the others involved in seizure propagation may change linearly with the duration of disease.
Materials and Methods
Subjects
The subjects in this study are a part of an ongoing investigation. Here, we include the first 12 TLE patients recruited in the first 2 years, excluding patient #1 who underwent a different imaging protocol (5 male, 11 right handed, aged 36±13 years, and range 18–54 years). The main inclusion criterion for the study was unilateral TLE by standard clinical evaluations, including unilateral ictal onsets and interictal discharges on EEG, with or without unilateral temporal hypometabolism on PET, and with or without hippocampal sclerosis. All patients subsequently underwent temporal lobe surgical resection. The intent was to enroll those TLE patients with seizures originating from the left or right hippocampus, so no patients with other structural or vascular abnormalities of the temporal lobe suspected of being the seizure focus were included. Patient #10 had bilateral mesial temporal ictal discharges, but was unilateral in other evaluations. See Table 1 for detailed information on all subjects. We included the left and right hippocampal gray matter volumes as determined by FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) image segmentation in the table, as qualitative image evaluation of hippocampal sclerosis was sometimes questionable.
Table 1.
Clinical Characteristics of Subjects
| ID | Patient age; control age (years) | M/F | Hand | Duration (years) | Hippocampal volume: L, R (mm3)aPatient; control | EEG ictal | EEG interictal | PET hypometabolism | Surgery | Time since surgery (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 49; 50 | F | R | 20 | 2711, 2631; 3720, 4023 | R Mes Ant Temp | R Mes Ant Temp | R Temp | R Temp Lob | 6 | Sz free |
| 3 | 51; 54 | F | R | 41 | 4408, 3144; 4155, 4201 | R Mes Ant Temp | R Mes Ant Temp | R Ant Temp | R Sel Amyg | 18 | Bilateral EEG spiking |
| 4 | 18; 18 | F | R | 16 | 3059, 3563; 4116, 4061 | L Mes Ant Temp | L Mes Ant Temp | L Temp | L Sel Amyg | 16 | Sz free |
| 5 | 39; 38 | F | R | 39 | 3426, 2828; 4199, 4318 | R Ant Temp | none | R Mes Temp | R Sel Amyg | 14 | Sz free |
| 6 | 28; 27 | M | R | 4 | 4145, 4432; 4129, 4429 | L Temp | L, rare R Temp | L Mes Temp | L Sel Amyg | 12 | Sz free |
| 7 | 54; 56 | F | R | 19 | 4079, 2955; 4195, 4607 | R Ant Temp | R Mes Ant Temp | R Mes Temp | R Temp Lob | 12 | No follow up |
| 8 | 18; 18 | M | R | 6 | 2988, 3923; 4350, 4451 | none | L Mes Ant Temp | L Mes Temp | L Sel Amyg | 8 | Sz days after surgery, now Sz free |
| 9 | 43; 41 | M | R | 10 | 4150, 3685; 3905, 3807 | R Mes Ant Temp | R Temp | R Mes Temp | R Sel Amyg | 6 | N/A |
| 10 | 24; 23 | M | R | 23 | 3837, 2294; 4299, 4334 | L, R Mes Temp | R Mes Ant Temp | R Temp | R Sel Amyg | 4 | N/A |
| 11 | 23; 23 | M | L | 4 | 3288, 3141; 5022, 5275 | R Mes Ant Temp | R Mes Ant Temp | R Ant Temp | R Temp Lob | 1 | N/A |
| 12 | 41; 42 | F | R | 18 | 4119, 2007; 4610, 4611 | R Mes Ant Temp | R Mes Ant Temp | R Temp | R Temp Lob | 1 | N/A |
| 13 | 38; 38 | F | R | 5 | 4046, 3138; 4173, 4148 | R Mes Ant Temp | L, R Mes Ant Temp | R Temp | R Sel Amyg | <1 | N/A |
Measure of gray matter volume from FreeSurfer segmentation of T1-weighted images.
M, male; F, female; L, left; R, right; Mes, mesial; Ant, anterior; Temp, temporal; Sel Amyg, selective amygdalohippocampectomy; Lob, lobectomy; Sz, seizure; N/A, too early for seizure outcome; EEG, electroencephalography; PET, positron emission tomography.
