Abstract
Improvement in angiogenesis using mesenchymal stem cells (MSCs) is evolving as an option in patients with vascular insufficiencies. The paracrine factors secreted by MSCs have been attributed to the angiogenic response. This study was conducted to identify the factors secreted by umbilical cord-derived MSCs (UCMSCs) that might play a role in angiogenesis. To this aim, we evaluated the presence of well known proangiogenic factors in the conditioned media (CM) derived from UCMSCs by ELISA. While vascular endothelial growth factor (VEGF), a well known angiogenic factor, was not detected in the CM, gene expression was nevertheless detected in these cells. Further investigations revealed the presence of soluble VEGF receptors (sVEGF-R1 and R2) that were capable of neutralizing exogenous VEGF. Human umbilical cord vein-derived endothelial cells exposed in vitro to CM, in comparison to control media, showed improved migration (P<0.007) and capillary-like network formation (P<0.001) with no significant change in endothelial cell proliferation. The angiogenic response observed with the paracrine factors secreted by UCMSC could be due to the presence of significant levels of a metalloprotease and matrix metalloproteases-2 (237.4±47.1 ng/106 cells). Data suggest that a VEGF-independent pathway is involved in the angiogenic response observed with endothelial cells in the presence of UCMSC-CM.
Introduction
Angiogenesis, the sprouting of new vasculature from a pre-existing network is a highly complex process involving a range of cell types and signaling pathways. Activation and proliferation of endothelial cells and smooth muscle cells are required to form neovasculature or remodel existing collaterals in multiple conditions, including local ischemia. Activation of endothelial cells by factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) has resulted in new blood vessel formation [1]. The potential of these growth factors to induce neovascularization is well established in both in vitro as well as in vivo studies [2–4]. Development of collateral vessels to supplement perfusion is critical for the survival of ischemic tissues such as in the case of critical limb ischemia and cardiac ischemia. Direct administration of the single angiogenic factor to improve neovascularization to treat diseases such as coronary artery disease and peripheral vascular disease [5–7] has shown only modest success, suggesting that the complex angiogenesis process may likely require multiple growth factors acting in concert.
It is well established that factors released upon tissue damage mobilize and recruit stem and progenitor cells to the damaged site, wherein they help in replacing the damaged tissues [8–10]. Ischemic tissue induces in its vicinity an increase in chemokines such as VEGF, thus promoting the migration of VEGF-R1 and VEGF-R2 expressing progenitor cells to the ischemic territory [11]. Several cell types such as endothelial progenitor cells and mesenchymal stem cells (MSCs) are known to respond to the ischemic stimuli and migrate to the affected area and secrete factors that stimulate angiogenesis.
Stem cell-based therapies have been attempted to improve neovascularization in patients suffering from ischemic diseases. The therapeutic potential of MSCs for the treatment of ischemic conditions such as peripheral vascular disease, stroke, and coronary artery disease has been tested in preclinical and clinical trials [12,13]. MSCs are defined as multipotent stem cells that are capable of self-renewal and can give rise to a number of cell types [14]. MSCs have been identified from a variety of tissues, including bone marrow, muscle, connective tissue, skin, adipose tissue, perichondrium, trabecular bone, placenta, and umbilical cord (UC) blood [15,16].
Although the exact mechanism of action of MSCs on endothelial cells is not clear, it has been suggested that the effect seen with MSCs can be attributed, at least in part, to its paracrine, immunomodulatory, anti-inflammatory properties [17,18], or its direct differentiation to endothelial cells [19,20]. While MSCs contributed to the recovery of tissues in models of myocardial infarction [21] and limb ischemia [22], the percentage of engrafted MSCs was low in comparison with the recipient tissue cells [23], suggesting that their efficacy might rely upon actions other than direct differentiation. Boomsma and Geenen have demonstrated that paracrine factors secreted by MSCs were responsible for inducing angiogenesis and cellular migration, suggesting that the soluble factors were responsible for recovery of tissue following ischemic heart injury [24,25]. Recent evidences showing secretion of multiple angiogenic factors like VEGF, bFGF, TGF-β, matrix metalloproteases (MMPs) (eg, MMP-2 and MMP-9), and others by MSCs suggest that a complex set of trophic factors secreted by these cells could be responsible for angiogenesis [26–28].
As angiogenesis plays a crucial part in the biological process of regeneration, and the soluble factors secreted by MSCs are thought to be responsible for the outcome following MSC treatment, it is important to identify the effect of these soluble factors on endothelial cells. Several studies have reported the effect of trophic factors released by MSCs derived from bone marrow and adipose tissue on endothelial cells. In these studies, the angiogenic response has been attributed to several proangiogenic cytokine expressions, including VEGF, PDGF, IGF-1, GM-CSF, and HGF [29–31]. Differences in gene expression as well as therapeutic potential of MSCs derived from varied sources have been reported [32,33]. Although several evidences demonstrate the proangiogenic property of MSCs, a greater understanding of the mechanisms by which MSCs derived from various sources induce angiogenesis need to be evaluated to increase the effectiveness of cell therapy.
