Abstract
The host response to both synthetic and biologically derived biomaterials is a temporally regulated, complex process that involves multiple interacting cell types. This complexity has classically limited the efficacy of in vitro assays for predicting the in vivo outcome, necessitating the use of costly animal models for biomaterial development. The present study addressed these challenges by developing an in vitro assay that characterized the dynamic inflammatory response of human monocyte-derived-macrophages to biomaterials, coupled with quasi-mechanistic analysis in silico analysis: principal component analysis (PCA) and dynamic network analysis (DyNA). Synthetic and extracellular matrix (ECM)–derived materials were evaluated using this method, and were then associated with the in vivo remodeling and macrophage polarization response in a rodent skeletal muscle injury model. PCA and DyNA revealed a distinct in vitro macrophage response to ECM materials that corresponded to constructive remodeling and an increased M2 macrophage presence in vivo. In contrast, PCA and DyNA suggested a response to crosslinked ECM and synthetic materials characteristic of a foreign body reaction and dominant M1 macrophage response. These results suggest that in silico analysis of an in vitro macrophage assay may be useful as a predictor for determining the in vivo host response to implanted biomaterials.
Introduction
Surgical reconstruction of soft tissues, which includes hernia repair, breast reconstruction, and pelvic organ prolapse repair, routinely uses implantable mesh devices. The contribution of these biomaterials to achieving the desired outcome depends upon complex host–material interactions, which in turn are dictated by the material's composition and structure. Traditional biomaterials used in soft tissue reconstruction have been composed of synthetic polymers, such as polypropylene, polyglycolide, and polyethylene, among others.1–3 Synthetic materials usually possess consistent and robust mechanical properties, highly tunable structure, and modifiable surface characteristics, thereby allowing for the manufacture of a wide array of devices.
Many synthetic materials, however, elicit a foreign body reaction, with the associated deposition of fibrotic scar tissue.4–6 This adverse host response has spurred the development of new materials aimed at abrogating the foreign body reaction, including naturally derived materials composed of extracellular matrix (ECM). ECM devices are manufactured by the decellularization of source tissues, such as dermis, urinary bladder, small intestine, and pericardium.7 The tissue decellularization process removes the majority of antigenic cellular material while retaining ECM architecture and biologically active components, such as glycosaminoglycans, growth factors, proteoglycans, and collagens.8,9 The physical and biologic properties of ECM are influenced by many factors, including source tissue, the species from which the source tissue is obtained (xenogeneic or allogeneic), and decellularization/postprocessing methods, such as chemical treatment, crosslinking, and sterilization.10–14
Both synthetic and naturally occurring biomaterial development presents design and processing considerations, which may affect the clinical outcome following implantation. Preclinical animal testing is the standard method for evaluation of biomaterials, but efficient screening of the many potential biomaterial design combinations is challenging in terms of both cost and time.15 A high-throughput in vitro assay and analysis methodology that could be used to predict in vivo host response, or at least to allow for down selection (i.e., narrowing down) of those constructs that are subjected to in vivo testing, would be an invaluable tool in biomaterial development. A primary obstacle for the development of a predictive in vitro model has been lack of an effective method that captures the complexity of cell–biomaterial interactions that are relevant in vivo.
The host response to biomaterials in either normal or injured tissue is complex and not fully understood, though the immune/inflammatory response is a critical determinant in the process.4,6 Multiple inflammatory cell types are involved in the response to biomaterials, and monocyte-derived macrophages, in particular, have several important functions,16 including the secretion of biochemical signals and chemokines/cytokines that directly influence tissue remodeling and proteases that actively remodel degradable biomaterials.17–19 Macrophages also contribute to the formation of multinucleate foreign body giant cells, the sine qua non of the foreign body reaction that characteristically develops in response to nondegradable materials.4,20 Therefore, the macrophage–material interaction may be a relevant predictor of the response to implanted biomaterials. This interaction has been modeled in vitro in several studies that quantified changes in gene expression, surface markers, and/or protein secretion.21–26 However, conclusions based on these in vitro assays are limited and have not been predictive of in vivo outcome due to the inherent complexity, dynamics, and donor variability of macrophage activation. In silico modeling techniques are a potentially effective methodology to characterize these complex macrophage responses. Data-driven modeling techniques, specifically principal component analysis (PCA)27–30 and dynamic network analysis (DyNA),28,31 provide an unbiased analysis of complex multidimensional data sets without mechanistic assumptions, and potentially reveal predictors of in vivo outcome. Further, these predictors may provide a basis for mechanistic investigation of the macrophage response at the biomaterial interface.
The purpose of the present study was to use these data-driven in silico techniques to identify the determinants of the in vitro macrophage response to synthetic and ECM biomaterials (Fig. 1). We hypothesized that these determinants would be associated with the in vivo remodeling outcome. Our findings indeed suggest that in silico methods such as these can aid in linking in vitro data with in vivo outcomes in the setting of biomaterial implantation.
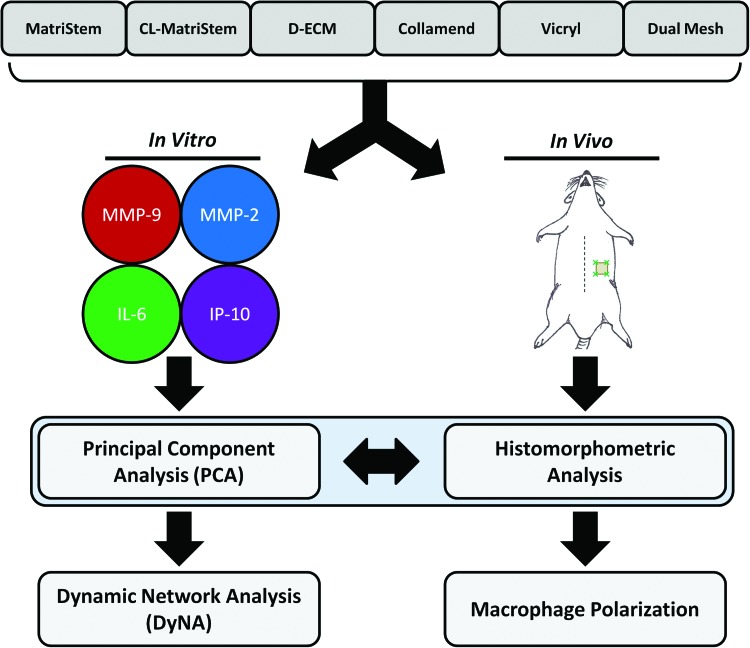
FIG. 1.
