Abstract
microRNAs (miRNAs) constitute complex regulatory network, fine tuning the expression of a myriad of genes involved in different biological and physiological processes, including stem cell differentiation. Mesenchymal stem cells (MSCs) are multipotent stem cells present in the bone marrow stroma, and the stroma of many other tissues, and can give rise to a number of mesoderm-type cells including adipocytes and osteoblasts, which form medullary fat and bone tissues, respectively. The role of bone marrow fat in bone mass homeostasis is an area of intensive investigation with the aim of developing novel approaches for enhancing osteoblastic bone formation through inhibition of bone marrow fat formation. A number of recent studies have reported several miRNAs that enhance or inhibit adipogenic differentiation of MSCs and with potential use in microRNA-based therapy to regulate adipogenesis in the context of treating bone diseases and metabolic disorders. The current review focuses on miRNAs and their role in regulating adipogenic differentiation of MSCs.
Introduction
Recent years have witnessed immense interest in studying mesenchymal stem cells (MSCs) and harnessing their unique differentiation capabilities for tissue engineering and regenerative medicine applications. While there are a myriad of molecular mechanisms that regulate stem cell differentiation, a new class of epigenetic regulators “microRNAs” have emerged as key player during stem cell differentiation including MSC. The role of microRNAs (miRNAs) in regulating MSC differentiation are currently being unraveled using integrated, experimental, and bioinformatics approaches. Our understanding of miRNAs and how they regulate MSC differentiation will have significant impact on their therapeutic potential. In this review, we will provide an overview of MSC differentiation into adipocytes and an up-to-date analysis of published data implicating miRNAs in regulating the adipogenic differentiation of MSCs.
Adipocytic Differentiation of MSCs
MSCs are described as adult progenitor multipotent stromal cells found and isolated from multiple tissues, including among others bone marrow [1], adipose tissue [2], umbilical cord [3], and skin [4]. MSCs have been shown to differentiate into several mesenchymal lineages including osteoblast, chondrocytes, and adipocytes to give rise to bone, cartilage, and adipose tissue, thus representing a possible use in cell therapy and regenerative medicine protocols [1,5].
The process of adipogenesis includes two major phases; the determination phase and the maturation phase. During the phase of determination, multipotent MSCs become incapable of differentiation into other mesenchymal lineages as they commit only to adipocytic lineage [6]. At this point, both adipocyte-committed MSCs (preadipocytes) and their precursors have a similar morphological phenotype. Later on, and in the maturation phase, these preadipocytes are transformed into mature adipocytes, which take part in synthesizing and the transportation of lipid, secretion of adipocyte-specific proteins and possessing the machinery that is required for insulin sensitivity [6]. The process of adipogenesis revealed a mark shift in the pattern of gene expression observed in undifferentiated MSC compared to mature adipocyte, which promotes and terminates the phenotypic and molecular characteristics that identify mature adipocytes [7]. A complex and well organized signaling cascades appear to be involved in regulating adipogenesis, which includes the expression of several transcription factors such as peroxisome proliferator-activated receptor-γ (PPARγ) and members of the CCAAT/enhancer-binding family of proteins (C/EBPs) (reviewed in Rosen et al. [7]). Bone marrow adipocytes appear to play significant role in bone metabolism [8], therefore, better understanding of stromal adipocyte commitment and maturation and identifying the molecular mechanisms that regulate their formation will assist in developing novel therapeutic modalities to regulate osteogenesis and hematopoiesis.
microRNAs and Regulation of MSC Differentiation
miRNAs are short single-stranded RNA sequences (usually 19–23 nucleotides), which are derived from ∼70 nucleotide precursors, and play a critical role in the post-transcriptional regulation of gene expression in a broad range of biological systems varying from insects to humans [9–12], through controlling a wide range of physiological and developmental processes [13]. Changes in microRNAs have been associated with many human diseases such as cancer [14–16], myocardial infarction and cardiovascular diseases [17,18], diabetes, and obesity [19–21]. miRNAs have been identified to act in functional networks linked to several genes as potential targets; so far, an almost 2,578 miRNAs have been identified in human cells, which apparently can affect multiple physiological and biological functions, such as stem cell differentiation, neurogenesis, hematopoiesis, immune response, and skeletal and cardiac muscle development [22–27]. While several reviews has covered the role of miRNAs in regulating osteoblastic differentiation of MSCs [28,29], the focus of this review is to highlight the regulation of adipogenic differentiation of MSCs by miRNAs.
microRNAs and Regulation of Adipogenic Differentiation of MSCs
A cascade of transcriptional events that occurs during adipocyte maturation, including the expression of PPARγ and CCAAT/enhancer-binding protein-α (C/EBPα), which are key factors regulating a myriad of adipocyte-related enzymes and proteins involved in generating and sustaining adipocyte phenotype [30–32]. Furthermore, there are other factors that can directly or indirectly interact with PPARγ, such as adipocyte determination and differentiation-dependent factor 1 (ADD1/SREBP-c1), a homolog of sterol regulatory element-binding proteins (SREBP), which was initially cloned as a basic helix-loop-helix (bHLH) protein involved in early adipogenesis, and another binding protein, a sterol response element (SRE) [33,34]. In addition, Krox20, Krüppel-like factors, and signal transducers and activators of transcription have all been shown to be tightly relevant to adipocyte differentiation [35–37]. All these transcription factors share a common characteristic as they regulate adipocyte differentiation by regulating the activity of PPARγ and C/EBP family. Adipocyte differentiation is regulated by the activity of various growth factors and hormones. Recent data suggested that miRNAs could be involved in human adipocyte maturation [38]. Tables 1 and 2 summarize the currently described microRNAs involved in adipocyte differentiation of MSCs derived from different sources.