We also enrolled an age- and gender-matched healthy control subject for each patient enrolled (5 male, 12 right handed, aged 36±14 years, and range 18–56 years). In all but one case, the age of the control was±2 years that of the matched patient. Control #3 was 3 years older than patient #3.
Imaging protocol
All MRI was performed using a Philips Achieva 3T MRI scanner (Philips Healthcare, Inc., Best, Netherlands) using a 32-channel head coil. Informed consent was obtained before scanning as per Institutional Review Board guidelines. The imaging protocol for each subject included the following scans: (1) Three-dimensional (3D), T1-weighted high-resolution image series across the whole brain for inter-subject normalization (1×1×1 mm3) and FreeSurfer segmentation; (2) two-dimensional (2D), T1-weighted high-resolution axial full brain image series in the same slice locations as the fMRI scans for functional to 3D data registration (1×1 mm2) with 3.5 mm slice thickness with 0.5 mm gap; (3) fMRI T2* weighted gradient echo, echo-planar image series at rest with eyes closed–matrix 80×80, field of view (FOV)=240 mm, 34 axial slices, echo time (TE)=35 ms, repetition time (TR)=2 sec, slice thickness=3.5 mm with 0.5 mm gap, and 300 volumes. The fMRI scan was acquired twice sequentially for a total of 600 volumes (20 min). Other language-based fMRI scans and diffusion-weighted MRI scans were also acquired, but not analyzed as a part of this study. Physiological monitoring of cardiac and respiratory fluctuations was performed at 500 Hz throughout all scanning using the MRI scanner integrated pulse oximeter and the respiratory belt.
fMRI image processing
The fMRI images were corrected for slice timing effects and motion occurring between the fMRI scans using SPM8 software (www.fil.ion.ucl.ac.uk/spm/software/spm8/). The six motion time series representing the x, y, and z translations and rotations of each volume acquisition were saved as potential confounds in the functional connectivity computation. The images were then corrected for physiological noise using a RETROICOR protocol (Glover et al., 2000) using the measured cardiac and respiratory time series. The corrected fMRI images were spatially normalized to the Montreal Neurological Institute (MNI) template using coregistration to the 3D and 2D T1-weighted structural image sets as intermediate steps, and then they were spatially smoothed with a 6×6×6 mm3 full-width, half maximum kernel. This resulted in a spatially normalized functional image series of 61×73×61 voxels (3×3×3 mm3) and a spatially normalized T1-weighted 3D image. The normalized fMRI time series were then low pass filtered at a cutoff frequency of 0.1 Hz (Cordes et al., 2001).
The temporal signal-to-noise ratio of each voxel in the brain was measured as the mean intensity divided by the standard deviation of the low pass filtered time series. This value was averaged across all voxels in the brain, and across the two fMRI series to compute a subject specific temporal signal-to-noise ratio. These were compared between the TLE and control groups using a paired t-test.
The normalized T1-weighted image was segmented into its gray matter, white matter, and cerebrospinal fluid components using SPM8. These component images were then resampled into the same resolution as the fMRI images for later use as potential confounds for the functional connectivity analyses.
In order to compare the head movement between the two groups of subjects, a summary measure of motion was computed for each subject. The motion for each volume acquisition was determined for each voxel as the geometric displacement in mm from the previous volume acquisition. The 95th percentile of this measure over all voxels was computed for each fMRI series. Since there were two fMRI series per subject, the median of the values from each of the two series was used as the subject's motion measure. The median of this measure across all TLE subjects was compared with the median measure across all controls using the bootstrap method with 1000 samples.
Regions of interest
FreeSurfer was used to segment the original T1-weighted 3D image of each subject into its cortical and some subcortical regions. This resulted in a T1-weighted brain image and a segmented brain image in the same space in which each voxel is given a value based on the corresponding cortical or subcortical region of which it is a part. Eight regions of interest were identified in each subject. The regions and the corresponding FreeSurfer value from the segmented FreeSurfer image are the following: left and right hippocampus (17, 53), left and right insula (1035, 2035), left and right thalamus (10, 49), precuneus (1025 and 2025), and mid cingulate. In order for the mid cingulate region to be consistent with the region identified in the literature (Blumenfeld et al., 2009; Fahoum et al., 2012; Holmes et al., 2013), we combined the caudal anterior cingulate (1002 and 2002) and the anterior half of the posterior cingulate (1023 and 2023).