Overview of the experimental approach. Six surgical mesh biomaterials were evaluated in both in vitro and in vivo contexts: MatriStem, crosslinked MatriStem (CL-MatriStem), dermal extracellular matrix (D-ECM), Collamend, Vicryl, and Dual Mesh. Monocyte-derived macrophages were cultured on the surface of each biomaterial, and the supernatants were repeatedly sampled over a 2–48-h time course to quantify matrix metalloproteinase 9 (MMP-9), matrix metalloproteinase 2 (MMP-2), interleukin-6 (IL-6), and interferon gamma-induced protein 10 (IP-10). These secretion profiles were analyzed using in silico methods: principal component analysis (PCA) and dynamic network analysis (DyNA). The ranked PCA scoring analysis provided distinct responses that were then associated with the in vivo responses to these materials in a skeletal muscle injury model in the rat. Implanted devices were analyzed for histologic remodeling outcome using histomorphometric scoring analysis as well as macrophage phenotype via immunolabeling. Color images available online at www.liebertpub.com/tec
Materials and Methods
All animal experiments were conducted in accordance to University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) regulations and guidelines.
Device preparation
Six materials were evaluated in this study: MatriStem™ (ACell, Inc., Columbia, MD), carbodiimide crosslinked MatriStem (CL-MatriStem), Collamend™ (C.R. BARD-Davol, Inc., Providence, RI), dermal ECM (D-ECM), Vicryl™ (Ethicon, Inc., Somerville, NJ), and Dual Mesh™ (W. L. Gore & Associates, Inc., Flagstaff, AZ).
MatriStem (noncrosslinked urinary bladder ECM), Collamend (crosslinked dermal ECM), Vicryl (degradable synthetic mesh composed of polyglactin 910), and Dual Mesh (nondegradable synthetic mesh composed of expanded polytetrafluoroethylene [ePTFE]) were used as provided by the manufacturer.
CL-MatriStem was prepared by chemically crosslinking MatriStem with 10 mM ethyldimethylaminopropyl carbodiimide (EDAC; Sigma, St. Louis, MO) for 24 h under agitation, followed by extensive rinsing and lyophilization. D-ECM was prepared as previously described10 from porcine dermis mechanically isolated from full-thickness skin. Dermis was treated with 0.25% trypsin (Thermo Fisher Scientific, Waltham, MA) for 6 h, 70% ethanol for 10 h, and 3% hydrogen peroxide for 15 min, 1% Triton X-100 in 0.26% EDTA/0.69% Tris for 6 h with fresh solution for an additional 16 h, and 0.1% peracetic acid/4% ethanol for 2 h. Dermis was rinsed with water between each treatment step and after the final step, and lyophilized.
Devices used for in vitro cell culture experiments were cut into 2-cm-diameter discs, and devices used for in vivo implantation were cut into 1×1-cm2 coupons. All devices were terminally sterilized with ethylene oxide (16-h cycle at 50°C in a Series 3plus EOGas Sterilizer; Anderson Sterilizers, Inc., Haw River, NC) prior to use, or used as supplied by the manufacturer when applicable.
In vivo response to implanted devices
The in vivo host response to each material was evaluated via implantation in an established partial-thickness, abdominal-wall-defect model in female Sprague–Dawley rats (250–300 g; Charles River Laboratories, Wilmington, MA).20,32 A ventral abdominal incision was made ∼2 cm lateral to the midline and a paramedian 1×1 cm2 partial-thickness abdominal wall defect was created. The external and internal oblique muscles were excised while leaving the underlying transversalis fascia intact. Size-matched device coupons (1×1 cm2) were inlaid within the defect and fixed to adjacent muscle at the four corners of the device with nondegradable 4-0 polypropylene (Prolene; Ethicon) interrupted sutures. Dual Mesh was implanted such that the textured surface was in contact with the defect. The skin incision was closed with degradable 4-0 Vicryl (Ethicon) sutures. Animals were sacrificed after 14 or 35 days of implantation (n=2 per device and time point), and implants with surrounding tissue were collected and fixed with neutral buffered formalin. Tissue was dehydrated, embedded in paraffin, and cut into 5-μm sections for histologic evaluation.
The host tissue remodeling response and macrophage phenotype were determined by histologic evaluation and immunofluorescent labeling, respectively. The response to the marker sutures at the four corners of the device was ignored for the purposes of this study. Tissue remodeling was characterized histomorphologically from hematoxylin-and-eosin-stained sections as previously described,20 and the scoring criteria are detailed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tec). In brief, histologic remodeling characteristics were scored semiquantitatively on a scale from 0 to 3 for foreign body giant cell formation, degradation, connective tissue organization, encapsulation, and muscle ingrowth. The sums of these scores represent the total remodeling score in which a higher score is associated with more favorable remodeling (e.g., fewer giant cells and greater connective tissue organization) than that associated with a lower score. All histologic scoring was performed by a blinded observer trained in evaluating the histologic response to implanted biomaterials in soft tissue locations, and the senior author for this article is a pathologist with more than 30 years of experience in designing and implementing such scoring metrics.
Immunofluorescent labeling was performed for macrophage markers associated with M1 (classical activation: proinflammatory) and M2 (alternative activation: tissue remodeling) phenotypic states.20 Sections were deparaffinized and placed in citrate antigen retrieval buffer (pH=6) heated to 95–100°C for 20 min (Three 5-min washes in phosphate-buffered saline [PBS] were performed between each step unless otherwise indicated.). Nonspecific antibody binding was blocked with 1% bovine serum albumin (Sigma) and 2% normal donkey serum (Invitrogen, Carlsbad, CA) in PBS for 1 h at room temperature. Immediately after blocking, sections were incubated with primary monoclonal antibodies diluted in blocking solution overnight at 4°C. The macrophage markers and dilutions utilized in this study were as follows: the M1 marker CCR7 (goat anti-rat, 1:100, ED1; Abd Serotec, Oxford, United Kingdom) and the M2 marker CD206 (rabbit anti-rat 1:100, ED1; Abd Serotec). Conjugated secondary antibodies diluted in PBS were added for 2 h at room temperature: AlexaFluor-594 donkey anti-rat (1:100) and AlexaFluor-488 donkey anti-rat (1:200). Nuclei were stained with DAPI (Invitrogen) and slides were coverslipped with fluorescent mounting media (Dako, Glostrup, Denmark). The host tissue–device interface was imaged and the number of cells expressing CCR7 (M1) or CD206 (M2) was quantified and presented as an M2/M1 ratio of CD206+/CCR7+ cells.