Table 1.
Mammalian microRNAs Involved in Adipogenesis in Bone Marrow-Derived Mesenchymal Stem Cells
| miRNA | Cell | Target gene(s) | Related process | Reference |
|---|---|---|---|---|
| miR-204 | ST2 and C2C12 | RUNX2 | ↑ Adipogenesis | [63] |
| mBMSCs | ||||
| hMSCs | ||||
| miR-637 | Bone marrow-derived MSCs | Osterix | ↑ Adipogenesis | [67] |
| miR-320 | Bone marrow-derived MSCs | RUNX2 | ↑ Adipogenesis | [64] |
| miR-378/378* | Bone marrow-derived ST2 cell line | AGO2 | ↑ Adipogenesis | [88] |
| KLF15, FABP4, FAS, SCD-1, and resistin | ||||
| miR-8, miR-200c, -141, -200b, -200a, -429 | Mouse ST2 marrow-derived stromal cells (MSCs ST2) | wntless (wls) and CG32767 genes | ↑ Adipogenesis | [49] |
| miR-199, and miR-346 | hMSCs from bone marrow | LIF | ↑ Adipogenesis | [55] |
| miR-31 | Murine mesenchymal stem cell line C3H10T1/2 (MSCs) | CEBPA | ↓ Adipogenesis | [78] |
| miR-24 | Murine mesenchymal stem cell line C3H10T1/2 (MSCs) | Unknown | ↑ Adipogenesis | [78] |
| miR-335 | Bone marrow-derived hMSCs | RUNX2 | ↓ Adipogenesis | [66] |
hMSCs, human mesenchymal stem cells; mBMSCs, mouse bone marrow-derived MSCs; ↑, promote; ↓, inhibit.
Table 2.
Mammalian microRNAs Involved in Adipogenesis in Adipose-Derived Mesenchymal Stem Cells and Preadipocytes
| miRNA | Cell | Target gene(s) | Related process | Reference |
|---|---|---|---|---|
| miR-17-92 | 3T3-L | Rb/p130 | ↑ Adipogenesis | [43] |
| miR-642a-3p | hAD-MSC | Unknown | ↑ Adipogenesis | [65] |
| miR-30a and 30d | hAD-MSC | RUNX2 | ↑ Adipogenesis | [65] |
| miR-21 | hAD-MSC | TGF-B1 | ↑ Adipogenesis | [44] |
| 3T3-l1 | AP1 | ↑ Adipogenesis | [46] | |
| miR-30c | hAD-MSC and MEFs | PAI-1 and ALK2 | ↑ Adipogenesis | [87] |
| miR-210 | 3T3-L1 | Tcf7l2 | ↑ Adipogenesis | [48] |
| miR-143 | Human pre-ad | ERK5 | ↑ Adipogenesis | [39] |
| MAPK7 | ||||
| miR-375 | 3T3-L | ERK1/2 | ↑ Adipogenesis | [42] |
| miR-27b | hAD-MSC | PPARγ and C/EBPα | ↓ Adipogenesis | [75] |
| miR-27a | 3T3-L | PPARγ | ↓ Adipogenesis | [76] |
| miR-130 | Human and mouse pre-ad | PPARγ | ↓ Adipogenesis | [77] |
| miR-138 | hAD-MSCs | EID-1 | ↓ Adipogenesis | [79] |
| miR-448 | 3T3-L1 | KLF5 | ↓ Adipogenesis | [57] |
| miR-103 and miR-107 | Primary adipocytes and the stromalvascular fraction from subcutaneous and visceral fat | CAV1 | ↓ Adipogenesis | [85] |
| miR-146b | 3T3-L1 | SIRT1 | ↑ Adipogenesis | [72] |
| miR-155 | hMSC-TERT20 | CEBPB | ↓ Adipogenesis | [86] |
| miR-221/222 | CDKN1B | |||
| miR-26 | hAD-MSC | ADAM17 | ↑ Adipogenesis | [90] |
hAD-MSCs, human adipose tissue-derived mesenchymal stem cells; MEFs, mouse embryonic fibroblasts; Pre-ad, preadipocytes; TERT, telomerase reverse transcriptase.
microRNAs targeting cell cycle and self-renewal-related genes
miRNAs are able to indirectly regulate adipogenic differentiation of MSCs by targeting various genes that may be involved in balancing self-renewal and stem cell differentiation, as shown with miR-143, which was reported as the first miRNA to regulate adipogenesis [39]. Elevated levels of miR-143 were detected in differentiated white adipocytes and its inhibition resulted in reduced adipocytic differentiation. miR-143 was found to target extracellular signal-regulated kinase 5 (ERK5), also known as mitogen-activated protein kinase 7 (MAPK7) gene, which is involved in promoting cell growth and proliferation in response to tyrosine kinase signaling, where its activation resulted in boosting adipocyte differentiation [40,41]. While the role of ERK5 in regulating adipocyte differentiation directly has not been confirmed, the authors suggested that it may play a role in balancing the proliferation and differentiation of adipocytes. Another member of ERK family was also studied as a target for miR-375 in 3T3-L1 cells [42]. The authors demonstrated that ERK1/2 pathway negatively regulate adipocyte differentiation, and suggested this reduction in adipogenesis is mediated through miR-375. Wang et al. found that all members of miR-17-92 cluster were significantly upregulated after hormonal induction of adipogenesis and based on those data, they concluded that overexpression of miR-17-92 cluster, with hormonal induction, may play a role in the positive regulation of adipocyte differentiation through targeting the tumor suppressor Rb/p130 gene, resulting in acceleration of adipogenic differentiation [43].