In a review article, Hass et al. have compared the characteristics of MSCs derived from various tissues and summarized that multifunctional MSCs derived from birth-associated tissues have better advantages [34]. We have previously reported the potential of MSCs derived from UC to improve angiogenesis in a preclinical model of ischemic limb disease [35]. Kim et al. had shown that the transplantation of UCMSCs in patients with Buerger's disease showed improvement in the symptoms along with increase in the number and size of capillaries [36]. Other similar studies have implicated paracrine factors secreted by UCMSCs to be responsible for improved angiogenesis [37,38]. In these reported studies, the paracrine factors responsible for the angiogenic response have not been elucidated.
In our study, experiments were performed to identify and understand the role of paracrine factors secreted by UCMSCs on angiogenesis. In this study, we report the absence of VEGF and presence of soluble VEGF receptors (sVEGF-R) in the conditioned media (CM). The significant levels of MMP-2 present in the CM could be responsible for the increased endothelial cell migration and capillary network formation observed in endothelial cells.
Materials and Methods
UCMSC culture and its characterization
Human UC tissues were obtained postpartum from mothers after informed written consent, the protocol of which was approved by the Institutional Stem Cell Committee. Cord tissue processing and UCMSC isolation and its propagation were carried out using our previously reported method [39,40]. Briefly, UCs were decontaminated and cells isolated by explant culture in Dulbecco's modified Eagle's medium F-12 (DMEM/F12) (Lonza) supplemented with 10% fetal bovine serum (Hyclone), 2 ng/mL bFGF (R&D Systems), and 1× antibiotics–antimycotic solution (Invitrogen). Cells were used from passages 2 to 6 in the experiments.
To prepare the CM, UCMSCs from passage 3–6 (5×104 cells/cm2) were seeded in 175-cm2 tissue culture flasks (Nunc). After 3–4 days (70% confluency), the media were replaced with 30 mL serum-free DMEM/F12 media and incubated for 72 h under normoxic condition. The CM were then collected and filtered through a 0.22-μM filter and frozen at −20°C till further use. CM, except when specified, were used directly (without concentrating) in all experiments.
For immunophenotypic characterization, the cells (passage 4) from three different cord tissue samples cultured in serum-free media were used. The cells were harvested, washed, pelleted, and resuspended in phosphate-buffered saline (PBS) at 106 cells/mL. The cell suspension (0.1 mL) was blocked with a Mouse BD Fc Block and then incubated with FITC-conjugated monoclonal antibodies specific for CD45, CD73, HLA-DR, CD29, CD44, CD105, and SSEA4 (BD Pharmingen and R&D Systems) on ice for 30 min. After washing with PBS, the cells were acquired on a FACS Calibur instrument (Becton-Dickinson) using Cell Quest software.
Human umbilical cord vein-derived endothelial cell culture and its characterization
Human umbilical cord vein-derived endothelial cells (HUVECs) were obtained from Lonza. The cells were plated at 5×104 cells/cm2 in tissue culture flasks and maintained in endothelial cell growth media (EGM-2) (Lonza) supplemented with growth factors as recommended by the manufacturer. In the experiments, cells were used from passages 2–6.
Reverse transcription-polymerase chain reaction
Total cellular RNA was isolated from UCMSCs (1×106 cells) cultured either in normal or serum-free conditions using an RNAse Miniprep kit (Qiagen). The RNA was treated with RNase-free DNase I (Invitrogen) to prevent genomic DNA contamination. cDNA was synthesized using total RNA (1 μg), oligo(dT)12–18 primer, and superscript RT (Invitrogen) as per the manufacturer's protocol. Amplification of the cDNA with the primers listed in Table 1 was performed in the MyCycler PCR machine (Bio-Rad) under the following conditions: denaturing at 94°C (30 s), annealing at 55°C (30 s), and extension at 72°C (60 s) for 30 cycles for each primer, except in 18S where the gene was amplified in 22 cycles.
Table 1.