Peripheral blood monocyte differentiation to macrophages
Human peripheral blood monocytes were isolated from the buffy coat of a healthy donor. The buffy coat was diluted (1:3 v/v) with RPMI media (Gibco, Grand Island, NY) and added to lymphocyte separation media (1:4 v/v) for centrifugation at 2000 rpm. The mononuclear cell layer was collected and washed twice with RPMI media. Monocytes were isolated via negative selection using magnetic activated cell sorting (Miltenyi Biotec, Auburn, CA). The cell suspension was labeled with biotinylated antibodies against CD3, CD7, CD16, CD19, CD56, CD123, and glycophorin diluted in FcR blocking reagent. The cell suspension was washed and incubated with magnetic anti-biotin microbeads and flowed through a magnetic column to bind and remove contaminating cell populations from the effluent. Mature macrophages were derived from freshly isolated monocytes by culturing for 7 days in RPMI media supplemented with 100 ng/mL macrophage colony stimulating factor and 20% fetal bovine serum (FBS).33
Analysis of macrophage-secreted proteins and viability
The in vitro macrophage secretory response during culture on the surface of six unique biomaterial scaffold conditions was compared by repeated media sampling over five time points. Each material was evaluated in triplicate. Macrophage viability was determined on the surface of each device at the final time point. All devices were initially placed in 12-well plates and acclimated in macrophage media (RPMI media supplemented with 5% FBS) for 20 min prior to macrophage seeding. Two million macrophages in 2 mL of macrophage media were seeded within annular stainless steel rings (1.3 cm2 seeding area) resting on top of devices in triplicate. The rings were removed after 1 h. Macrophage-secreted proteins were analyzed after 2, 6, 12, 24, and 48 h of culture by sampling 50 μL of media from each well after gentle mixing. Each sample was immediately frozen at −80°C and analyzed via the SearchLight® Multiplex Immunoassay (Aushon Biosystems, Billerica, MA) for the concentration (pg/mL) of soluble matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9), and for the cytokines interleukin-6 (IL-6) and interferon gamma-induced protein 10 (IP-10, which is also known as CXCL10). All values are reported as the mean±one standard deviation.
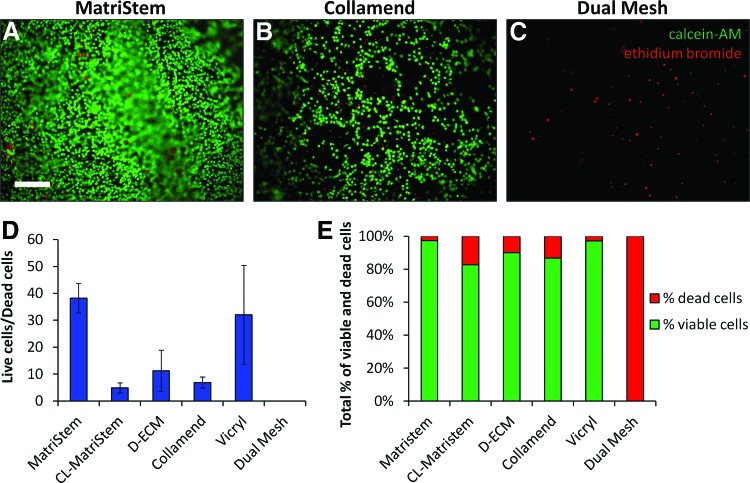
Cell viability was determined for each device after the final 48-h time point. Culture media were replaced with 1 μM calcein AM and 1 μM ethidium bromide in PBS (live/dead assay; Invitrogen) for 20 min at 37°C to label viable and dead macrophages. Devices with labeled macrophages were covered with a glass coverslip and the material surface was imaged via epifluorescence. The ratio of viable to dead macrophages was quantified from 100×images using ImageJ software (National Institute of Health, Bethesda, MD).
Statistical analysis and data-driven modeling
Statistical analysis of macrophage protein secretion was conducted using SPSS software (IBM SPSS Statistics; IBM, Armonk, NY) via a mixed-model analysis of variance (ANOVA). Each secreted protein was individually analyzed to determine within-subject effects (i.e., differences over time), between-subject effects (i.e., differences due to scaffold type), and interaction effects. A post hoc Tukey's test was conducted to make individual comparisons between each scaffold type. Statistical significance was defined as p<0.05. This traditional statistical analysis was followed by data-driven modeling approaches: PCA and DyNA.
Principal component analysis
The primary drivers of the response to each material were determined by performing weighted PCA from the dynamic protein secretion profile using Matlab® software (The Mathworks, Inc., Natick, MA), as previously described.28,29 Protein concentration values were normalized between 0 and 1 since individual proteins may act according to different concentration scales. Normalization for each protein's concentration was carried out across the entire data set (i.e., across time point and device) by the following equation:
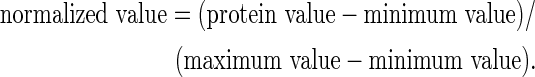 |
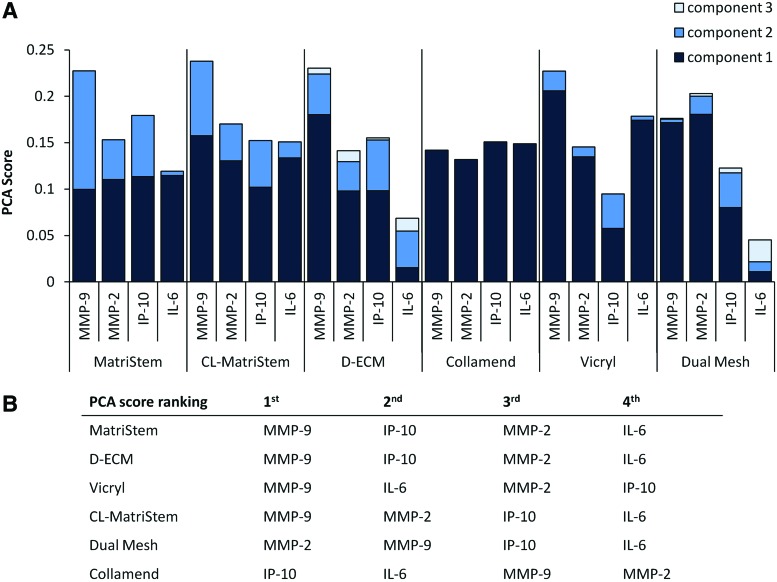
PCA was performed for each individual device response to derive the principal components of each secreted protein as previously described.28 Only the components that accounted for 95% of the variance in the data were considered for analysis, and these components were weighted by multiplying by the associated eigenvalue. The weighted components were summed to provide a “PCA score.” The PCA score provides a quantitative metric for defining the primary drivers of the response; that is, the primary drivers are the proteins with highest PCA score and account for the greatest variance in the dynamic response.28,30 The PCA scores derived for each device were ranked to define the protein responders that were most influential in the macrophage response to a device. Devices were clustered into groups that shared similar PCA score rankings when proteins were ordered from highest to lowest PCA score.