Kim et al. investigated the role of miR-21 in adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells (hAD-MSC) and its potential targets [44]. Their data showed that miR-21 can positively regulate adipogenic differentiation of hAD-MSC by targeting transforming growth factor-beta 1 (TGF-β1), which is known to inhibit adipogenesis in vitro and in vivo [45]. In another study, Kang et al. showed that 3T3-L1 cells that were transfected with miR-21 showed higher level of adipogenic differentiation [46], these results were indicated by the morphological changes in miR-21-transfected adipocytes that expressed higher levels of adiponectin. However, their data showed that miR-21 may regulate adipogenic commitment of preadipocytes by directly targeting AP1 gene, activating protein-1, resulting in inducing adipocyte differentiation.
Wingless-type MMTV integration family (Wnts) has been shown to suppress adipocyte differentiation by blocking the expression of PPARγ and CEBPA, which are essential transcription factors in adipogenesis [47]. These data were supported by different studies that were conducted to explore the role of different miRNAs in regulating adipogenesis via modulation of the Wnt pathway [48,49]. Kennell et al. first studied miRNA-8 in drosophila Kc167 cells and revealed that Wnt signaling pathway is regulated by miRNA-8 at different levels via inhibition of transcription factor (TCF) protein expression and direct targeting of wntless (wls) and CG32767 genes, which positively regulate Wnt signaling pathway, based on these data, they concluded that mammalian homologues of miRNA-8, miR-200c/141, and miR-200b/200a/429 clusters have a potential role in regulating adipocyte differentiation in ST2 marrow stromal cells [49]. Stable expression of miR-200c/141 and/or miR-200b/200a/429 clusters induced the differentiation of those cells into adipocytes, which was indicated by the elevated levels of fatty acid-binding protein 4 (FABP4), and an increase in lipid accumulation. In a similar manner, Qin et al. performed miRNA expression profiling during adipocyte differentiation and identified 18 miRNAs, including miR-210, miR-148a, miR-194, and miR-322, which could promote adipocyte differentiation via inhibition of Wnt signaling [48]. Overexpression of miR-210 in 3T3-L1 cells resulted in enlarged cells, with distinctive lipid droplets, while its inhibition led to diminished adipogenesis. The authors identified Tcf7l2, T-cell-specific transcription factor 7 like 2, a member of LEF/TCF family, which plays a role in triggering the downstream responsive genes of Wnt signaling [50], as bona fide target for miR-210.
Leukemia inhibitory factor (LIF) is an inflammatory cytokine that plays a significant role in regulating multiple biological activities such as cell survival, proliferation, and cell differentiation [51]. It was shown that LIF expression declines, in association with decreased differentiation plasticity of hMSCs [52–54]. Oskowitz et al. identified two miRNAs, miR-199 and miR-346, which can synergistically function by targeting LIF during hMSC differentiation, resulting in enhanced adipocyte and osteoblast differentiation [55].
Kruppel-like factor 5 (KLF5), a transcription factor, which function as signaling modulator for various cellular processes including cell proliferation, cell cycle, migration, apoptosis, and cell differentiation [56], was recently investigated as potential target for miR-448 [57]. The authors demonstrated that serotonin (5-HT) is a novel autocrine/paracrine regulator of adipocytic cell differentiation [57]. Interestingly, the authors found miR-448, which is located in the fourth intron of 5-HT(2C)R, to suppress adipocyte differentiation by targeting KLF5 in the 3T3-L1 model.
microRNAs targeting osteoblast-related genes
Runt-related transcription factor 2 (RUNX2) is a master transcription factor known to regulate osteoblast and chondrocyte differentiation [58,59]. In undifferentiated cells, RUNX2 and PPARγ are expressed at low level to sustain differentiation potential of MSC [6,60]. It has been shown that reduction in the level of RUNX2 in chondrocytes enhanced their adipogenic commitment [61], while deficiency in PPARγ stimulates osteogenesis and enhanced bone formation [62]. Thus, Huang et al. intended to study whether RUNX2 regulation by miR-204/211 has an effect on adipogenic differentiation [63]. Many adipogenic genes such as AP2, adipsin, and PPARγ were found to be upregulated when miR-204 was overexpressed in ST2 cells, whereas upregulation of RUNX2 was observed in miR-204-sponge-transfected cells. As a result, it has been revealed that miR-204 can positively regulate adipogenic commitment, probably through downregulation of RUNX2. In an independent study, Hamam et al. performed global microRNA and mRNA expression profiling during adipocytic differentiation of human bone marrow-derived MSCs (hBM-MSCs). While several differentially expressed miRNAs were identified in that study, the authors reported proadipogenic function for miR-320 family in hBM-MSCs via targeting multiple genes involved in cell differentiation and cell cycle regulation. The author subsequently validated RUNX2 as bona fide target for miR-320 family using the luciferase reporter system [64]. Zaragosi et al. reported miR-642-3p as a highly adipo-specific miRNA, and additionally studied the involvement of miR-30 family in the regulation of adipogenesis in hAD-MSC [65]. Their data showed that during adipogenesis, miR-642a-3p, miR-378, miR-30a, miR-30b, miR-30c, miR-30d, miR-30e, and miR-193b were strongly upregulated. In addition, a direct link between miR-30a and miR-30d and adipogenesis by targeting the activity of the transcription factor RUNX2, resulted in enhancing adipogenesis. RUNX2 has also been studied as a target gene for miRNAs involved in the regulation of adipogenesis in hMSCs derived from bone marrow, subcutaneous adipose tissue, and from articular cartilage [66]. Tome et al. reported that all MSC populations were found to express higher levels of miR-335 compared with dermal fibroblasts, and in addition, BM-MSCs showed the highest level of miR-335 expression among all examined MSC populations. Hence, miR-335 was reported to negatively regulate both adipogenic and osteogenic differentiation of hMSCs, as indicated by the reduction in PPARγ and osteopontin, respectively. The authors' data revealed RUNX2 as one target for miR-355, through direct binding to its 3′ untranslated region (UTR) and reduced level of RUNX2 protein after miR-355 overexpression.