Sequence of Primers Used for Reverse Transcription-Polymerase Chain Reaction Studies
| No. | Gene name and accession no. | Sequence | Size of PCR product (bp) |
|---|---|---|---|
| 1 | VEGF NM_001025366 | F 5′-CTACCTCCACCATGCCAAG-3′ | 513 |
| R 5′-CACATCTGCAAGTACCTTCG-3′ | |||
| 2 | bFGF J04513.1 | F 5′-TTCTTCCTGCGCATCCAC-3′ | 354 |
| R 5′-CAGCTCTTAGCAGACATTGG-3′ | |||
| 3 | PDGF X03795.1 | F 5′-GGAGGAAGAGAAGCATCGAG-3′ | 340 |
| R 5′-CTCACATCCGTGTCCTCTTC-3′ | |||
| 4 | IL-6 NM_000600.3 | F 5′-ATCCTCGACGGCATCTCAGC-3′ | 350 |
| R 5′-AGCAGGCTGGCATTTGTGGT-3′ | |||
| 5 | IL-8 BC013615.1 | F 5′-TCTGCAGCTCTGTGTGAAG-3′ | 192 |
| R 5′-GTCCAGACAGAGCTCTCTTC-3′ | |||
| 6 | MMP-2 NM_004530.4 | F 5′-AGAGTTGGCAGTGCAATACC-3′ | 371 |
| R 5′-TCATGATGTCTGCCTCTCC-3′ | |||
| 7 | MMP-9 NM_004994.2 | F 5′-GCAGAGGAATACCTGTACC-3′ | 240 |
| R 5′-CCAATAGGTGATGTTGTGGTG-3′ | |||
| 8 | sVEGF-R1 U01134 | F 5′-ACAATCAGAGGTGAGCACTGCAA-3′ | 180 |
| R 5′-TCCGAGCCTGAAAGTTAGCAA-3′ | |||
| 9 | VEGF-R1 AF063657 | F 5′-TCCCTTATGATGCCAGCAAGT-3′ | 79 |
| R 5′-CCAAAAGCCCCTCTTCCAA-3′ | |||
| 10 | sVEGF-R2 NM_002253 | F 5′-CTTGGCCCACAGCCTCTGCC-3′ | 285 |
| R 5′-GGCATTCCAACTGCCTCTGCAC-3′ | |||
| 11 | VEGF-R2 NM_002253 | F 5′-CTTGGCCCACAGCCTCTGCC-3′ | 634 |
| R 5′-GTTCCCCTCCATTGGCCCGC-3′ | |||
| 12 | 18S NM_022551.2 | F 5′-TCGAAGACGATCAGATACC-3′ | 354 |
| R 5′-GCATGCCAGAGTCTCGTTC-3′ |
bFGF, basic fibroblast growth factor; MMP, matrix metalloprotease; PCR, polymerase chain reaction; sVEGF-R, soluble VEGF receptors; VEGF, vascular endothelial growth factor.
ELISA
The CM were analyzed for angiogenesis influencing factors like VEGF-A (Invitrogen), bFGF, PDGF, EGF, IL-6, IL-8, MMP-2, sVEGF-R1, and sVEGF-R2 (R&D Systems) using protocols specified in their respective kits. Data are expressed as mean±SEM nanograms of the secreted factor per 106 cells at the time of harvest.
To determine VEGF neutralizing property, the CM (200 μL) were incubated with recombinant human VEGF-A (rhVEGF-A, 100–1,500 pg/mL; Promokine) for 30 min and the residual VEGF activity was assessed using the VEGF-A kit (Invitrogen). A VEGF antibody, bevacizumab (250 μg/mL) (Avastin; Genentech), known to inhibit the VEGF activity was used as control.
Zymography
Zymography was performed using 8% Tris-glycine sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel containing 1 mg/mL gelatin (type A; Sigma). CM (20 μL) containing 1 mg/mL protein were mixed with a sample buffer (10% SDS, 4% glycerol, 0.25 M Tris-HCl, and 0.1% bromophenol blue) and separated at 4°C. The gels were rinsed with 2.5% Triton X-100 and incubated overnight (O/N) at 37°C in Triton X-100 solution. Bands were visualized by staining the gels with a solution containing 0.25% Coomassie blue R250, 40% methanol, and 10% acetic acid for 2 h at room temperature and destained with 40% methanol and 10% acetic acid. Gelatinolytic activities appear as clear zones against a blue background.