Dynamic network analysis
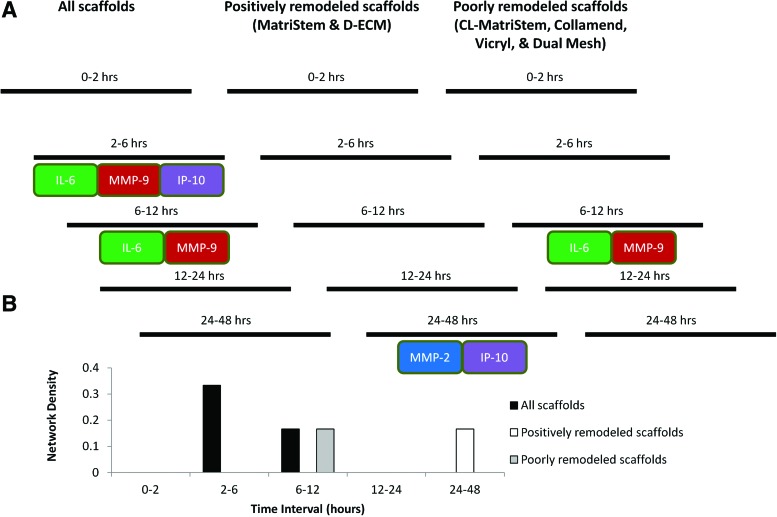
DyNA was performed as described previously28,31 to determine the dynamic connectivity between the normalized protein values (defined in the previous section, Principal component analysis) using Matlab and Inkscape software (http://inkscape.org/). Devices were placed into three analysis groups based upon in vivo remodeling results (Fig. 2): degradable, effectively remodeled ECM devices (MatriStem and D-ECM), poorly remodeled devices that elicited a foreign body response (CL-MatriStem, Collamend, Vicryl, and Dual Mesh), and a group that included all devices. Network connections and network nodes were defined for each consecutive time interval (i.e., 0–2, 2–6 h, etc.). Network nodes were defined as protein values that had significantly increased (p<0.05) from baseline (t=0 h) using a Student's t-test and network connections between nodes were defined as correlations (positive or negative correlations above 0.8) between the nodes within each time interval. Network density was found for each time interval to represent the total degree of connectivity in the system and was defined as the following: network density=number of connections/number of possible connections.
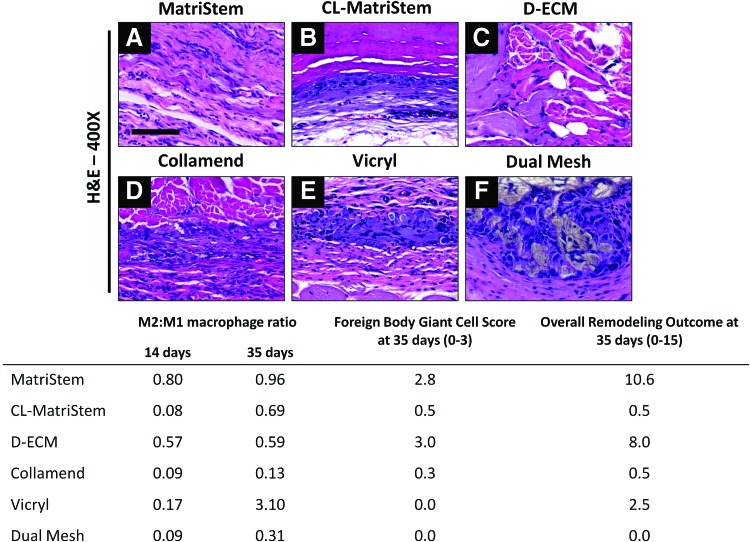
FIG. 2.
In vivo remodeling and macrophage polarization in response to each biomaterial in a skeletal muscle injury. Representative hematoxylin and eosin (H&E) images (A–F) at the host–material interface after 35 days of implantation (400×, scale bar represents 100 μm). The ratio of M2:M1 polarized macrophages was quantified after 14 and 35 days using immunofluorescent labeling for CD206+ M2 and CCR7+ M1 macrophages. The host remodeling response was evaluated from H&E images after 35 days. Histomorphometric scoring analysis was conducted to describe the prevalence of foreign body giant cells and the final remodeling outcome (scoring criteria detailed in Supplementary Table S1), in which a higher score indicates fewer giant cells and more positive remodeling characteristics, respectively. Color images available online at www.liebertpub.com/tec
Results
In vivo response to implanted devices
The histomorphometrically assessed remodeling response after 35 days as well as the macrophage polarization state for each implanted device after 14 and 35 days are shown in Figure 2. The noncrosslinked biologic materials MatriStem (Fig. 2A and Supplementary Fig. S1A, C) and D-ECM (Fig. 2C) had the highest total remodeling scores at 35 days concomitant with the fewest number of foreign body giant cells and highest M2:M1 ratio at day 14. Collamend (Fig. 2D and Supplementary Fig. S1B, D) and Dual Mesh (Fig. 2F) elicited a classic foreign body reaction with the formation of multinucleate foreign body giant cells by 35 days. These materials also had the lowest remodeling scores and lowest M2:M1 ratio at 14 days, though the M2:M1 ratio at 35 days had increased substantially for several devices, including CL-MatriStem and Vicryl. The materials analyzed in this study followed a correlation of total remodeling score at 35 days that was associated with a higher M2:M1 ratio at 14 days. The materials were ranked in the relative order of higher to lower remodeling scores as follows: MatriStem>D-ECM>CL-MatriStem>Vicryl>Collamend>Dual Mesh.