Balancing between adipogenic and osteogenic differentiation in MSC, Zhang et al., demonstrated a role for miR-637 in maintaining the balance between these two lineages by targeting Osterix (Osx) mRNA [67]. Osx, a zinc finger-containing transcription factor, which plays significant role in bone and osteoblast formation and its transcription could be induced by bone morphogenetic protein 2 (BMP2) in hMSCs [68–71]. However, their data showed that Osterix is a direct target for miR-637 and its inhibition can positively regulate adipocyte differentiation, which was indicated by the elevated levels of PPARγ, C/EBPα, and SREBP-1c in de novo adipose tissue. Furthermore, the authors went to explore the effect of miR-637 on adipogenic differentiation in vivo using a de novo adipogenic mouse model. The authors noticed remarkable enhancement in adipose tissue formation after injection of Lv-miR-637-transduced hMSCs. Interestingly, the osteogenic differentiation potential of Lv-miR-637-transduced cells was apparently diminished, which was associated with lower alkaline phosphatase activity.
microRNAs targeting adipocyte-related genes
Recently, miR-146b was studied for its role in regulating adipocytic differentiation [72], miR-146b was also revealed to be a positive regulator of adipogenesis in 3T3-L cells, and this effect was first indicated by the positive correlation between the expression level of this miRNA and adipose tissue volume in obese mice. miR-146b was reported to positively regulate adipogenic differentiation by targeting Sirtuin 1 (SIRT1) gene, which has been found to inhibit PPARγ and stimulates lipolysis, resulting in delaying adipogenic commitment of 3T3-L1 cells and reducing fat production [73]. Additionally, miR-27 family was shown to play a role in adipogenic regulation by directly targeting adipogenic regulatory genes [74]. PPARγ and C/EBPα were reported to be key regulators of adipogenic differentiation and targeted by miR-27b, which suppresses their expression and negatively affect the adipogenic commitment in hAD-MSC cells, delaying the accumulation of triglyceride to late stages in these cells [75]. Moreover, and in a similar manner, Kim et al. demonstrated that miR-27a, another member of the miR-27 family, negatively regulated adipogenesis [76]. Their data showed that miR-27a was downregulated in mature adipocytes in the fraction of high-fat diet-fed obese mice. Their data showed that during adipocyte differentiation, overexpression of miR-27a in 3T3-L1 cells resulted in reduction of lipid accumulation, downregulation in PPARγ levels and reduced protein level of adiponectin. Moreover, they revealed that miR-27a overexpression represses many adipogenic marker genes such as adipocyte lipid-binding protein 2 (AP2), which is also known as FABP4, adiponectin, CD36, and lipoprotein lipase (LPL). Collectively, these results implied that miR-27a can target PPARγ directly, resulting in repression of adipogenesis suggesting that downregulation of miR-27a might be relevant to adipose tissue dysregulation in obesity.
In another study, PPARγ was also found to be targeted by miR-130 in both human and mouse preadipocytes, and was revealed to bind to both PPARγ mRNA coding and its 3′ UTR [77]. In the same study, the authors reported lower levels of miR-130 in obese compared to lean women, which was linked to increased levels of PPARγ among relatively young and healthy populations. These lower expression levels of miR-130 in obese individuals could possibly reflect a reduced numbers of preadipocytes due to their conversion into mature adipocytes in the adipocyte pool. C/EBPα was found to be targeted by miR-31 in MSCs at both the transcriptional and translational levels, resulting in negative regulation of adipocyte differentiation [78]. The authors also revealed proadipogenic role for miR-24, via enhanced BMP2-induced commitment to adipocytes. On the other hand, a study conducted by Yang et al. on hAD-MSCs revealed that miR-138 overexpression in these cells suppress the expression of PPARγ and C/EBPα, and other adipocyte differentiation markers, such as FABP4 and lipoprotein 4, coupled with reduction in the accumulation of lipid droplets [79]. Furthermore, the adenovirus early region 1-A-like inhibitor of differentiation (EID-1) was predicted to be targeted by miR-138, which was previously reported to be involved in adipogenesis by promoting small heterodimer partner, an endogenous enhancer of PPARγ, and TGF-β signaling pathways. The authors succeeded to find a link between miR-138 and EID-1 gene and reveal that miR-138 can negatively regulate adipogenesis by the inhibition of EID-1 gene [80–82].