Western blot
To identify the response of UCMSCs to VEGF treatment, the cells were incubated with or without rhVEGF (25 ng/mL) for 72 h. The CM derived from treated and untreated cells were concentrated to 1/10th of its initial volume by spinning in a Concentrator (Eppendorf) and resolved by electrophoresis on 8% SDS-PAGE gels using standard techniques under a denaturing nonreducing condition. Briefly, CM samples containing equal proteins were separated for 1.5 h with 4:1 nonreducing sample loading dye [62.5 mM Tris-HCl (pH 6.8), 25% glycerol, 10% SDS, and 0.01% bromophenol blue] without heating and transferred to a nitrocellulose membrane at 4°C for 1 h. Blots were then blocked in 5% bovine serum albumin (Amresco) in a TBST buffer [100 mM Tris-HCl (pH 7.5), 1.5M NaCI, and 0.1% Tween 20] O/N. Blots were then washed thrice for 10 min each at room temperature with a TBST buffer and incubated with either 0.1 μg/mL anti-sVEGF-R1 or anti-sVEGF-R2 monoclonal antibody (R&D Systems), respectively, for 2 h at room temperature in a TBST buffer. The above blots were rinsed with TBST and immersed in HRP-conjugated goat-anti-mouse lgG (R&D Systems) 1:5,000 dilution. Immunoreactivity was visualized by the addition of Sigma FAST™ and 3,3′-diaminobenzidine (Sigma). To identify VEGF, the above blots were stripped in a buffer containing1.5% glycine, 0.1% SDS, and 1% Tween 20 and reprobed with a VEGF antibody (0.1 μg/mL) (Avastin; Genentech), as described above.
Cell migration assay
The in vitro cell migration assay was performed in 24-well plates by using cell culture inserts (3 μm; Nunc) as described previously with modifications [41]. A single cell suspension of HUVECs (15,000) suspended in 250 μL of control media (DMEM/F12+0.2% serum) was seeded into each culture insert. Seven hundred microliters of either CM or control media containing 0.2% serum and supplemented with or without rhVEGF (25 ng/mL) or bevacizumab (250 μg/mL) was added into the 24-well plate in triplicate. After O/N incubation, the media inside the insert were carefully aspirated, and nonmigrated cells on the upper side of the membrane were removed with a swab. The migrated cells located on the bottom side of the membrane were washed and fixed with cold methanol and incubated for 20 min at room temperature. Methanol was then aspirated and the membranes were stained with 0.1% crystal violet solution for 30 min, washed twice in PBS, and observed under a microscope (Axio Observer Z1; Carl Zeiss). Cell migration was determined by counting the cells that have migrated through the filter in six random fields by two independent observers.
Cell proliferation assay
Cellular proliferation was assessed using the CyQuant Cell Proliferation Assay Kit (Invitrogen) according to manufacturer's instructions. HUVECs were plated at 5×103/well in 96-well plates in EGM-2 O/N. The following day, the media was replaced with 0.2% serum-supplemented DMEM/F12 to condition the cells. The next day, media were replaced with 0.2% serum containing control or CM and treated with or without rhVEGF (25 ng/mL) and bevacizumab (250 μg/mL) in triplicate. After incubation for 72 h, the media were removed from the wells and the plates were frozen O/N at −80°C. The next day, the plates were thawed and the cells were incubated with a CyQuant GR dye/cell-lysis buffer for 5 min at room temperature. Total cellular DNA was quantified using a fluorescence microplate reader (Polarstar Optima; BMG Labtech) at 480 nm excitation/520 nm emissions and the cells quantified using a standard curve.
In vitro tube formation assay
The effect of CM on capillary network formation was studied on Matrigel using HUVECs. Briefly, 60 μL of growth factor-reduced Matrigel (BD Biosciences) was added to each well of a 96-well plate and incubated for 1 h at 37°C. HUVEC (15,000 cells) were seeded per well in media containing control media or UCMSC-CM supplemented with or without VEGF (25 ng/mL) and/or bevacizumab (250 μg/mL). The plates were incubated for 4 h at 37°C and capillary network formation was assessed by two independent observers under a light microscope (Carl Zeiss). The length of the capillary network captured from five random fields in each well was quantitated using ImageJ software (NIH).
To check the effect of MMP-2 present in the CM on endothelial cells, CM or control media were incubated with a broad-spectrum inhibitor of MMPs, GM6001 (0–150 μM) (Chemicon), for 1 h at 37°C. The effect of GM6001 was assessed on capillary network formation and quantified as mentioned above.
Statistical analysis
All experiments were performed in triplicate with UCMSCs derived from three different donors. The data are expressed as mean±SEM. Statistical significance was determined using either one-way or two-way ANOVA using GraphPad Prism software (GraphPad). P value of less than 0.05 was considered to be statistically significant.
Results
Characterization of UCMSCs
UCMSCs were cultured either in regular growth media or in serum-free media for 72 h and characterized using flow cytometry. FACS data show high expression of CD73, CD105, CD44, and CD29 and in contrast no expression of CD45 and HLA-DR, showing the specific characteristics of MSCs. Data obtained from a representative UCMSC sample cultured in serum-free media is shown in Fig. 1. A similar pattern of expression was observed in cells cultured in the presence of serum (data not shown).
FIG. 1.