Analysis of macrophage-secreted proteins and viability
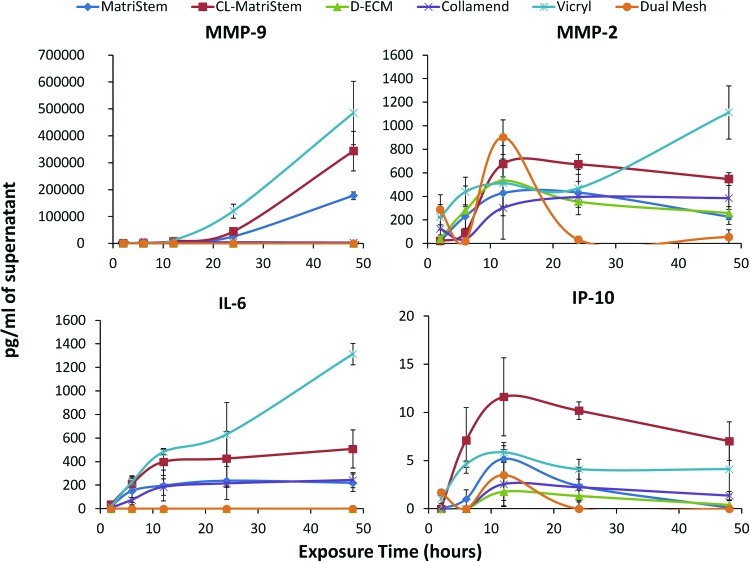
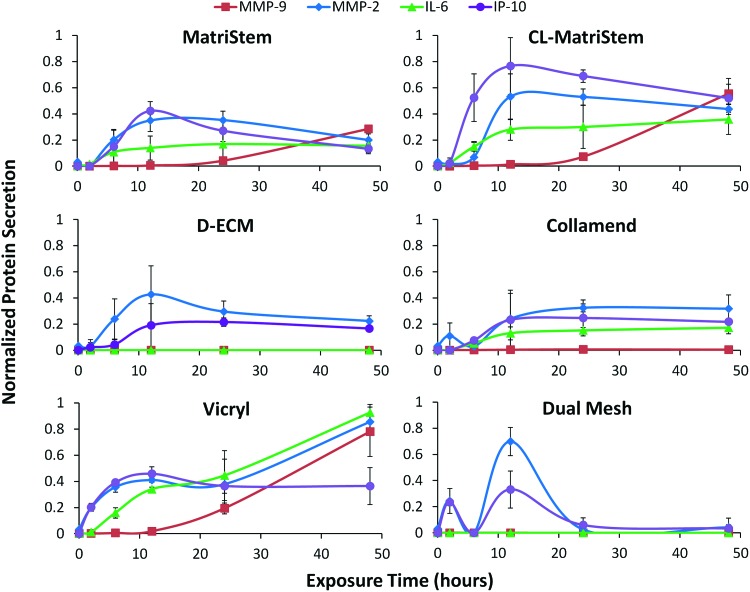
Protein secretion profiles (Fig. 3) varied considerably during the 48-h time course with each device eliciting a unique dynamic profile. Statistical analysis of protein secretion using a mixed-model ANOVA (Supplementary Fig. S2) shows that all of the analyzed proteins were affected (p<0.05) by scaffold exposure time and scaffold type (Supplementary Fig. S2, shaded blue boxes). Post hoc analysis (Supplementary Fig. S2, orange and yellow highlighted p-values) showed that MMP-9 displayed the greatest variability between scaffold types, and Vicryl elicited the most consistent differences from other scaffolds regardless of protein analyte assessed. Each protein's secretion profile was normalized between 0 and 1 to compare the dynamics of each secretion profile in response to different biomaterials (Fig. 4).
FIG. 3.
Multiplex immunoassay of dynamic macrophage protein secretion in response to each scaffold. MMP-9, MMP-2, IP-10, and IL-6 concentrations (pg/mL) were quantified from culture media supernatants after 2, 6, 12, 24, and 48 h of macrophage exposure (error bars represent±one standard deviation, n=3). Color images available online at www.liebertpub.com/tec
FIG. 4.
Normalized multiplex immunoassay macrophage protein secretions in response to each scaffold. Media supernatant protein concentrations (MMP-9, MMP-2, IP-10, and IL-6) following macrophage culture were normalized between 0 (no increase) and 1 (maximum protein concentration across all scaffolds and time points). The normalized concentration values of each protein were compared for each scaffold condition (error bars represent±one standard deviation, n=3). Color images available online at www.liebertpub.com/tec
Macrophage viability was also affected by the type of biomaterial. MatriStem supported a nearly confluent layer of viable macrophages (Fig. 5A), whereas fewer total cells were visible on Collamend (Fig. 5B) and there were no viable cells by 48 h in the Dual Mesh cultures (Fig. 5C). The ratio of live/dead cells and relative percentages of live/dead cells (Fig. 5D, E) confirmed that macrophage viability was greatest when cultured on MatriStem and lowest when cultured on Dual Mesh.
FIG. 5.
Cell viability of macrophages seeded on the surface of each scaffold. Live/dead viability staining was performed after 48 h of culture to label the cytoplasm of viable cells with calcein-AM (green) and the nuclei of dead or dying cells with ethidium bromide (red) (A–C, scale bar represents 200 μm). Total viability was quantified as a ratio or viable/dead cells (D) and as the percentage of total cells that are viable and dead (E) (error bars represent±one standard deviation, n=3). Color images available online at www.liebertpub.com/tec
Principal component analysis
We hypothesized that the principal drivers of the inflammatory responses of macrophages cultured on biomaterials would differ based on the biomaterial. To test this hypothesis, the dynamic macrophage secretory profile was used to find a weighted PCA score to determine the primary drivers in the response to each material; that is, the mediators that are responsible for the most variance in the overall response are associated with a higher PCA score. The relative PCA scores for each material are presented in Figure 6A. The drivers for each material were ranked according to their relative PCA score (Fig. 6B). MMP-9 was the mediator with the highest-ranking PCA score for MatriStem, D-ECM, Vicryl, and CL-MatriStem. The remaining PCA score rankings for MatriStem and D-ECM were identical in the order of IP-10>MMP-2>IL-6 while CL-MatriStem and Vicryl rankings diverged with MMP-2>IP-10>IL-6 and IL-6>MMP-2>IP-10, respectively. The PCA score profile for Collamend was nearly identical for each protein with IP-10 greater than the remaining proteins. PCA scores for Dual Mesh were also unique with MMP-2>MMP-9>IP-10>IL-6.
FIG. 6.
PCA of the normalized protein secretion profile. Individual PCA was performed on the total normalized responses to each material. Weighted PCA scores were obtained that represent the contribution of each protein to the macrophage response to each individual scaffold (A). PCA scores were ranked for each scaffold to determine the primary drivers of the response, and grouped according to similarities in the relative order of each driver (B). Color images available online at www.liebertpub.com/tec
Dynamic network analysis
We hypothesized that different dynamic networks of inflammation would be induced in macrophages cultured on the different biomaterials. Accordingly, DyNA was performed to characterize the connectivity of the macrophage response over the entire time course using the normalized protein values as summarized in Figure 7A. An example of the DyNA output between the 2- and 6-h time points when analyzing all scaffolds is shown in Supplementary Figure S3. DyNA performed on data for all devices showed positive correlations between both IL-6 and IP-10 to MMP-9 during the 2–6-h interval as well as between IL-6 and MMP-9 over the 6–12-h interval. The positively remodeled device group showed a positively correlated network between MMP-2 and IP-10 during the 24–48-h interval, whereas the poorly remodeled device group showed a positive correlation between IL-6 and MMP-9 during the 6–12-h interval, as was seen when all scaffolds were analyzed together.
FIG. 7.