Concordant with previous studies implicating miR-103 and miR-107 in adipogenesis [83], Li et al. linked miR-103 to brain development, adipogenesis, lipid metabolism, immunity, and hematopoiesis [84]. Trajkovski et al. have also examined the effect of miR-103 and miR-107 during adipocyte differentiation of isolated stromal-vascular cells from visceral and subcutaneous fat [85]. Their data revealed increased number of adipocyte when miR-103 was inactivated, while overexpression of miR-107 resulted in reduced adipogenesis, which implicated miR-103 and miR-107 in the negative regulation of adipocyte differentiation, via modulation of caveolin-1. Skarn et al. identified 12 miRNAs that were differentially expressed during adipocytic differentiation of hBM-MSC line (hMSC-TERT) and functionally validated miR-155, miR-221, and miR-222 as negative regulators of adipocytic differentiation of hMSCs via targeting CEBPB and CDKN1B [86].
microRNAs targeting other pathways
Karbiener et al. performed microarray on hAD-MSC and on mouse embryonic fibroblasts and found miR-30c to be upregulated during adipocytic differentiation [87]. Two genes, plasminogen activator inhibitor 1 (PAI-1) and activin receptor-like kinase 2 (ALK2), were identified as bona fide targets for miR-30c. Although these two targeted genes are so far not interconnected, the authors found that co-silencing, not single silencing of PAI-1 or ALK2 has a significant impact on adipogenesis as measured by elevated levels of triglycerides and lipid accumulation and the increased expression of adipogenic marker genes [PPARγ, C/EBPα, FABP4, fatty acid synthase (FASN), and glucose transporter type 4 (GLUT4)].
Gerin et al. investigated the role of miR-378/378* in adipocyte differentiation and metabolism, via knocking down Argonaute2 (Ago2), which plays a key role in miRNA processing, to study the potential role of different miRNAs in adipocyte differentiation and/or metabolism [88,89]. Although the authors observed no remarkable differences in adipogenesis between control and Ago2 knockdown samples of 3T3-L1 cells, incorporation of [14C] glucose or acetate into triacylglycerol showed reduced levels, which suggests that miRNAs may play a role in adipocyte metabolism. Moreover, they focused their studies to investigate the role of certain microRNAs in adipogenesis via screening differentially expressed microRNAs between preadipocytes and adipocytes in the 3T3-L1 and bone marrow-derived stroma (ST2) cell lines. miR-378/378* were highly induced during adipogenesis, and when they were overexpressed in ST2 MSC precursors, an increased level of lipid droplets, upregulation in several adipogenic markers [KLF15, FABP4, FASN, stearoyl-Coenzyme A desaturase 1 (SCD-1), and resistin], and increase in the incorporation of [14C] acetate into triacylglycerol was observed. In contrast, knocking down miR-378 and/or miR-378* caused reduction in the accumulation of triacylglycerol. Interestingly, and in a traditional search for potential targets for specific miRNAs, Gerin et al. found that none of the predicted targets of miRNA-378 or 378* were downregulated in response to these miRNAs. Unexpectedly, some of the suggested target genes exhibited an increase in reporter gene expression. In particular, C/EBPα and C/EBPβ activity on the GLUT4 promoter was increased in the presence of miRNA-378/378*, suggesting miRNAs might exert their effects in adipocytes through an atypical mechanism, such as transcriptional coactivation.
Recently, Karbiener et al. reported miR-26a family as a positive regulator of adipogenic differentiation in hAD-MSC [90]. Their data showed a significant increased in adipocytes formation assessed by oil red staining and high expression of adipogenic markers upon overexpression of miR-26a and miR-26b. Transcriptomatic analysis revealed enriched TGF-β and Notch signaling pathways among miR-26a-suppressed mRNA. Furthermore, miR-26a showed significant effect on the expression level of various genes involved in different cellular pathways, such as pyruvate metabolism, tricarboxylic acid (TCA) cycle, and fatty acid metabolism suggesting increased de novo synthesis of lipids combined with an increase level of triglyceride accumulation upon miR-26 at the early white stage. However, In their functional studies, knocking down of several mRNAs (SMURF2, ATPAF1, ADAM17, and PLOD2) showed a positive effect on adipogenic differentiation as indicated by lipid accumulation, however, induction of UCP1 was only observed upon knocking down of ADAM metallopeptidase domain 17 (ADAM17), which also was shown to be the most downregulated transcriptom by miR-26a. Karbiener et al. revealed the direct miR-26-ADAM17 interaction indicating that ADAM17 is a direct target for miR-26 family with an antiadipogenic and antibrowning effect [90].