Flow cytometry analysis of cell surface markers in UCMSCs cultured in serum-free conditions. The cells are negative for CD45, weakly positive for HLA-DR, and strongly positive for CD73, CD105, CD44, and CD29. Cells counts are shown in y-axis and relative fluorescence in x-axis. Isotype control is shown in gray color and samples incubated with specific antibodies are represented in black. n=3. UCMSCs, umbilical cord-derived mesenchymal stem cells.
rhVEGF in the presence of CM does not increase EC proliferation
One of the first steps in angiogenesis is endothelial cell proliferation. Endothelial cells derived from human UC are normally cultured in EGM-2 media containing serum. To overrule the effect of serum, we had conducted proliferation studies in media containing 0.2% serum. In the proliferation study, an angiogenic stimulator rhVEGF-A (25 ng/mL) and an anti-VEGF molecule, bevacizumab (250 μg/mL), were used as controls. Data represented in Fig. 2 show that the proliferation of endothelial cells almost doubled on rhVEGF treatment as compared to control media (P<0.01). This increase in proliferation was blocked in the presence of bevacizumab. In comparison to control media, no significant difference in the proliferative response was seen in endothelial cells treated with CM alone (P<0.15). Further supplementation of CM with either rhVEGF or bevacizumab showed no change in the proliferative response as compared to CM alone.
FIG. 2.

The effect of UCMSC-CM on endothelial cell proliferation. Proliferation is represented as a percentage of cells on day 3 versus the initial number on day 0 (mean±SEM). Endothelial cells cultured in control media and exposed to VEGF show a significant increase in cell proliferation (P<0.01), which is reversed on exposure to bevacizumab. In comparison to control media, no significant change in proliferation is seen with P>0.15. A similar observation is seen in CM treated with either VEGF or bevacizumab as compared with CM. Results represent an average of data obtained from CM derived from three different donors, each experiment was performed in triplicate. CM, conditioned media; VEGF, vascular endothelial growth factor.
Identification of angiogenic factors in CM
UCMSCs derived from three different donors were characterized for proangiogenic markers, and the data obtained are provided in Fig. 3. Molecular transcripts of factors like VEGF, PDGF, bFGF, IL-6, IL-8, and metalloproteases (MMP-2 and MMP-9) were detected using RT-polymerase chain reaction (PCR) studies. The three UCMSCs (UC-1, -2 & -3) tested, expressed the above genes with no significant difference in expression between the three samples tested either in the absence or presence of serum. Comparison of protein expression in similar samples using ELISA showed no detection of VEGF, very low concentrations of PDGF, bFGF, and IL-6, and a very high concentration of MMP-2. Data represented in Table 2 are expressed as mean±SEM nanograms of the secreted proteins per 106 cells at the time of harvest.
FIG. 3.

RT-PCR analysis of transcripts involved in angiogenesis expressed in UCMSCs derived from three different donors cultured in serum-free conditions (UC-1, UC-2, and UC-3) as well as a same sample cultured in regular growth media containing serum (UC-N).
Table 2.
Quantitative Analysis of Angiogenic Factors Secreted by UCMSCs in the Conditioned Media Was Measured by ELISA
| Test | UC-S | UC-SF |
|---|---|---|
| VEGF | N/D | N/D |
| bFGF | 0.078±0.04 | 0.156±0.01 |
| PDGF | 41.5±5.9a | 4.7±3.2 |
| IL-6 | 0.098±0.01 | 0.068±0.02 |
| IL-8 | 17.6±2.8b | 7.9±1.8 |
| MMP-2 | 337.9±62.5 | 237.4±47.1 |
P<0.005; bP<0.04.
N/D, not detected; UCMSCs, umbilical cord-derived mesenchymal stem cells.
Inhibition of exogenous rhVEGF by USMSC-CM
Although mRNA transcripts of VEGF were detected in UCMSCs, the protein was not detected in CM. In addition, the lack of a proliferative response of endothelial cells in CM treated with rhVEGF-A prompted us to further investigate the role of CM on VEGF. The data represented in Fig. 4 indicate a dose-dependent reduction in the detection of rhVEGF-A added to the CM. The percentage of rhVEGF-A neutralized by CM was 92±5 (100 pg/mL), 83±7(500 pg/mL), 61±4 (1,000 pg/mL), and 35±2 (1,500 pg/mL), respectively. On similar treatment of rhVEGF-A with a high concentration of bevacizumab (250 μg/mL), no VEGF activity was detected.
FIG. 4.