Each of the eight regions of interest was applied to each of the two fMRI processed image series to yield an average time series for each region for each fMRI series for each subject. In addition, the average time series in the white matter as determined by the segmentation of the normalized T1-weighted image was used to create a whole brain white matter time series for each fMRI series for each subject. Each time series was high pass filtered at 0.0067 Hz and converted to a percent signal change by dividing by the whole brain mean and multiplying by 100. In order to combine the right TLE and the left TLE patients, the regions were converted from designations of left and right to ipsilateral and contralateral referring to the side of seizure focus. Next, an 8×8 matrix of pair-wise functional connectivity measures was created by performing the partial correlation of each pair of regions while controlling for the signal in the white matter time series and the six motion time series for each fMRI series. The correlation coefficients were converted to Z values using the Fisher Z transform (Fisher, 1915).
Functional connectivity within the network
The 8×8 functional connectivity matrix of Z values was averaged between the two fMRI series for each subject. Four networks were identified based on a priori hypotheses (Fig. 1). The functional connectivity of the network of hypothesized seizure propagation ipsilateral to the seizure focus (IPSI) was determined as the average connectivity of the three paths between the ipsilateral hippocampus, the ipsilateral insula, and the ipsilateral thalamus for each subject. The connectivity of the network of the same regions contralateral to the seizure focus (CONTRA) was determined as the average connectivity of the three paths between the contralateral hippocampus, the contralateral insula, and the contralateral thalamus for each subject. The connectivity of the cross-hemisphere network of seizure propagation (CROSS) was determined as the average connectivity of the ipsilateral to contralateral hippocampus, insula, and thalamus for each subject. Finally, the connectivity of the cingulate midline network (MID) was determined as the average connectivity of the cingulate to each of the ipsilateral thalamus, contralateral thalamus, and the precuneus for each subject (Fig. 2A). These network functional connectivity measures were compared between the TLE and the controls group using the paired t-test, and their relationship with the duration of disease was determined via linear Pearson's correlation coefficient.
FIG. 1.
Network diagram. The eight regions of interest are shown as circles, and each label is provided. The four networks identified from these regions in one individual subject are each shown in a different color: IPSI in blue and the regions on the coronal brain slice are also shown in blue, CONTRA in red with regions on the coronal brain slice shown in red, CROSS in purple, and MID in green with two of the four nodes (cingulate and precuneus) shown in green on the axial brain slice. See “Regions of interest” section for more detailed descriptions of regions and networks. HIPI, HIPC: hippocampus ipsilateral, contralateral; INSI, INSC: insula ipsilateral, contralateral; THALI, THALC: thalamus ipsilateral, contralateral; CING: mid cingulate gyrus; PREC: precuneus; IPSI: ipsilateral network; CONTRA: contralateral network; CROSS: cross hemispheric network; MID: midline cingulate network.
FIG. 2.
Network functional connectivity processing steps beginning with the eight region by eight region correlation matrix derived from each of the two 10 min fMRI imaging series (A). Dynamic and between network functional connectivity processing steps beginning with the eight region by eight region correlation matrix derived from each minute of the 20 min of fMRI acquisition (B). These steps represent processing as performed on each individual subject. fMRI, functional magnetic resonance imaging.
Dynamic functional connectivity within networks
We hypothesized that TLE would alter the dynamic nature of the functional connectivity of seizure propagation networks. We quantified this by parsing the region of interest time series into 20 sequential, nonoverlapping 1 min windows. Then, twenty 8×8 functional connectivity matrices of Z-scores, and thus 20 sets of four network connectivity values (IPSI, CONTRA, CROSS, and MID) were determined for each subject (Fig. 2B).