Graphical representation of the DyNA of the normalized protein secretion profile. The presence of dynamic inflammatory networks was assessed between each time interval for three scaffold groups as determined from the histomorphometric remodeling score: all scaffolds evaluated in this study, the positively remodeling scaffolds (MatriStem and D-ECM), and the poorly remodeling scaffolds (CL-MatriStem, Collamend, Vicryl, and Dual Mesh). Protein-labeled boxes in contact with each other are correlated (i.e., connected) to each other during their respective time intervals (A), and the total number of connections over each time interval is shown by the network density (B). Time intervals without boxes lack significant connections (correlation threshold for significance=0.8). Color images available online at www.liebertpub.com/tec
We next hypothesized that in addition to observing different secretory profile networks induced by macrophages cultured on the different biomaterials, the total network connectivity would differ as well. Total network density, however, was similar in both positively and poorly remodeled device groups (Fig. 7B).
Discussion
Implantable surgical mesh materials and medical devices composed of various biomaterials are used for tissue reconstruction and functional tissue replacement. Though some of these devices have remained relatively unchanged over the last 50 years,3,5,34 new devices are continuously being developed to address evolving clinical needs. Next-generation devices have attempted to reduce the incidence of clinical complications, such as excessive tissue fibrosis and stress shielding,35,36 or to impart new functionalities, such as electrical conductivity and drug-delivery vehicles.37,38 Consequently, the number and diversity of clinically available devices has increased rapidly during that time as has the number of procedures utilizing implantable biomaterials. The list of biomaterials has thus expanded from a few “inert” plastics and metals, to a wide variety of synthetic polymers and biologically derived materials intended to elicit host interaction and integration with the device. The processes by which such implantable biomaterials are evaluated, however, have not kept pace with the rate of new biomaterial development. Traditional in vivo testing methods are limited in their ability to rapidly characterize complex biologic processes such as the host tissue response after biomaterial implantation, thereby necessitating alternative approaches for screening biomaterials.
The present study sought to develop an in vitro assay and concomitant in silico analysis modality to characterize and predict the in vivo host response following exposure to a biomaterial. The biomaterials selected for this study are clinically relevant and represent a range of compositions and in vivo responses. Thus, the method described herein would be applicable to a wide spectrum of material classifications. The tissue-derived ECM materials (MatriStem and D-ECM) and the synthetic Vicryl devices in this study are biodegradable in vivo, and molecules released during degradation may affect the host response directly.39 The surface chemistries and compositions of biologic materials are heterogeneous and diverse9; in contrast, the synthetic materials are homogenous with relatively few surface groups. These differences in material properties translate into highly diverse in vivo host responses, which were then captured in a temporally dynamic in vitro assay of macrophage–material interaction. PCA and DyNA were conducted on macrophage protein secretion profiles to determine the protein drivers and connectivity of the macrophage response. These outcome measures were then associated with the in vivo tissue responses to obtain a predictive model of implant performance.
Tissue remodeling after biomaterial implantation is the sum result of several interconnected processes, but the innate immune response is universally involved after material implantation in vivo.2,17 Macrophages in particular interact with biomaterials soon after implantation and continue to participate in the material host response over the lifetime of the implant. Recently, different functional macrophage phenotypes (M1 and M2) have been described and characterized. The M1 phenotype is classically associated with host defense and proinflammatory functions while the alternative M2 phenotype is generally involved with tissue remodeling and constructive functions.40 Recent reports suggest that a dominant and persistent M1 macrophage response to an implanted material is correlated with fibrosis and poor tissue remodeling, whereas greater M2 macrophage involvement supports constructive remodeling with less scarring and site-specific functional tissue formation.20,41,42 The central role of macrophages does not imply that other cell types, such as neutrophils, platelets, and others, are not relevant to host–biomaterial immune interactions. However, given their importance, diversity in activation in vivo, and plasticity, macrophages were selected as the in vitro model system for biomaterial interaction. Similar to previous studies, macrophages showed a unique protein secretion profile in response to different biomaterials in vitro.22,23,25,26 Unlike these previous studies however, the present study analyzed a dynamic response profile using in silico methods to classify distinct patterns of responses. The central goal was to develop an assay and analysis paradigm for biomaterial scaffolds, based exclusively on in vitro data combined with in silico analysis of principal drivers (PCA) and interconnected networks of inflammation (DyNA), as a screening tool that might be used prior to testing these materials in vivo.
PCA of in-vitro-secreted protein concentrations suggested response patterns that were not apparent from the raw data, and these patterns corresponded to distinct in vivo responses. MatriStem and D-ECM were both characterized by identical PCA score rankings in vitro, with MMP-9 as the primary characteristic of the inflammatory response. Both MatriStem and D-ECM correspondingly showed the most favorable in vivo host response of all the evaluated materials, with the fewest foreign body giant cells and highest remodeling scores. When this approach is applied to the remaining materials, PCA score rankings placed Vicryl and CL-MatriStem into a similar in vitro category, and these materials were characterized with intermediate remodeling characteristics. Dual Mesh and Collamend diverged completely from each of these groups, and showed poor remodeling characteristics compared with the other materials, as evidenced by the lowest M2:M1 ratio and remodeling score. These in vitro group rankings were indeed associated positively with their predicted in vivo remodeling characteristics. The results of the present study suggest that, in developing new implantable biomaterials, some attention should be focused on driving the type of in vitro inflammatory response that in silico analyses suggest is associated with favorable in vivo outcomes.
Data-driven approaches, such as PCA, have the potential to facilitate the discovery of unique inflammatory response characteristics, such as the response to Collamend. The results of the present study show that each protein possesses very similar PCA scores, suggesting that there is no primary characteristic or driver for the Collamend-induced inflammatory response. The direct interpretation of this finding is that each protein contributes similarly to the overall variance during the full time course of the study. The system may be described as being in a state of “shock” without clear inflammatory drivers of healing. Multiple inflammatory mediators are elevated, but are not progressing toward resolution or increased inflammation, a state analogous to some chronic, nonhealing wounds. Devices yielding constant PCA scores should be analyzed carefully as a ranking system may not be meaningful, and may be more susceptible to detection noise.
DyNA is an alternative data-driven method for characterizing network dynamics. Unlike PCA in the present study, which considers the entirety of dynamic response (i.e., all time points are analyzed), DyNA discerns which mediators are statistically correlated between individual time intervals and are thus potentially connected.28,31 The number of connections to a particular mediator may indicate the importance of that protein over specific intervals of time. The number of connections, or network density, can also be interpreted as an indicator of the complexity of the response or as the amount of regulation involved in the response. DyNA performed on individual response groups is therefore not intended to be a predictive tool, but was performed to gain additional insights into differences between positive and poor responders. DyNA performed on the entire data set implicated MMP-9 as a mediator in the response, which was consistent with PCA, and that it was an important node during the early and middle phases of the response. This early-mid time period had the greatest network connectivity, suggesting that it is most relevant in regulating the response. DyNA also showed that the responses between positively remodeled scaffolds (e.g., MatriStem) and poorly remodeled scaffolds (e.g., Collamend) are different, though additional interpretations for this data set are challenging.