Neutralization of exogenous rhVEGF by UCMSC-CM. rhVEGF (100–1,500 pg/mL) was added to 200 μL of UCMSC-CM and incubated for 30 min and the residual VEGF activity was assessed using the VEGF ELISA kit. VEGF was similarly analyzed after treatment with bevacizumab (250 μg/mL). The data represent the mean value±SEM obtained from two independent experiments conducted using the CM derived from the UCMSCs derived from three different donors. Each experiment was conducted in triplicate. rhVEGF, recombinant human VEGF.
Identification of VEGF neutralizing factors
To identify the factor(s) that might be responsible for neutralizing rhVEGF-A, we passed the CM through various molecular weight (300, 100, and 10 kDa) cutoff filtration devices (JumboSep centrifugal devices; Pall). To the filtrate and concentrate fractions that were collected from the devices, rhVEGF (1,000 pg/mL) was added and incubated for 30 min and the fractions were analyzed for VEGF using ELISA. It was observed that VEGF activity was reduced only in the 100–300 kDa fraction, indicating that VEGF neutralizing factor(s) were in this molecular range (Fig. 5A). While VEGF-R are found in a cell membrane-bound form, alternative splicing of the VEGF-R mRNA results are found in a soluble truncated form. These soluble isoforms of VEGF-R named sVEGF-R1 (molecular weight 110 kDa) and sVEGF-R2 (molecular weight 160 kDa) contain the ectodomain of their corresponding full-length isoforms that are capable of not only sequestering VEGF but also bind and inactivate membrane-bound VEGF-R1 and R2 receptors [42,43].
FIG. 5.

Identification of VEGF neutralizers. (A) UCMSC-CM was passed through various molecular weight (300, 100, and 10 kDa) cutoff filtration devices. To the filtrate and concentrate fractions collected from the respective devices, rhVEGF-A (1 ng/mL) was added and incubated for 15 min. VEGF activity in these fractions was analyzed by ELISA, and the fractions showing inhibition in VEGF activity were assumed to have VEGF inhibitors/neutralizers. The experiment was conducted using CM derived from three different UCMSCs. (B) Transcripts of VEGF-R1 and -R2 and its soluble forms (sVEGF-R1 & -R2) expressed in UCMSCs were analyzed using RT-PCR studies. The housekeeping gene, 18S, was used as an internal control. The cells were derived from three different donors cultured in serum-free conditions (UC-1, UC-2, and UC-3) or in regular growth media containing serum (UC-N). sVEGF-R, soluble VEGF receptor.
Based on the molecular size fraction (100–300 kDa) of the CM in which inhibition of VEGF was observed, we hypothesized that VEGF neutralization could be due to the presence of sVEGF-R. To verify if VEGF-R (including membrane bound or its soluble forms) are expressed by UCMSCs, we determined the expression of VEGF-R1 & R2 and sVEGF-R1 & R2 in UCMSCs. RT-PCR studies (Fig. 5B) showed high levels of VEGF-R1 expression as compared with VEGF-R2. The expression of sVEGF-R1 and sVEGF-R2 was detected in all samples suggesting that these soluble receptors might be responsible for sequestering VEGF and preventing the latter's detection in ELISA. Expression levels quantified using ELISA revealed a high expression of sVEGF-R1 (3,058±679 pg/106 cells) and a relatively lower expression of sVEGF-R2 (47±25 pg/106 cells) in the CM.
VEGF-challenged UCMSCs neutralize higher VEGF concentrations
To evaluate UCMSCs response to increasing concentrations of rhVEGF-A, we incubated rhVEGF-A (1–25 ng/mL) for 72 h in the presence and absence of cells. At the end of treatment, VEGF was quantified in the incubated media and the data were calculated as a percentage of residual activity obtained in comparison with the activity obtained in the absence of cells (to compensate for any loss of VEGF activity as a result of its incubation). As represented in Fig. 6A, no VEGF was detected in the media derived from UCMSCs treated with 1 ng/mL rhVEGF, indicating 100% neutralization (P<0.002). On treating the same number of cells with 5 ng/mL or 25 ng/mL rhVEGF, the residual VEGF activity was reduced by 33.2±3.4% (P<0.006) and 28.5±8.8% (P<0.05), respectively. This significant reduction in VEGF activity indicates that UCMSCs respond to the increasing VEGF challenge by secreting more VEGF neutralizers. To determine the effect on sVEGF-R in the presence of increasing VEGF treatments, we quantified the sVEGF-R in the cell supernatants by ELISA. Data revealed that while sVEGF-R1 was present in all conditions with no significant change between treatments (Fig. 6B), sVEGF-R2 was not detected in either of the samples. To confirm the binding of soluble receptors with VEGF, we performed western analysis. The analyzed blots are shown in Fig. 6C. Data revealed high levels of sVEGF-R1 in supernatants derived from untreated cells (A) as compared with VEGF-treated cells (B). The expression of sVEGF-R2 was however reversed, with higher levels expressed in VEGF-treated cells (B) as compared with nontreated cells (A). To identify the presence of VEGF in the supernatant, the above blots were stripped and reprobed with bevacizumab, a VEGF antibody. In the blots, VEGF was detected only in supernatants of cells that were treated with VEGF and not in untreated cells, further confirming the absence of VEGF secretion by UCMSC.