Conclusion Remarks
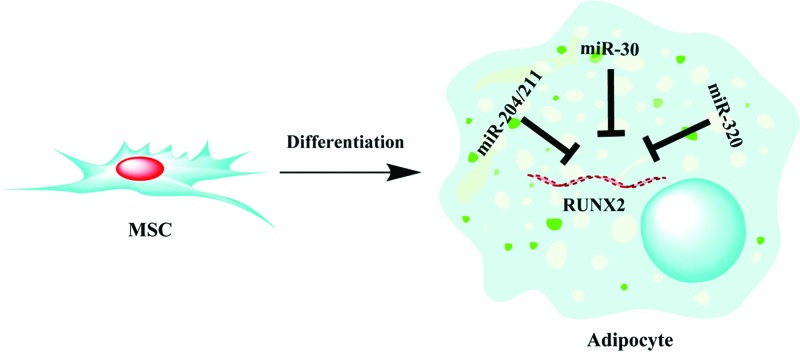
The role of miRNA-mediated post-transcriptional regulation in adipogenesis has been studied particularly to identify the schematic mechanism of microRNA and gene regulation of adipogenic differentiation of stem cells. Thus far, several microRNA families has been identified using high-throughput screen, computational and experimental approaches and were found to regulate adipogenesis by targeting key pathways involved in stem cell differentiation and proliferation. Of particular interest, we and others have identified RUNX2 to be a favorite hub for microRNA-mediated gene silencing during adipogenesis. RUNX2 has been shown to be targeted by miR-204/211 and miR-30 families, and most recently we found RUNX2 to be highly targeted by miR-320 family during adipocytic differentiation of hMSCs (Fig. 1) [64]. The findings from miRNA investigations during adipogenesis suggest the potential utilization of miRNA mimics/inhibitors or sponge to treat bone diseases, metabolic disorders, and obesity. As proof of principle, several preclinical data have shown the feasibility of utilizing miRNA-based therapies in vivo in various disease models [17,91–93]. The only miRNA-based therapy in clinical trials thus far is miravirsen, which is a miR-122 inhibitor previously shown to reduce HCV viral load in a primate HCV disease model [93]. Santaris Pharma A/S has completed phase I and phase II clinical trials using this agent in healthy subject and patients with chronic hepatitis C infection (http://clinicaltrials.gov). Therefore, the translation of in vitro and preclinical findings in this field into clinical trials is just beginning to unfold. Determining biodistribution, specificity, pharmacokinetics, and safety have to be addressed before the successful utilization of miRNA-directed therapies in the clinic.
FIG. 1.
Regulation of runt-related transcription factor 2 (RUNX2) by miR-204/211, miR-30, and miR-320 family. During adipocytic differentiation of mesenchymal stem cells (MSCs), members of the miR-204/211, miR-30, and miR-320 family are upregulated, which subsequently repress RUNX2 and promote adipogenesis. Color images available online at www.liebertpub.com/scd
Acknowledgment
This work was supported by the National Science Technology and Innovation Plan (NSTIP) strategic technologies program, grant number (11-BIO-1941-02) in the Kingdom of Saudi Arabia.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P. and Hedrick MH. (2002). Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erices A, Conget P. and Minguell JJ. (2000). Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109:235–242 [DOI] [PubMed] [Google Scholar]
- 4.Vishnubalaji R, Manikandan M, Al-Nbaheen M, Kadalmani B, Aldahmash A. and Alajez NM. (2012). In vitro differentiation of human skin-derived multipotent stromal cells into putative endothelial-like cells. BMC Dev Biol 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G. and Rogister B. (2005). Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 23:392–402 [DOI] [PubMed] [Google Scholar]
- 6.Rosen ED. and MacDougald OA. (2006). Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896 [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Walkey CJ, Puigserver P. and Spiegelman BM. (2000). Transcriptional regulation of adipogenesis. Genes Dev 14:1293–1307 [PubMed] [Google Scholar]
- 8.Gimble JM, Robinson CE, Wu X. and Kelly KA. (1996). The function of adipocytes in the bone marrow stroma: an update. Bone 19:421–428 [DOI] [PubMed] [Google Scholar]
- 9.Baek D, Villen J, Shin C, Camargo FD, Gygi SP. and Bartel DP. (2008). The impact of microRNAs on protein output. Nature 455:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS. and Johnson JM. (2005). Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433:769–773 [DOI] [PubMed] [Google Scholar]
- 11.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R. and Rajewsky N. (2008). Widespread changes in protein synthesis induced by microRNAs. Nature 455:58–63 [DOI] [PubMed] [Google Scholar]
- 12.Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, Yue S, Huang SH, Xu W, et al. (2011). MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res 71:2381–2391 [DOI] [PubMed] [Google Scholar]
- 13.Bushati N. and Cohen SM. (2007). microRNA functions. Annu Rev Cell Dev Biol 23:175–205 [DOI] [PubMed] [Google Scholar]
- 14.Calin GA. and Croce CM. (2006). MicroRNA signatures in human cancers. Nat Rev Cancer 6:857–866 [DOI] [PubMed] [Google Scholar]
- 15.Alajez NM, Shi W, Hui AB, Bruce J, Lenarduzzi M, Ito E, Yue S, O'Sullivan B. and Liu FF. (2010). Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98. Cell Death Dis 1:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alajez NM. (2011). Cancer stem cells. From characterization to therapeutic implications. Saudi Med J 32:1229–1234 [PubMed] [Google Scholar]
- 17.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S. and Giacca M. (2012). Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492:376–381 [DOI] [PubMed] [Google Scholar]
- 18.Quiat D. and Olson EN. (2013). MicroRNAs in cardiovascular disease: from pathogenesis to prevention and treatment. J Clin Invest 123:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Lin EJ, Cahill MC, Wang C, Liu X. and During MJ. (2009). Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med 15:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilton C, Neville MJ. and Karpe F. (2012). MicroRNAs in adipose tissue: their role in adipogenesis and obesity. Int J Obes (Lond) 37:325–332 [DOI] [PubMed] [Google Scholar]
- 21.Dehwah MA, Xu A. and Huang Q. (2012). MicroRNAs and type 2 diabetes/obesity. J Genet Genomics 39:11–18 [DOI] [PubMed] [Google Scholar]
- 22.Krichevsky AM, Sonntag KC, Isacson O. and Kosik KS. (2006). Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL. and Wang DZ. (2006). The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38:228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Samal E. and Srivastava D. (2005). Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436:214–220 [DOI] [PubMed] [Google Scholar]