The standard deviation across the 20 functional connectivity values of a given network was used as a measure of dynamic variability of that network during the scan acquisition. This measure was compared between TLE and controls using a paired t-test. We were most interested in whether decreases (or increases) in average static functional connectivity between networks were related to increases (or decreases) in network variability given by the standard deviation, both between groups and as the duration of disease increased.
Dynamic network functional connectivity between networks
With regard to dynamic changes in inter-network functional connectivity, we proposed that an increase in the functional interaction between two networks may be detected as an increase in the positive or negative covariance of minute-to-minute functional connectivity of the two networks (i.e., covariance of twenty 1-min IPSI functional connectivity measures and twenty 1-min CONTRA functional connectivity measures; Fig. 2B) (Allen et al., 2014). This covariance was compared between patients and controls using paired t-tests, and linearly correlated with the duration of disease. We focused our investigation on the relationship between the ipsilateral network and other networks, and on any networks found to have significant changes in dynamic network connectivity variability measured by the standard deviation.
Results
The temporal signal-to-noise ratio analysis showed no important difference between the two groups (mean±SD; TLE=141±36; controls=144±26). Head motion was also minimally different between the two groups: median displacement from the previous volume in the TLE subjects was 0.36 mm versus 0.28 in the controls. The 95% confidence interval of the difference between the two groups was [−0.05, 0.16] (p=0.26, Wilcoxon rank sum test).
Functional connectivity within the network
The average static functional connectivity (Z value) within the CROSS network was reduced in TLE compared with controls: TLE=11.86, control=15.47; 95% confidence interval for controls-TLE [2.2, 5.0], p=0.0001 (Fig. 3). While there was no linear relationship between this decrease across all TLE subjects, in those with a duration greater than 5 years the functional connectivity increased linearly with an increase in the duration of disease (r=0.6, p=0.04; Fig. 3). The MID network had a trend toward reduced connectivity in TLE (p=0.09). No other networks showed changes in within-network static functional connectivity between TLE and controls.
FIG. 3.
Functional connectivity within the CROSS network versus duration of disease in years. The TLE subjects are shown in closed diamonds and the controls are indicated in open circles. The healthy controls are plotted at the same duration of disease as their corresponding age- and gender-matched TLE patient. The trendline represents the linear fit of the TLE data of patients with duration of disease greater than 5 years. TLE, temporal lobe epilepsy.
The functional connectivity within the IPSI network decreased linearly with an increase in the duration of disease, considering the patient group alone (r=−0.58, p=0.05), and when matched controls' values were subtracted (r=−0.61, p=0.03; Fig. 4A). No other static network connectivity changed linearly with the duration of disease.
FIG. 4.
The functional connectivity within the IPSI network versus duration of disease in TLE (A). Linear trendline is included. The functional connectivity over the 20 min fMRI acquisition is shown in (B) for two different pairs of subjects: TLE with 4 years' duration and matched control (top, point in A indicated by *) and TLE with 41 years' duration and matched control (bottom, point in A indicated by **).
Dynamic functional connectivity within networks
When comparing the standard deviation of the twenty 1-min network functional connectivity measures, no networks showed a significant difference between the two groups. Interestingly, the CROSS network, which showed a significant decrease in functional connectivity in TLE compared with controls, did not have a corresponding increase in standard deviation over time in TLE (p=0.35). Nor did the IPSI network have increased standard deviation of functional connectivity, even though the functional connectivity within the network decreased with an increase in the duration of disease (p=0.80; Fig. 4B). Similarly, the MID network that had a trend toward reduced connectivity in TLE also did not have a corresponding increase in standard deviation of connectivity in TLE (p=0.29), but had a significant linear increase in standard deviation of functional connectivity as the duration of disease increased in TLE (r=0.92, p=0.00002; Fig. 5).
FIG. 5.
The variability of the functional connectivity of the MID network over the 20 min fMRI acquisition versus duration of disease in TLE. Linear trendline is included.