FIG. 6.
Response of UCMSCs to VEGF challenge. UCMSCs (1×106 cells/well) were incubated with VEGF (0–25 ng/mL) for 72 h and the supernatant was tested for VEGF activity (A) and sVEGF-R1 (B) using respective ELISA kits, as mentioned under the Materials and Methods section. Data represent the mean±SD of experiments conducted in triplicate from samples derived from three different donor-derived UCMSCs. *P<0.006, **P<0.05. (C) The CM obtained from UCMSCs incubated with or without 25 ng/mL VEGF for 72 h were concentrated 10-fold and resolved on 8% SDS-PAGE gels using denaturing nonreducing conditions. The individual gels were blotted and probed with either sVEGF-R1 or -R2 antibodies, respectively. The same blots were stripped and reprobed with the VEGF antibody, as described in the Materials and Methods section. In (C), lane A represents CM obtained from untreated cells and lane B represents CM derived from cells incubated with 25 ng/mL rhVEGF. VEGF, represented as open arrow is detected only in supernatants of cells that were treated with VEGF and not in untreated cells, further confirming the absence of VEGF secretion by UCMSC. The closed arrow represents the corresponding VEGF receptor's.
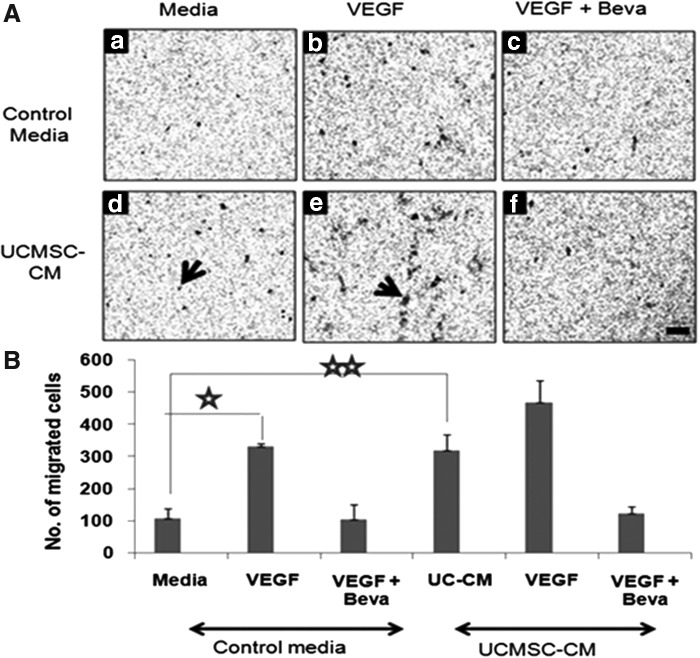
Effect of CM on endothelial migration
Since endothelial cell migration is important in angiogenesis, we evaluated the effect of CM on HUVEC migration using cell culture inserts. Data represented in Fig. 7 show that VEGF, as expected, increased migration of HUVEC significantly (P<0.003), which was reversed on treatment with bevacizumab. While CM alone significantly increased endothelial cell migration as compared with control media (P<0.007), the extent of migration was almost similar to that of control media containing VEGF. No significant change in cell migration was observed on further addition of VEGF to CM (P<0.15). This increase in cell migration was reversed in the presence of bevacizumab.
FIG. 7.
Migration of endothelial cells in response to UCMSC-CM. Cell migration studies were conducted by incubating endothelial cells in the upper chamber of transwell cell culture inserts. The number of cells that had migrated across 3-μm pore size filter membranes in response to either control media or UCMSC-CM present in the lower chamber was analyzed after fixing and staining the cells with crystal violet. Representative photographs of cells that had migrated across the filter after an 18-h incubation are shown in (A). The respective media (a, d) were treated either with rhVEGF (25 ng/mL) (b, e) and/or bevacizumab (250 μg/mL), (c, f) respectively. Arrows indicate migrated cells and the scale indicates 100 μm. Photographs were taken of each filter in six random areas and the mean number±SEM of cells migrated per filter was counted by two random observers. The total number of migrated cells per filter is reported in (B). Each experiment was carried out in triplicate using cells derived from three individual donors (⋆P<0.003, ⋆⋆P<0.007).