- 25.Pedersen I. and David M. (2008). MicroRNAs in the immune response. Cytokine 43:391–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD. and Plasterk RH. (2007). Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, Liu R, George J, Ng HH, et al. (2008). MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells 26:17–29 [DOI] [PubMed] [Google Scholar]
- 28.Taipaleenmaki H, Bjerre Hokland L, Chen L, Kauppinen S. and Kassem M. (2012). Mechanisms in endocrinology: micro-RNAs: targets for enhancing osteoblast differentiation and bone formation. Eur J Endocrinol 166:359–371 [DOI] [PubMed] [Google Scholar]
- 29.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T. and Zhang Y. (2012). MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol 8:212–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregoire FM, Smas CM. and Sul HS. (1998). Understanding adipocyte differentiation. Physiol Rev 78:783–809 [DOI] [PubMed] [Google Scholar]
- 31.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A. and Evans RM. (1999). PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–595 [DOI] [PubMed] [Google Scholar]
- 32.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM. and Mortensen RM. (1999). PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617 [DOI] [PubMed] [Google Scholar]
- 33.Tontonoz P, Kim JB, Graves RA. and Spiegelman BM. (1993). ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol 13:4753–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL. and Brown MS. (1993). SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 75:187–197 [PubMed] [Google Scholar]
- 35.Chen Z, Torrens JI, Anand A, Spiegelman BM. and Friedman JM. (2005). Krox20 stimulates adipogenesis via C/EBPbeta-dependent and -independent mechanisms. Cell Metab 1:93–106 [DOI] [PubMed] [Google Scholar]
- 36.Birsoy K, Chen Z. and Friedman J. (2008). Transcriptional regulation of adipogenesis by KLF4. Cell Metab 7:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanbu-Wakao R, Morikawa Y, Matsumura I, Masuho Y, Muramatsu MA, Senba E. and Wakao H. (2002). Stimulation of 3T3-L1 adipogenesis by signal transducer and activator of transcription 5. Mol Endocrinol 16:1565–1576 [DOI] [PubMed] [Google Scholar]
- 38.Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, Rodriguez-Hermosa JI, Ruiz B, Ricart W, Peral B. and Fernandez-Real JM. (2010). MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One 5:e9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, et al. (2004). MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 279:52361–52365 [DOI] [PubMed] [Google Scholar]
- 40.Kim SW, Muise AM, Lyons PJ. and Ro HS. (2001). Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. J Biol Chem 276:10199–10206 [DOI] [PubMed] [Google Scholar]
- 41.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ. and Lee JD. (1998). Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395:713–716 [DOI] [PubMed] [Google Scholar]
- 42.Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS, Yao CH, Zhu BY, Gao ZP, Zhang L. and Liao DF. (2011). MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin Exp Pharmacol Physiol 38:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ. and Li X. (2008). miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A 105:2889–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YJ, Hwang SJ, Bae YC. and Jung JS. (2009). MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27:3093–3102 [DOI] [PubMed] [Google Scholar]
- 45.Ignotz RA. and Massague J. (1985). Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A 82:8530–8534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang M, Yan LM, Zhang WY, Li YM, Tang AZ. and Ou HS. (2013). Role of microRNA-21 in regulating 3T3-L1 adipocyte differentiation and adiponectin expression. Mol Biol Rep 40:5027–5034 [DOI] [PubMed] [Google Scholar]
- 47.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL. and MacDougald OA. (2000). Inhibition of adipogenesis by Wnt signaling. Science 289:950–953 [DOI] [PubMed] [Google Scholar]
- 48.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, Li A, Xie Y, Li J, et al. (2010). A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics 11:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennell JA, Gerin I, MacDougald OA. and Cadigan KM. (2008). The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci U S A 105:15417–15422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koslowski MJ, Kubler I, Chamaillard M, Schaeffeler E, Reinisch W, Wang G, Beisner J, Teml A, Peyrin-Biroulet L, et al. (2009). Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn's disease. PLoS One 4:e4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G. and Schaper F. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, Yang YS, Im HJ, Kim KH, et al. (2005). Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine 31:119–126 [DOI] [PubMed] [Google Scholar]
- 53.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP. and Gerson SL. (2000). Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 9:841–848 [DOI] [PubMed] [Google Scholar]
- 54.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM. and Lee T. (2005). Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol 205:194–201 [DOI] [PubMed] [Google Scholar]
- 55.Oskowitz AZ, Lu J, Penfornis P, Ylostalo J, McBride J, Flemington EK, Prockop DJ. and Pochampally R. (2008). Human multipotent stromal cells from bone marrow and microRNA: regulation of differentiation and leukemia inhibitory factor expression. Proc Natl Acad Sci U S A 105:18372–18377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong JT. and Chen C. (2009). Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci 66:2691–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y, Takanabe-Mori R, Hasegawa K, Kita T. and Kimura T. (2010). Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol 24:1978–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, et al. (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- 59.Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y. and Komori T. (2004). Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18:952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD. and MacDougald OA. (2005). Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A 102:3324–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enomoto H, Furuichi T, Zanma A, Yamana K, Yoshida C, Sumitani S, Yamamoto H, Enomoto-Iwamoto M, Iwamoto M. and Komori T. (2004). Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci 117:417–425 [DOI] [PubMed] [Google Scholar]