The data-driven approaches applied in the present study (PCA and DyNA) do not imply a mechanistic understanding of the response. For example, MMP-9 possesses multiple roles in tissue remodeling and homeostasis,43,44 and MMP-9 expression is necessary for macrophage fusion into the foreign body giant cells that characterize a mature foreign body response.45 Having such diverse roles, MMP-9 has been reported in both M1 and M2 polarized macrophages,43,44,46,47 and therefore the significance of its expression is highly dependent on kinetics and context. The present study showed that CL-MatriStem and Vicryl exposure both elicited highly elevated MMP-9 levels in vitro and correspondingly were shown to develop an M1 response and multiple foreign body giant cells in vivo. However, both Collamend and Dual Mesh also induced a similar M1 and foreign body reaction in vivo, but secrete negligible amounts of MMP-9 in vitro. These data indicate that there is a discontinuity between the in vitro results and in vivo outcome and/or several divergent functions of MMP-9 that challenges a simplistic mechanistic approach, thus necessitating these in silico methods. These complex, dynamic expression patterns in macrophages are not limited to MMP-9, but extend to the other molecules tested in this study. MMP-2 has been associated with an M2 response,48 though it can be induced by other classically proinflammatory signals, such as IL-6.49 IL-6 is an M1 cytokine in which strict temporal regulation is necessary for wound healing; IL-6 inhibition prevents proper healing, though persistent expression is associated with scar formation.50 IP-10 function is classically regarded as a potent proinflammatory factor that is upregulated in M1 macrophages and downregulated in M2 macrophages. However, clinical studies of trauma suggest that IP-10 coordinates a beneficial, self-limiting inflammatory response.51 The overlapping and redundant functions of these proteins complicate the interpretation of the inflammatory response, as do biological characteristics of the proteins themselves. Each of the analyzed proteins has different biological half-lives and proteases, such as MMPs, may directly influence the release and stability of the other analytes. Rapid release of vesicle sequestered proteins via exocytosis, membrane shedding, and protein translation are highly regulated events that convolute a causal relationship between the analyzed proteins in a cell culture supernatant. The in silico data-driven approaches in the present study utilize the complexity of the system, rather than overinterpreting raw data.
The present study was performed in the context of skeletal muscle injury, an experimental paradigm in which macrophage participation and polarization has been studied extensively. The methods presented herein may also have applications to other injury locations, such as adipose, bone, or cardiac tissues. Macrophage involvement is critical to these tissues as well, but remodeling outcomes following injury and scaffold implantation vary considerably. Therefore, further studies are necessary to determine the universal applicability of this approach.
Though the results of the present study are encouraging, certain factors within an in vitro culture system may present a challenge to this approach. Cell isolation and culture artifacts are a potential limitation associated with such assays. Though a negative selection isolation protocol was used to circumvent the influence of antibody binding to monocytes, some degree of cell injury and/or activation with concomitant release of M1 inflammatory mediators cannot be ruled out. The macrophage maturation protocol, though well established, has been shown to introduce polarization bias toward an M2 phenotype.52 There is also the potential for macrophages to interact with other elements in a culture system, such as the polystyrene well plate or the brief initial exposure to the stainless steel seeding rings. Minute quantities of leachable cytotoxic compounds from synthetic materials, endotoxin, or residual decellularization chemicals and cryopreservatives in ECM devices could substantially alter protein expression and/or cell viability. Chemical contaminants would likely sustain an effect within a closed, small-volume system such as an in vitro well plate, but may be tolerated and effectively cleared from the local environment in vivo, and thereby minimize any influence on responder cells. Other limitations of the present study are the relatively low number of devices and secreted factors investigated, and the use of a single blood donor. The present study showed that analyzing a select group of secreted factors is sufficient to approximate a predictive model, though additional analytes may be added in the future to refine and optimize this modality. Donor variability is frequently a limitation for immunological studies. However, the dynamic data-driven approaches in the present study focused on relative changes in protein responses over time, and may be less susceptible to differing baseline inflammatory responses.
Conclusions
The present study describes an in vitro macrophage–biomaterial interaction assay that, when analyzed using in silico methods (PCA and DyNA), is able to predict the host immune and remodeling responses in vivo. This methodology enables the analysis and interpretation of complex macrophage behavior when exposed to biomaterials. The ranked PCA method successfully grouped ECM devices that have positive remodeling characteristics, such as MatriStem, apart from poorly performing devices. These groupings were based entirely on in vitro macrophage secretions, which was not otherwise apparent from the raw data. Such an approach may be used as a screening assay for biomaterial development to reduce the burden of preclinical animal testing by predicting in vivo response in vitro.
Supplementary Material
Acknowledgments
M.T.W. was partially supported by the NIH-NHLBI training grant (T32-HL76124-6) entitled “Cardiovascular Bioengineering Training Program,” and B.N.B. was partially supported by the NIH K12 Award “Interdisciplinary Research Careers in Women's Health” (HD043441). The authors would like to thank Deanna Rhoads and the McGowan Histology Center for histologic section preparation, and Hongbin Jiang for assistance with animal care.
Disclosure Statement
Dr. Stephen F. Badylak currently conducts sponsored research for ACell, Inc. and C.R. Bard, Inc. Dr. Stephen F. Badylak receives no financial gains for any of the products sold by either company.