Dynamic network functional connectivity between networks
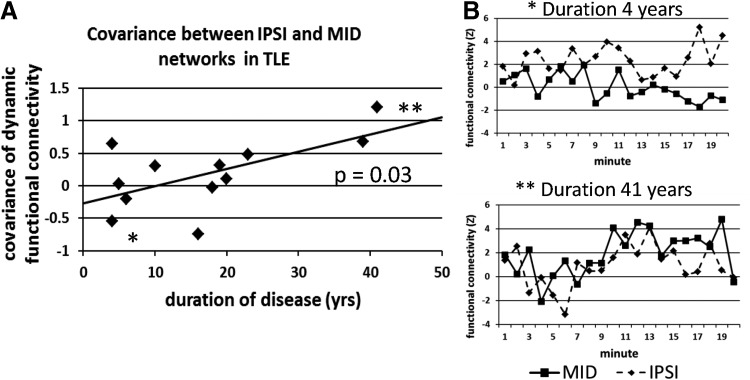
The linear increase in variability of the functional connectivity in the MID network as the duration of disease increased (Fig. 5) suggested that another network may be interacting with this network over the years of duration. To quantify this nondirectional influence, we used the covariance of the 20 functional connectivity measures in two networks as a measure of the dynamic functional relationship between the networks (Allen et al., 2014). We found that the covariance of the IPSI and MID networks increased linearly as the duration of disease increased (r=0.62, p=0.03; Fig. 6A, B), but that the covariance was not different between the TLE and controls groups (p=0.93). There was no linear relationship between the covariance of the MID and CONTRA networks and the duration of disease (r=0.41, p=0.18).
FIG. 6.
The covariance of the functional connectivity of the IPSI and MID networks versus duration of disease in TLE (A). Linear trendline is included. The functional connectivity over the 20 min fMRI acquisition in each network is shown in (B) for two different subjects: TLE with 4 years' duration (top, point in A indicated by *), and TLE with 41 years' duration (bottom, point in A indicated by **).
Discussion
In this study, we investigated TLE from the viewpoint of functionally adapting brain networks. In doing so, we present one of the earliest applications of dynamic functional connectivity methodologies to this disorder, and we also develop novel extensions of these techniques to quantify the relationship between networks evolving over minutes and years. Specifically, we have three main findings. First, we show that static functional connectivity between the proposed seizure propagation network and its contralateral homologue (CROSS) is decreased in TLE, even in the shortest duration patients in our study; but this decrease is not due to or correlated with increased temporal variability in the functional connectivity measurement. Second, the functional connectivity of the ipsilateral seizure propagation network (IPSI) decreased linearly with an increase in the duration of disease, even without an accompanying measurable increase in temporal variability. Third, we found that variability of the functional connectivity over the 20 min in the midline cingulate network (MID) was increased as the duration of disease increased; and that this increase in variability was associated with an increase in covariance with the IPSI network over the years. Secondarily, we did not find changes in the functional connectivity of the CONTRA network itself, over the duration of disease, or in its temporal relationship with the MID network.
Dynamic functional connectivity
One method for computing dynamic functional connectivity involves parsing the acquisition time series into windows of a fixed length over which individual measures of functional connectivity can be calculated (Hutchison et al., 2013). The optimal length of the window and the amount of overlap of consecutive windows is not known for specific applications. In our case, we chose to use 1 min windows that would target changes in functional connectivity of ∼0.016 Hz. These fluctuations in connectivity may reflect infra-slow fluctuations in EEG activity representing a slow modulation of cortical excitability (Hiltunen et al., 2014; Palva and Palva, 2012; Vanhatalo et al., 2004). These fluctuations were found to correspond to interictal events in epilepsy in sleep (Vanhatalo et al., 2004). We used the standard deviation as a measure of the variability of the functional connectivity time series over the 20 min acquisition. In addition, we used an approach similar to Allen and colleagues (2014) to compute dynamic functional connectivity between networks as the covariance of the functional connectivity time series between two networks.
Cross-hemispheric network
The only network we examined with a significant decrease in average static functional connectivity across all TLE subjects compared with controls was the CROSS network, consisting of cross-hemispheric connections. This network has not been studied as a whole, but the left to right hippocampal connection has been found to be decreased in TLE in some studies (Pereira et al., 2010; Pittau et al., 2012), but not in others (Haneef et al., 2014; Morgan et al., 2011). However, the pattern of the decrease in TLE is neither constant nor linear with the duration of disease; instead, it has a minimum of around 5 years' duration (Fig. 3) and increases linearly toward the controls value with an increase in the duration of disease. This is consistent with our previous findings in the hippocampus connection in a different group of TLE subjects (Morgan et al., 2011). In that study, we found that cross-hippocampal functional connectivity increased linearly with the duration of disease after ∼10 years' duration.
