Abstract
The Brazilian AIDS epidemic is characterized by significant geographic contrasts: a reduction in incidence and mortality in the epicenter (southeast) and an increase in the northeast. HIV-1-transmitted drug resistance (TDR) and genetic diversity were investigated among 106 antiretroviral (ARV)-naive patients from Maranhão State, northeast. The HIV-1 protease (PR) and reverse transcriptase (RT) regions were sequenced; subtypes were assigned by REGA/phylogenetic analysis. TDR to the nucleoside/nonnucleoside reverse transcriptase inhibitor (NRTI/NNRTI) and protease inhibitor (PI) was identified by the Calibrated Population Resistance tool (Stanford). The median age was 31 years (range 18–72), with 54.7% women, 78.3% heterosexual transmission, and 17.9% men who have sex with men (MSM). Around 30% had <350 CD4+ T cells/μl and 47.2% had plasma viral loads ≤10,000 copies/ml. The TDR rate was 3.8% (4/106; CI 95%, 1.2–8.9%) (three males, two of them MSM). Only single class mutations to NRTI (M184V; T215S) or NNRTI (K103S/N) were detected. Subtype B represented 81.1% (86/106), F1 1.9% (2/106), and C 2.8% (3/106); 14.2% were mosaics: 13 BF1 and 2 BC. Surveillance of TDR and HIV-1 genetic diversity is important to improve control strategies regionally.
Striking regional differences have been described in the dynamic of the Brazilian AIDS epidemic, which has stabilized in the most populated and industrialized southeast/south regions where cases are strongly concentrated. In the past decade, a reduction of 18.6% in the AIDS detection rate has taken place in the southeast, contrasting with a 62.6% increase in the northeast region, with high rates of HIV-1-infected young individuals in the 15–24 year age range.1 Since the 1990s the Brazilian public health program has provided free antiretroviral drugs (ARV) to more than 313,000 HIV-1/AIDS patients, promoting a 14% reduction in AIDS-related mortality.1 However, during the past decade, a 33.3% growth in AIDS-related mortality was reported in the northeast region.1
One of the drawbacks of the widespread use of ARV is the selection of mutations associated with drug resistance, which can be transmitted to uninfected patients. Transmitted drug resistance (TDR) represents an important public health issue that can impact initial treatment options including highly active antiretroviral therapy (HAART) with nucleoside and nonnucleoside reverse transcriptase inhibitors (NRTI/NNRTI) and protease inhibitors (PI).2 Genotyping tests have been employed to orient treatment options by identifying mutations that may confer resistance to ARV drugs.
Since the beginning of the AIDS epidemic in Brazil, Maranhão State (6,794,301 inhabitants) in northeast Brazil has identified 11,460 cases. São Luís, the State capital, is a port city and Itaqui harbor exports iron ore, pig iron, soy, petroleum derivatives, copper, and aluminum. In the northeast, São Luís has the highest incidence of AIDS cases (42.5/100,000 inhabitants) among municipalities with over 50,000 inhabitants. From 2009 to 2012, 10 referral centers for AIDS patients' care were available in Maranhão State, where most of the HIV-1-infected patients were symptomatic and had AIDS-defining illness at diagnosis.3
Low to moderate levels of TDR have been reported in patients from the most densely populated southeast/south regions in Brazil.4–7 However, there are very limited data from regions where the epidemic is growing, including Maranhão State in northeast Brazil, our study area, which has recently reported a 106.2% increase in AIDS cases rate. This is the first detailed molecular epidemiological investigation of TDR and HIV-1 genetic diversity among ARV-naive patients from Maranhão. Surveillance of TDR and HIV-1 diversity is important, particularly in areas of rising incidence such as northeast Brazil, and may contribute to improved regional prevention strategies to control the epidemic.