References
- 1.Weyhe D., Schmitz I., Belyaev O., Grabs R., Muller K.M., Uhl W., et al. . Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg 30,1586, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ozawa T., Mickle D.A., Weisel R.D., Koyama N., Wong H., Ozawa S., et al. . Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg 124,1157, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Leber G.E., Garb J.L., Alexander A.I., and Reed W.P.Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133,378, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Anderson J.M., Rodriguez A., and Chang D.T.Foreign body reaction to biomaterials. Semin Immunol 20,86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klinge U., Klosterhalfen B., Muller M., and Schumpelick V.Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165,665, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Luttikhuizen D.T., Harmsen M.C., and Van Luyn M.J.Cellular and molecular dynamics in the foreign body reaction. Tissue Eng 12,1955, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Badylak S.F., Freytes D.O., and Gilbert T.W.Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater 5,1, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hodde J.P., Badylak S.F., Brightman A.O., and Voytik-Harbin S.L.Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 2,209, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Brown B., Lindberg K., Reing J., Stolz D.B., and Badylak S.F.The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 12,519, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Reing J.E., Brown B.N., Daly K.A., Freund J.M., Gilbert T.W., Hsiong S.X., et al. . The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31,8626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeken C.R., Melman L., Jenkins E.D., Greco S.C., Frisella M.M., and Matthews B.D.Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair. J Am Coll Surg 212,880, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valentin J.E., Stewart-Akers A.M., Gilbert T.W., and Badylak S.F.Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A 15,1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodde J., Janis A., Ernst D., Zopf D., Sherman D., and Johnson C.Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med 18,537, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Akhyari P., Aubin H., Gwanmesia P., Barth M., Hoffmann S., Huelsmann J., et al. . The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods 17,915, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Rashid S.T., Salacinski H.J., Hamilton G., and Seifalian A.M.The use of animal models in developing the discipline of cardiovascular tissue engineering: a review. Biomaterials 25,1627, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Brunelli S., and Rovere-Querini P.The immune system and the repair of skeletal muscle. Pharmacol Res 58,117, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Di Vita G., D'Agostino P., Patti R., Arcara M., Caruso G., Davi V., et al. . Acute inflammatory response after inguinal and incisional hernia repair with implantation of polypropylene mesh of different size. Langenbecks Arch Surg 390,306, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Di Vita G., Patti R., D'Agostino P., Ferlazzo V., Angileri M., Sieli G., et al. . Modifications in the production of cytokines and growth factors in drainage fluids following mesh implantation after incisional hernia repair. Am J Surg 191,785, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Jansen P.L., Kever M., Rosch R., Krott E., Jansen M., Alfonso-Jaume A., et al. . Polymeric meshes induce zonal regulation of matrix metalloproteinase-2 gene expression by macrophages and fibroblasts. FASEB J 21,1047, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., et al. . Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8,978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariganello M.B., Labow R.S., and Lee J.M.In vitro response of monocyte-derived macrophages to a decellularized pericardial biomaterial. J Biomed Mater Res A 93,280, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Ariganello M.B., Simionescu D.T., Labow R.S., and Lee J.M.Macrophage differentiation and polarization on a decellularized pericardial biomaterial. Biomaterials 32,439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schachtrupp A., Klinge U., Junge K., Rosch R., Bhardwaj R.S., and Schumpelick V.Individual inflammatory response of human blood monocytes to mesh biomaterials. Br J Surg 90,114, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Weyhe D., Belyaev O., Buettner G., Mros K., Mueller C., Meurer K., et al. . In vitro comparison of three different mesh constructions. ANZ J Surg 78,55, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Orenstein S.B., Qiao Y., Klueh U., Kreutzer D.L., and Novitsky Y.W.Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia 14,401, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Weyhe D., Hoffmann P., Belyaev O., Mros K., Muller C., Uhl W., et al. . The role of TGF-beta1 as a determinant of foreign body reaction to alloplastic materials in rat fibroblast cultures: comparison of different commercially available polypropylene meshes for hernia repair. Regul Pept 138,10, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Janes K.A., and Yaffe M.B.Data-driven modelling of signal-transduction networks. Nat Rev Mol Cell Biol 7,820, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mi Q., Constantine G., Ziraldo C., Solovyev A., Torres A., Namas R., et al. . A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS One 6,e19424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namas R.A., Namas R., Lagoa C., Barclay D., Mi Q., Zamora R., et al. . Hemoadsorption reprograms inflammation in experimental gram-negative septic peritonitis: insights from in vivo and in silico studies. Mol Med 18,1366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieman G., Brown D., Sarkar J., Kubiak B., Ziraldo C., Dutta-Moscato J., et al. . A two-compartment mathematical model of endotoxin-induced inflammatory and physiologic alterations in swine. Crit Care Med 40,1052, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziraldo C., Vodovotz Y., Namas R.A., Almahmoud K., Tapias V., Mi Q., et al. . Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PLoS One 8,e79804, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F.Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 33,2916, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recalcati S., Locati M., Marini A., Santambrogio P., Zaninotto F., De Pizzol M., et al. . Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol 40,824, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Usher F.C.Hernia repair with knitted polypropylene mesh. Surg Gynecol Obstet 117,239, 1963 [PubMed] [Google Scholar]
- 35.Wolf M.T., Carruthers C.A., Dearth C.L., Crapo P.M., Huber A., Burnsed O.A., et al. . Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. J Biomed Mater Res A 102,234, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce R.A., Perrone J.M., Nimeri A., Sexton J.A., Walcutt J., Frisella M.M., et al. . 120-Day comparative analysis of adhesion grade and quantity, mesh contraction, and tissue response to a novel omega-3 fatty acid bioabsorbable barrier macroporous mesh after intraperitoneal placement. Surg Innov 16,46, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Nelson D.M., Baraniak P.R., Ma Z., Guan J., Mason N.S., and Wagner W.R.Controlled release of IGF-1 and HGF from a biodegradable polyurethane scaffold. Pharm Res 28,1282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao I.C., Liu J.B., Bursac N., and Leong K.W.Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell Mol Bioeng 1,133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reing J.E., Zhang L., Myers-Irvin J., Cordero K.E., Freytes D.O., Heber-Katz E., et al. . Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A 15,605, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Mosser D.M., and Edwards J.P.Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8,958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badylak S.F., Valentin J.E., Ravindra A.K., McCabe G.P., and Stewart-Akers A.M.Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14,1835, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Sussman E.M., Halpin M.C., Muster J., Moon R.T., and Ratner B.D.Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng 42,1508, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Webster N.L., and Crowe S.M.Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol 80,1052, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Hanania R., Sun H.S., Xu K., Pustylnik S., Jeganathan S., and Harrison R.E.Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. J Biol Chem 287,8468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLauchlan S., Skokos E.A., Meznarich N., Zhu DH, Raoof S., Shipley J.M., et al. . Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol 85,617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee J.W., Lee K.W., Kim D., Lee Y., Jeon O.H., Kwon H.J., et al. . NF-kappaB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line RAW 264.7. J Biochem Mol Biol 40,88, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Lolmede K., Campana L., Vezzoli M., Bosurgi L., Tonlorenzi R., Clementi E., et al. . Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol 85,779, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Busch S.A., Horn K.P., Silver D.J., and Silver J.Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci 29,9967, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobara M., Noda K., Kitamura M., Okamoto A., Shiraishi T., Toba H., et al. . Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res 87,424, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Gallucci R.M., Simeonova P.P., Matheson J.M., Kommineni C., Guriel J.L., Sugawara T., et al. . Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J 14,2525, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Zaaqoq A.M., Namas R., Almahmoud K., Azhar N., Mi Q., Zamora R., et al. . Inducible protein-10, a potential driver of neurally controlled interleukin-10 and morbidity in human blunt trauma. Crit Care Med 42,1487, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaguin M., Houlbert N., Fardel O., and Lecureur V.Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol 281,51, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.