Using the standard deviation of the minute-to-minute functional connectivity, we did not find a corresponding increase in variability of functional connectivity in this network in TLE. This suggests that there is no minute-to-minute variation in electrical activity (interictal activity) across this network which transiently interrupts its connectivity. However, it is possible that the interruption may be at a different frequency not detected here.
Ipsilateral seizure network
The network ipsilateral to the seizure origin (IPSI), including the ipsilateral hippocampus, insula, and thalamus, was found to have a linearly decreasing static functional connectivity as the duration of disease increased (Fig. 4A). The group comparison did not show reduced IPSI connectivity in all TLE compared with the controls. This is evident in Figure 4A where the difference between the TLE subject and the matched control is positive in the early years of duration, indicating that the TLE patients had increased connectivity in this network (Fig. 4B, top), and negative in the later years of duration, indicating reduced connectivity in TLE compared with controls (Fig. 4B, bottom). These data suggest that the first seizures may be prompted by or associated with an increase in connectivity in this network, but the repeated seizures have a continuing detrimental effect on the network connectivity. The mechanism for this zero crossing point around 10 years is unknown, but may coincide, in part, with the reversal of the CROSS network connectivity from decreasing to increasing connectivity over the duration of disease. However, direct linear correlation of these two trends was not statistically significant.
Midline cingulate network
The dynamic functional connectivity analysis revealed a unique evolution in the midline (MID) network. While there was a nonsignificant trend of decreased functional connectivity in this network in TLE compared with the controls, the variability of the functional connectivity within this network increased linearly with the duration of disease in TLE (Fig. 5). When probed further, the dynamic connectivity between this network and the IPSI network increased significantly with the duration of disease as well (Fig. 6A). The results show that in the early years of duration, the IPSI and MID networks have a slightly negative covariance signifying the opposing functional connectivity over time (Fig. 6B, top). Conversely, at higher durations of disease, the covariance between the two networks becomes increasingly more positive, representing their more synchronous variations over the acquisition (Fig. 6B, bottom). We posit that this suggests an evolution from a system with a strong IPSI network functioning in a separate state from the MID network, to a weakening of the IPSI network becoming increasingly influenced by the MID network. This may coincide with when the connectivity in the IPSI network in TLE drops below that in the healthy controls (Fig. 3A) and/or when the CROSS network functional connectivity begins to increase. This is similar to our previous findings (Morgan et al., 2011) of an increase in influence of the contralateral hippocampus' fMRI signal on the ipsilateral hippocampus' fMRI signal as the duration of disease increased determined by Granger causality measures (Deshpande et al., 2009; Rogers et al., 2010), which we interpreted as evidence of a weakening of the ipsilateral hippocampus function over time.
Limitations and future work
One major limitation of this study is its relatively small sample size. This is primarily due to our stringent inclusion criteria to enroll only those TLE patients with a purely unilateral hippocampal seizure focus. We used the matched control pairwise method to increase the statistical power of these analyses, but an increase sample size will be required to confirm these findings. In addition, we have only two subjects with the duration of disease greater than 30 years.
Another limitation of this study is the large regions of interest and relatively general identifications of networks. The regions were chosen here for their supposed role in the seizure propagation network. However, while the chosen regions have been strongly implicated in TLE seizure propogation in individual studies, the network as a whole has not been clearly established as a unified functional network in TLE or otherwise. Another option would have been to identify functionally based regions in a data-driven way, for example, with independent component analysis or seed region connectivity mapping. However, these data-driven approaches are substantially less powerful in such a small sample, so we restrained our analysis to the prespecified regions derived independently from consideration of the literature. Future work could include the use of more precise sub-regions, defined from these data or others and applied in independent data to avoid bias.