A total of 138 consecutive cases of ARV-naive patients infected with HIV-1 living in Maranhão State were enrolled (January–June 2012): 97 were recruited in São Luís at the regional referral center for CD4+ cell counts and viral loads determinations (Public Health Central Laboratory/LACEN) and 41 patients were recruited in Teresina, Piauí State at the Hospital de Doenças Tropicais Dr. Natan Portela. According to official data approximately 10% of HIV-1/AIDS patients from Maranhão, especially the ones living near the Maranhão-Piauí border, attend the closest referral center, which is located in Teresina, Piauí. Age, sex distribution, and time since diagnosis among patients recruited in São Luís and Teresina were statistically similar. The 138 patients recruited represented 21.1% of the 502 AIDS cases reported in Maranhão State during the study period.3
Among 138 ARV-naive patients recruited, 53 lived in São Luís city while the remaining 85 patients lived in 44 different small interior villages scattered throughout the state. Sociobehavioral data were obtained by face-to-face interviews using standardized questionnaires. Clinical and laboratory data (plasma viral load-bDNA, Siemens Medical Solutions, Mississauga, Ontario, Canada, and CD4+ T cell counts, FACSalibur, Becton & Dickson, San Jose, CA) were retrieved in standardized questionnaires from the medical files at Hospital Presidente Vargas, São Luís, Maranhão and at Hospital de Doenças Tropicais Dr Natan Portela, Teresina, Piauí. This study was approved by the regional Institutional Review Boards (Comitê de Ética em Pesquisa da Faculdade de Ciências Médicas da Universidade Estadual do Piauí, protocol #022/2011, and Comitê de Ética de Pesquisa do Hospital de Doenças Tropicais Dr Natan Portela, protocol #16/2011). All participants were instructed about the study and signed a written informed consent form.
HIV-1 RNA was extracted from plasma (QIAamp Viral RNA Mini Kit/QIAGEN, Qiagen GmbH), reverse transcribed, and the complementary DNA (cDNA) was used as the target for a nested polymerase chain reaction (nested PCR) of the pol region as previously described.8 The entire protease (PR) and approximately 750 bp of the reverse transcriptase (RT) fragment were amplified employing K1/K2 as external primers and DP10/F2 as internal primers. After purification of amplicons (QIAquick PCR Purification Kit/QIAGEN, Qiagen GmbH), genomic sequencing was performed (BigDye Terminator Cycle Sequencing, Applied Biosystems, Foster City, CA; ABI Prism 3130 Genetic Analyzer, Applied Biosystems, Foster City, CA).
The HIV-1 subtypes were identified by REGA genotyping tool version 2.0 and by phylogenetic inference using reference sequences obtained from Los Alamos HIV. Trees for phylogenetic analysis were generated by the neighbor-joining method under Kimura's two-parameter correction model using MEGA5 software. Bootstrap values (1,000 replicates) above 70% were considered significant. SimPlot 3.5.1 was used to analyze intersubtype recombination in sequences with discordant subtypes in the PR and RT fragments.9
The GenBank accession numbers used in the comparative phylogenetic and SimPlot analyses are subtype B: U21135, FJ638434, AY173956, K03455, EF379194, EF379210, FJ548795, FJ548804, and U63632; subtype C: AF110967, U46016, FJ548791, FJ594149, AF286228, AF067155, and U52953; subtype F1: DQ358801, AF077336, and AF005494; and the simian immunodeficiency virus sequence from the chimpanzee (SIVcpz): X52154, used as an outgroup.
TDR was analyzed using the Calibrated Population Resistance–CPR tool (Stanford Surveillance Drug Resistance Mutation–SDRM). The ARV mutation susceptibility profile was analyzed by the Stanford HIV Drug Resistance Database.10
Continuous variables were described as medians with minimum and maximum range. Categorical variables were presented as frequency and percentage. The Chi-square or Fisher's exact test was used to analyze the differences among categorical variables, with a significance level of 5%. All descriptive analyses were performed using Epi Info version 7 (CDC, Atlanta).
In 106 out of 138 patients (76.8%) the PR and RT regions of HIV-1 were amplified, sequenced, and described in this study. The median age was 31 years (range: 18–72 years), with females representing 54.7; 78.3% reported heterosexual unprotected sex, 17.9% (19/106) were men who have sex with men (MSM), and two participants (one female, one male) were intravenous drug users (IDU). The median of CD4+ T cell count was 402 cells/μl (range: 8–1,193 cells/μl) and 34/106 (32.1%) had cell counts below 350 cells/μl. Plasma viral loads ranged from below 10,000 copies/ml (47.2%, 50/106) to over 100,000 copies/ml (6.6%, 7/106).
The TDR rate was 3.8% (4/106; CI 95%, 1.2–8.9%); three out of four were males and two out of three men were MSM (Table 1). The median of CD4+ T cell counts in TDR cases was 218.5 cells/μl (range 51–569 cells/μl) and the median of plasma viral load was 22,169 copies/ml (1,770–139,308 copies/ml range). Only singleton mutations associated with NRTI (M184V and T215S) or with NNRTI (K103S and K103N) were detected. No major mutation to PI was identified; however, two isolates presented PI accessory mutations (L10I and A71V).
Table 1.