- 62.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, et al. (2004). PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang J, Zhao L, Xing L. and Chen D. (2010). MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 28:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamam D, Ali D, Vishnubalaji R, Al-Nbaheen M, Aldahmash A, Kassem M. and Alajez NM. (2014). microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (Skeletal) stem cells. Cell Death Dis 5:e1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaragosi LE, Wdziekonski B, Brigand KL, Villageois P, Mari B, Waldmann R, Dani C. and Barbry P. (2011). Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol 12:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tome M, Lopez-Romero P, Albo C, Sepulveda JC, Fernandez-Gutierrez B, Dopazo A, Bernad A. and Gonzalez MA. (2011). miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ 18:985–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JF, Fu WM, He ML, Wang H, Wang WM, Yu SC, Bian XW, Zhou J, Lin MC, et al. (2011). MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell 22:3955–3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR. and de Crombrugghe B. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29 [DOI] [PubMed] [Google Scholar]
- 69.Celil AB, Hollinger JO. and Campbell PG. (2005). Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem 95:518–528 [DOI] [PubMed] [Google Scholar]
- 70.Lee MH, Kwon TG, Park HS, Wozney JM. and Ryoo HM. (2003). BMP-2-induced Osterix expression is mediated by Dlx5 but is independent of Runx2. Biochem Biophys Res Commun 309:689–694 [DOI] [PubMed] [Google Scholar]
- 71.Ryoo HM, Lee MH. and Kim YJ. (2006). Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene 366:51–57 [DOI] [PubMed] [Google Scholar]
- 72.Ahn J, Lee H, Jung CH, Jeon TI. and Ha TY. (2013). MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol Med 5:1602–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW. and Guarente L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Q, Gao Z, Alarcon RM, Ye J. and Yun Z. (2009). A role of miR-27 in the regulation of adipogenesis. FEBS J 276:2348–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ. and Scheideler M. (2009). microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun 390:247–251 [DOI] [PubMed] [Google Scholar]
- 76.Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS. and Kim JB. (2010). miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun 392:323–328 [DOI] [PubMed] [Google Scholar]
- 77.Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, et al. (2011). miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 31:626–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun F, Wang J, Pan Q, Yu Y, Zhang Y, Wan Y, Li X. and Hong A. (2009). Characterization of function and regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun 380:660–665 [DOI] [PubMed] [Google Scholar]
- 79.Yang Z, Bian C, Zhou H, Huang S, Wang S, Liao L. and Zhao RC. (2011). MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev 20:259–267 [DOI] [PubMed] [Google Scholar]
- 80.Bavner A, Johansson L, Toresson G, Gustafsson JA. and Treuter E. (2002). A transcriptional inhibitor targeted by the atypical orphan nuclear receptor SHP. EMBO Rep 3:478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Macchiarulo A, Rizzo G, Costantino G, Fiorucci S. and Pellicciari R. (2006). Unveiling hidden features of orphan nuclear receptors: the case of the small heterodimer partner (SHP). J Mol Graph Model 24:362–372 [DOI] [PubMed] [Google Scholar]
- 82.Nishizawa H, Yamagata K, Shimomura I, Takahashi M, Kuriyama H, Kishida K, Hotta K, Nagaretani H, Maeda N, et al. (2002). Small heterodimer partner, an orphan nuclear receptor, augments peroxisome proliferator-activated receptor gamma transactivation. J Biol Chem 277:1586–1592 [DOI] [PubMed] [Google Scholar]
- 83.Bengestrate L, Virtue S, Campbell M, Vidal-Puig A, Hadaschik D, Hahn P. and Bielke W. (2011). Genome-wide profiling of microRNAs in adipose mesenchymal stem cell differentiation and mouse models of obesity. PLoS One 6:e21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li G, Wu Z, Li X, Ning X, Li Y. and Yang G. (2011). Biological role of microRNA-103 based on expression profile and target genes analysis in pigs. Mol Biol Rep 38:4777–4786 [DOI] [PubMed] [Google Scholar]
- 85.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH. and Stoffel M. (2011). MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474:649–653 [DOI] [PubMed] [Google Scholar]
- 86.Skarn M, Namlos HM, Noordhuis P, Wang MY, Meza-Zepeda LA. and Myklebost O. (2012). Adipocyte differentiation of human bone marrow-derived stromal cells is modulated by microRNA-155, microRNA-221, and microRNA-222. Stem Cells Dev 21:873–883 [DOI] [PubMed] [Google Scholar]
- 87.Karbiener M, Neuhold C, Opriessnig P, Prokesch A, Bogner-Strauss JG. and Scheideler M. (2011). MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol 8:850–860 [DOI] [PubMed] [Google Scholar]
- 88.Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V. and MacDougald OA. (2010). Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab 299:E198–E206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diederichs S. and Haber DA. (2007). Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell 131:1097–1108 [DOI] [PubMed] [Google Scholar]
- 90.Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, Mossenbock K, Bernhardt GA, Mayr T, et al. (2014). MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells 32:1578–1590 [DOI] [PubMed] [Google Scholar]
- 91.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, et al. (2011). Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet 43:371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM. and Slack FJ. (2012). Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A 109:E1695–E704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S. and Orum H. (2010). Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]