Another potential issue regarding the regions of interest is the variability in size across subjects. No regions were linearly correlated with the duration of disease across the TLE patients. In addition, no volumes were linearly correlated with the functional connectivity measures shown in Figures 4A, 5A, or 6A. This suggests that while it is possible that the regional volume may play a role in the functional connectivity relationships discussed, it is not the primary driver of them.
The dynamic functional connectivity methods utilized here are not proved and have several possible limitations. The window approach is commonly used (Allen et al., 2014; Chang and Glover, 2010; Hutchison et al., 2013); however, the window size and amount of overlap of the window depends on the application, hypothesis, and noise in the data.
We did not measure simultaneous EEG during the MRI scanning session. Therefore, we cannot relate any changes in functional connectivity to transient EEG modulations that may have occurred. However, our assumption was that transient EEG changes would increase the variability of the functional connectivity in patients compared with the controls, but no increase in variability between groups was detected. While it is possible that transient changes in EEG which correlate linearly with the duration of disease may be partly responsible for our findings across the duration of disease, there is no literature or anecdotal evidence known to support this idea.
In this study, we used the duration of disease as a representation of seizure burden in the patient. This assumes that all patients have seizures (either clinical or below clinical threshold activity) at the same constant frequency, which is not likely. Ideally, we would prefer a direct quantification of electrical activity at the cortex across these networks. While it is possible to record this in short time periods, it is not feasible to acquire or to determine this information for the years of duration of the disease.
Conclusion
In this study, we applied the measurement of static and dynamic functional connectivity to TLE. By identifying four networks of potential seizure propagation across the brain, we quantified widespread network functional alterations in TLE and their evolution over the duration of the disease. Our data suggest an initial disruption of cross-hemispheric networks and an increase in functional connectivity in the ipsilateral temporal network accompanying the onset of TLE seizures. As seizures progress over years, the functional connectivity across the ipsilateral temporal network gradually diminishes, while the functional independence of this ipsilateral network from the network of midline regions of the cingulate declines. This can be interpreted as a gradual breakdown of the seizure onset and early propagation network involving the ipsilateral hippocampus and temporal lobe as it becomes more in synchrony with the network of regions believed to be responsible for secondary generalization of the seizures, a process that may facilitate the spread of seizures across the brain. These findings demonstrate that both static and dynamic functional connectivity measures can be sensitive to the alterations of networks in the progression of epilepsy, and form the foundation for future studies in this area. Ultimately, however, the significance of this research may be realized in relating it to symptoms and treatment outcomes of TLE.
Acknowledgments
The authors would like to thank Alexander Dagley and Benjamin Conrad for their work on the FreeSurfer image segmentation for hippocampal volume computation and region-of-interest identification. In addition, the authors are grateful to Robert Barry, Ph.D. for his assistance and programming of the RETROICOR physiological noise correction of the fMRI images. This work was supported in part by the NIH NS075270.
Author Disclosure Statement
No competing financial interests exist.
References
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. 2004. Whole-brain voxel-based statistical analysis of gray alter and white matter in temporal lobe epilepsy. Neuroimage 23:717–723 [DOI] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, et al. 2010. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 81:1147–1154 [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, et al. 2009. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30:1580–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwblomme T, David O, Minotti L, Job AS, Chassagnon S, Hoffman D, et al. 2013. Prognostic value of insular lobe involvement in temporal lobe epilepsy: a stereoelectroencephalographic study. Epilepsia 54:1658–1667 [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. 2009. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 132:999–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. 2002. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study. Ann Neurol 51:202–208 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Cavanna AE. 2007. The precuneus and consciousness. CNS Spectr 12:545–552 [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. 2010. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50:81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassoux F, Semah F, Bouilleret V, Landre E, Devaux B, Turak B, et al. 2004. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain 127:164–174 [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. 2001. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22:1326–1333 [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, James GA, Peltier S, Hu XP. 2009. Multivariate Granger causality analysis of fMRI data. Hum Brain Mapp 30:1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont P, Zaknun JJ, Maes A, Tepmongkol S, Vasquez S, Bal CS, et al. 2009. Dynamic perfusion patterns in temporal lobe epilepsy. Eur J Nucl Med Mol Imaging 36:823–830 [DOI] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. 2008. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci 28:9066–9081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahoum F, Lopes R, Pittau F, Dubeau F, Gotman J. 2012. Widespread epileptic networks in focal epilepsies: EEG-fMRI study. Epilepsia 53:1618–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. 1915. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 10:507–521 [Google Scholar]