Amino Acid Substitutions in Protease and Reverse Transcriptase Resistance-Related Codons of Patients from Maranhão State, Northeast Brazil
| Mutations | Resistance profile | |||||||
|---|---|---|---|---|---|---|---|---|
| GenBank accession number | Route of infection/ HIV-1 PR/RT subtype | Sex/age (years) | PI accessory | NRTI | NNRTI | Low | Intermediate | High |
| KF782694 | MSM B | M/29 | — | — | K103S | — | EFV | NVP |
| KF782711 | MSM B | M/35 | A71V | T215S | — | AZT, d4T | — | — |
| KF782723 | Hetero B | M/45 | L10I | — | K103N | — | — | EFV, NVP |
| KF782732 | Hetero B/BF1B | F/31 | — | M184V | — | ABC | — | FTC, 3TC |
PR, protease region; RT, reverse transcriptase region; MSM, men who have sex with men; Hetero, heterosexual; M, male; F, female; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; ABC, abacavir; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; NVP, nevirapine; 3TC, lamivudine. The resistance mutations are given in bold.
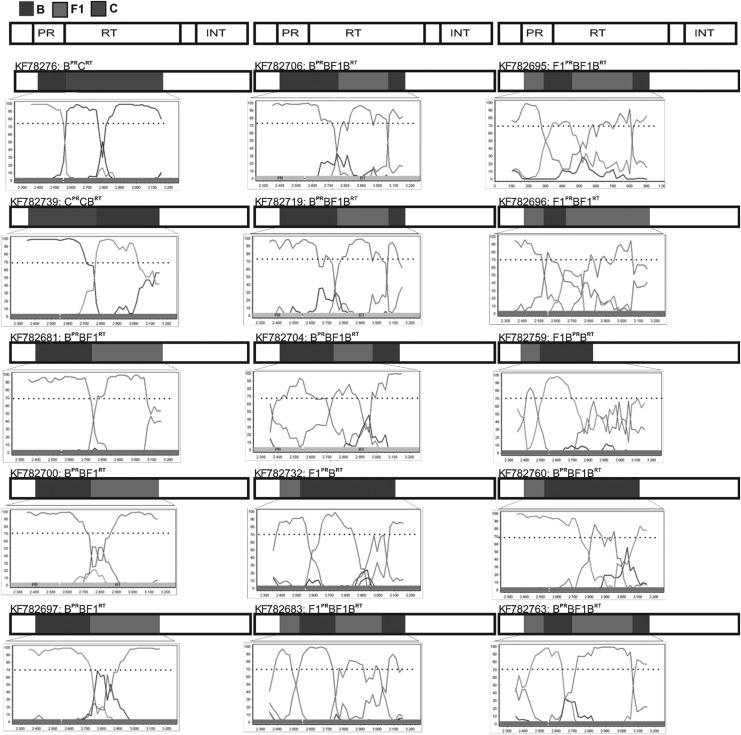
“Pure” non-subtype B isolates were rare: two subtype F1 (1.9%) and three subtype C (2.8%). “Pure” subtype B predominated (81.1%, 86/106) and most PR/RT sequences (85.8%, 91/106) clustered with a single HIV-1 subtype. However, 14.2% of the isolates were mosaic forms with discordant subtypes in the PR and RT regions (Fig. 1). Among 13 B/F1 recombinant mosaics, several different recombination patterns were observed: BPR/BF1BRT (n=6), BPR/BF1RT (n=3), F1PR/BF1BRT (n=1), F1PR/BF1RT (n=2), and F1BPR/BRT (n=1). One BPR/CRT and one CPR/CBRT recombinant were also detected.
FIG. 1.
Bootscanning analysis of HIV-1 protease (PR) and reverse transcriptase (RT) regions of 15 recombinant isolates from Maranhão State, northeast Brazil. The GenBank accession numbers used in the SimPlot analyses are subtype B: K03455, U63632, and FJ548804; subtype C: U52953, FJ594149, and FJ548791; subtype F1: DQ358801 and AF005494.
Phylogenetic analyses of pure and recombinant sequences indicated seven clusters with high support values (>70%) comprising 15 isolates (data not shown). Five of these clusters aligned within the HIV-1 subtype B clade and one comprised two isolates obtained from a heterosexual couple with a known epidemiological link (KF782707, KF782708). Two other clusters comprised the B/F1 mosaic viruses: one with isolates from a heterosexual couple (KF782705, KF782732) and the other with sequences from one MSM and two females, suggesting a possible link between these two groups (KF782706, KF782719, and KF782681).
The first detailed study on the molecular pattern of HIV-1 circulating among ARV-naive patients living in Maranhão State, northeast Brazil showed a low rate of TDR (3.8%) detected mostly in male patients, two MSM and one heterosexual. Despite the low TDR rate, resistance should be monitored in Maranhão State where a significant increase in AIDS cases and associated mortality has been reported in the past decade. Higher TDR rates regionally may represent additional public health challenges. The Brazilian AIDS epidemic is highly heterogeneous within its five geographic regions reflecting striking socioeconomic contrasts.
Although Brazil is among the countries with emerging economies, Maranhão ranks as one of the most resource-limited states in the country, presenting the second lowest human development index (HDI, a statistical tool developed by the United Nations to measure and rank levels of social and economic development based on life expectancy at birth, mean years of schooling, expected years of schooling, and gross national income per capita). Among the recent trends of the Brazilian AIDS epidemic is the increasing incidence among the poor and underprivileged.1 It is possible that the low access to health and education facilities faced by patients living in Maranhão may contribute to the regional spread of the virus. In this sense, the low TDR rate would not be a good public health indicator and might be associated with late diagnosis and probably low access to ARV treatment.11
As expected for TDR, only singleton mutations to either NRTI or to NNRTI were detected. The M184V NRTI resistance mutation detected is responsible for lamivudine (3TC) resistance, which also increases susceptibility to zidovudine (ZDV) and stavudine (d4T). The revertant T215S mutation is considered a “track” of the T215Y/F mutation transmitted to one ZDV-naive patient that reverted to a phenotype closer to the wild type. Previous Brazilian studies have described T215 revertant mutations in ARV-naive patients.8,12,13 The K103N NNRTI mutation causes high-level resistance to nevirapine (NVP) and efavirenz (EFV), which is an important component of the HAART regimen in Brazil. The K103S mutation causes intermediate/high-level resistance to NVP and low/intermediate-level resistance to EFV. Interestingly, in this study five patients had the common polymorphic accessory mutation E138A, which although not included in the CPR database, can be weakly selected by rilpivirine (RPV), a drug still not available in Brazil, decreasing its susceptibility by ∼2-fold.
In Maranhão State the HIV-1 epidemic is highly driven by subtype B. Subtype F1 was rare, different from previous reports from northeast Brazil where it is the second most prevalent subtype. Moreover, this study from Maranhão shows a low prevalence of subtype C, which is the most prevalent variant in south Brazil. Recent studies from central west Brazil indicate subtype C dissemination northward, mainly among young women.14
The low prevalence of “pure” F1 and C subtypes in this setting contrasts with the 14% prevalence of mosaic viruses: 13 BF1 and 2 BC recombinants. The rate of BF1 recombinants in southeast and in northeast Brazil is around 4%.15 Higher rates have been reported in central west Goiás State (7.2%), Mato Grosso State (12%), Mato Grosso do Sul State (8.2%), and Tocantins State (9.6%) in north Brazil.8,12,13,15 The cocirculation of different subtypes favors coinfection or superinfection, which is required for intersubtype recombination. In the context of rare “pure” subtype F1 and C viruses, the import of mosaic BF1 and BC viruses from other regions such as central Brazil or elsewhere is highly probable. Also, given the high diversity of BF1 mosaic patterns detected it is also possible that second- or third-generation BF1 recombinants are being produced by the recombination of imported BF1 viruses with subtype B, which is highly prevalent regionally. PR and RT fragments are considered hotspots for recombination and, more recently, increased genotyping to monitor ARV therapy has shown a growing rate of unrelated BF1 mosaic viruses in Brazil, which has been considered a geographic recombination hotspot. The relationship among these BF1 mosaic viruses from the northeast and other BF1 circulating recombinant forms (CRFs) already described in Brazil or elsewhere deserves further study.
Seaports represent important settings for the spread of HIV-1/AIDS and other sexually transmitted diseases as thousands of male sailors from around the world wait for ships to be unloaded and arriving truck drivers are usually served by a thriving sex industry. Therefore the regional AIDS epidemic is probably influenced by the Itaqui Port in São Luís, one of the most important seaports and the second largest in cargo volume in the country. In southeastern Brazil, one of the highest rates of AIDS cases is reported in Santos, the largest port in Latin America and a major transportation hub to all Brazilian regions and to the rest of South America. Also, the subtype C epidemic in Brazil was originally thought to have been initiated in the southern area in the city of Rio Grande, which has one of the largest seaports in the country, which serves as a southern transportation hub to neighboring countries in South America, and which receives ships from Africa and Asia.
In conclusion, the low level of TDR in individuals infected with HIV-1 living in Maranhão in the northeast, where late diagnosis has been reported, suggests that this rate may be higher among recently infected individuals. The genetic diversity observed indicates a probable insertion of recombinant viruses generated and circulating in other regions as well as local dissemination clusters. In this resource-limited setting with a rising incidence of HIV-1/AIDS and related mortality, HIV-1 molecular data provided in this study may contribute to better management, prevention, and control strategies.