- Frings L, Schulze-Bonhage A, Spreer J, Wagner K. 2009. Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol 256:2021–2029 [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. 2000. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167 [DOI] [PubMed] [Google Scholar]
- Goellner E, Bianchin MM, Burneo JG, Parrent AG, Steven DA. 2013. Timing of early and late seizure recurrence after temporal lobe epilepsy surgery. Epilepsia 54:1933–1941 [DOI] [PubMed] [Google Scholar]
- Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Stern JM. 2014. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen T, Kantola J, Abou Elseoud A, Lepola P, Suominen K, Starck T, et al. 2014. Infra-slow EEG fluctuations are correlated with resting-state network dynamics in fMRI. J Neurosci 34:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MJ, Gore JC, Folley BS, Abou-Khalil B, Sonmezturk HH, Morgan VL, 2011. Resting state functional connectivity correlated with neuropsychological tests in temporal lobe epilepsy patients. In: Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Montreal, Canada, p. 4136 [Google Scholar]
- Holmes MJ, Yang X, Landman BA, Ding Z, Kang H, Abou-Khalil B, et al. 2013. Functional networks in temporal-lobe epilepsy: a voxel-wise study of resting-state functional connectivity and gray-matter concentration. Brain Connect 3:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M. et al. 2013. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80:360–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard J, Guenot M, Ostrowsky K, Sindou M, Mauguiere F. 2000. The role of the insular cortex in temporal lobe epilepsy. Ann Neurol 48:614–623 [PubMed] [Google Scholar]
- Kim BJ, Hong SB, Seo DW. 2008. Differences in ictal hyperperfusion of limbic-related structures between mesial temporal and neocortical epilepsy. Epilepsy Res 81:167–175 [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Vire T, Remes J, Elseoud AA, Starck T, Tervonen O, et al. 2011. A sliding time-window ICA reveals spatial variability of the default mode network in time. Brain Connect 1:339–347 [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. 2006. Negative BOLD responses to epileptic spikes. Hum Brain Mapp 27:488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Gambardella A, Aguglia U, Quattrone A. 2008. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study. Neurology 71:1094–1101 [DOI] [PubMed] [Google Scholar]
- Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. 2007. Temporal lobe interictal epileptic discharges affect cerebral activity in “Default mode” brain regions. Hum Brain Mapp 28:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Zhang ZQ, Pan ZY, Mantini D, Ding JR, Duan XJ, et al. 2010. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One 5:e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. 2004. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage 23:167–174 [DOI] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Abou-Khalil B. 2010. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res 88:168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou-Khalil B. 2011. Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia 52:1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. 2002. The role of subcortical structures in human epilepsy. Epilepsy Behav 3:219–231 [DOI] [PubMed] [Google Scholar]
- Palva JM, Palva S. 2012. Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. Neuroimage 62:2201–2211 [DOI] [PubMed] [Google Scholar]
- Pereira FRS, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, et al. 2010. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. 2012. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 53:1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Katwal SB, Morgan VL, Asplund CL, Gore JC. 2010. Functional MRI and multivariate autoregressive models. Magn Reson Imaging 28:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. 2007. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25:1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. 2005. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 46:669–676 [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. 2004. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A 101:5053–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlooswijk MCG, Jansen JFA, Majoie HJM, Hofman PAM, de Krom MCTFM, Aldenkamp AP, et al. 2010. Functional connectivity and language impairment in cryptogenic localization-related epilepsy. Neurology 75:395–402 [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. 2001. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318 [DOI] [PubMed] [Google Scholar]
- Yu L, Blumenfeld H. 2009. Theories of impaired consciousness in epilepsy. Disorders of consciousness. Ann N Y Acad Sci 1157:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Chen Z, et al. 2009a. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J Neurol 256:1705–1713 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, et al. 2009b. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett 458:97–101 [DOI] [PubMed] [Google Scholar]