Sequence Data
The GenBank accession numbers of sequences analyzed in this study are KF782680–KF782785.
Acknowledgments
We are thankful to the medical staff of Dr. Costa Alvarenga Public Health Central Laboratory (LACEN) from Teresina City and Public Health Central Laboratory (LACEN) from São Luís City for support during recruitment. This study was supported by CAPES and PRONEX/FAPEG/CNPq-07/2012. Mariane Stefani is a recipient of a fellowship from CNPq (grant 304869/2008-2), Maria Edileuza Moura was supported by a fellowship from CAPES and FAPEMA (grant APP-UNIVERSAL-00222/12), Mônica Reis was supported by FAPEG (grant 201210267000386), Yanna Lima was supported by CAPES (grant 52001016), and Ludimila Cardoso was supported by CAPES (grant 02479/09-5).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Brazil Ministry of Health: National Department of STD, Aids and Viral Hepatitis: Boletim Epidemiológico AIDS/DST–Ano II, n. 1, Brasília, 2013 [Google Scholar]
- 2.WHO–World Health Organization: HIV Drug Resistance Report. World Health Organization, Geneva, 2012 [Google Scholar]
- 3.Secretaria Municipal de Planejamento e Avaliação de Teresina: Teresina 2000 à 2010: diagnóstico, avanços, desafios. 2013. www.pensarmaisteresina.com.br/wp-content/uploads/2013/08/AGENDA_2030-v30jul2013-v2.pdf Accessed December2013
- 4.Inocêncio LA, Pereira AA, Sucupira MCA, et al. : Brazilian Network for HIV Drug Resistance Surveillance: A survey of individuals recently diagnosed with HIV. J Int AIDS Soc 2009;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sprinz E, Netto EM, Patelli M, et al. : Primary antiretroviral drug resistance among HIV type 1-infected individuals in Brazil. AIDS Res Hum Retroviruses 2009;25(9):861–867 [DOI] [PubMed] [Google Scholar]
- 6.Sanabani SS, Pastena ER, da Costa AC, et al. : Characterization of partial and near full-length genomes of HIV-1 strains sampled from recently infected individuals in São Paulo, Brazil. PLoS One 2011;6(10):e25869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira JL, Rodrigues R, Lança AM, et al. : Transmitted drug resistance among people living with HIV/Aids at major cities of São Paulo State, Brazil. Adv Virol 2013;2013:878237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso LPV, Queiroz BB, and Stefani MMA: HIV-1 pol phylogenetic diversity and antiretroviral resistance mutations in treatment naïve patients from Central West Brazil. J Clin Virol 2009;46(2):134–139 [DOI] [PubMed] [Google Scholar]
- 9.Lole KS, Bollinger RC, Paranjape RS, et al. : Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 1999;73(1):152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanford HIV Drug Resistance Database: http://hivdb.stanford.edu Accessed November2013
- 11.Alves MTSSB, Silva AAM, Nemes MIB, and Brito LGO: Tendências da incidência e da mortalidade por Aids no Maranhão, 1985 a 1998. Rev Saude Publ 2003;37(2):177–182 [DOI] [PubMed] [Google Scholar]
- 12.Ferreira AS, Cardoso LPV, and Stefani MMA: Moderate prevalence of transmitted drug resistance and high HIV-1 genetic diversity in patients from Mato Grosso State, Central Western Brazil. J Med Virol 2011;83(8):1301–1307 [DOI] [PubMed] [Google Scholar]
- 13.Silveira AA, Cardoso LP, Francisco RB, and Stefani MM: HIV type 1 molecular epidemiology in pol and gp41 genes among naive patients from Mato Grosso do Sul State, central western Brazil. AIDS Res Hum Retroviruses 2012;28(3):304–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcântara KC, Lins JBA, Albuquerque M, et al. : HIV-1 mother-to-child transmission and drug resistance among Brazilian pregnant women with high access to diagnosis and prophylactic measures. J Clin Virol 2012;54(1):15–20 [DOI] [PubMed] [Google Scholar]
- 15.Cavalcanti AM, Lacerda HR, Brito AM, et al. : Antiretroviral resistance in individuals presenting therapeutic failure and subtypes of the human immunodeficiency virus type 1 in the northeast region of Brazil. Mem Inst Oswaldo Cruz 2007;102(7):785–792 [DOI] [PubMed] [Google Scholar]