Effect of CM on tube formation
Angiogenesis requires the assembly of endothelial cells into capillary-like structures. We investigated the role of UCMSC-derived paracrine factors in vessel assembly using the Matrigel assay. Through qualitative observation of tube formation (Fig. 8A) and data obtained from its quantitative analysis (Fig. 8B–D), it is seen that in comparison with control media, rhVEGF (25 ng/mL) increased the capillary network (P<0.02), branch points (P<0.0002), and number of rings (P<0.0003), which was reversed on addition of bevacizumab (250 μg/mL). A similar treatment of HUVEC in the presence of CM showed a significant increase in network formation (P<0.001), branch points (P<0.0004), and number of rings (P<0.002) as compared with control media. While the VEGF-induced capillary network is widely known, our observation of a similar response with CM in the absence of VEGF suggests the presence of other proangiogenic factors that could be responsible for this enhanced tube formation. Addition of VEGF to the CM, however, did not significantly increase either of the parameters as compared with CM alone (P>0.5).
FIG. 8.

Capillary network formation of HUVECs in the presence of UCMSC-CM. HUVECs were seeded on growth factor-reduced Matrigel. Control or CM were treated as follows for 1 h before its addition to the cells. (A) Representative photographs of tubes formed in 96-well plates. (a) Control media; (b) control media treated with rhVEGF; (c) control media treated with rhVEGF and bevacizumab; (d) CM; (e) CM treated with rhVEGF. Scale bar represents 200 μm. (B) Average tube length (⋆P<0.02; ⋆⋆P<0.001); (C) number of branch points (⋆P<0.0002, ⋆⋆P<0.0004); and (D) number of rings (⋆P<0.0003, ⋆⋆P<0.002) obtained from five representative areas of each well. n=3. HUVECs, human umbilical cord vein-derived endothelial cells.
Role of MMP-2 in CM-induced tube formation and migration
The high levels of MMP-2 expression observed in the CM as compared to other proangiogenic molecules (Table 2) prompted us to further investigate this enzyme's role in CM-induced angiogenesis. Breakdown of ECM by proteolytic enzymes is necessary to facilitate endothelial cell invasion, migration, and tube formation. MMPs are secreted in the extracellular fluid and known to degrade most basement membrane components. We studied the production and activity of secreted MMPs by UCMSCs using zymography, a technique that reveals both active MMP and latent Pro-MMP. Separation of bands on the basis of molecular weights during electrophoresis distinguishes pro-MMP and active MMP. Zymographic analysis revealed the presence of significant levels of pro-MMP-2 (72 kDa) and active MMP-2 (63 kDa) in all samples derived from UCMSCs obtained from three independent donors (Fig. 9A). The absence of additional proteolytic bands suggests that other metalloproteases are either not secreted/detected by this assay. To study the effect of VEGF on MMP secretion, we treated the cells with rhVEGF (1–25 ng/mL) for 72 h and analyzed MMP-2 by ELISA. Data represented in Fig. 9B. indicate very high basal levels of MMP-2, which further increased dose dependently up to 5 ng/mL VEGF treatment (P<0.015). A further increase in VEGF concentration (25 ng/mL) did not significantly increase MMP-2 expression as compared to nontreated cells (P<0.42).
FIG. 9.

Role of MMP-2 in UCMSC-CM-induced angiogenesis. (A) Zymographic analysis of MMP-2 expression in UCMSC-CM. Arrowheads indicate the latent and active forms of MMP-2 secreted in UCMSCs derived from three different donors. (B) UCMSCs incubated with VEGF (0–25 ng/mL) for 72 h and the supernatant was tested for MMP-2 expression by ELISA. The data represented as mean±SEM were obtained from three different experiments conducted in triplicate (⋆P<0.06; ⋆⋆ P<0.015). MMP, matrix metalloprotease.
To check if MMP-2 present in the CM was responsible for the latter's effect on endothelial cells, CM or control media were incubated with a wide acting MMP blocker, GM6001 (0–150 μM) (Calbiochem), for 1 h at 37°C and its effect was assessed on endothelial cell migration and capillary network formation.
To verify the effect of GM6001 on endothelial cell migration induced by CM, in vitro migration studies were performed using the protocol mentioned under the Materials and Methods section. Data in Fig. 10A show representative pictures of the migrated cells and in Fig. 10B the average number of cells that had migrated across the filter following various treatments. In cells treated with control media, a significant reduction in the number of migrating endothelial cells was observed with 100 μM (P<0.007) and 150 μM (P<0.03) as compared to no treatment. In presence of CM, the cells treated with a similar concentration of GM6001 showed significant inhibition in cell migration at 150 μM (P<0.05) as compared to untreated cells. With 100 μM, however, no change in response was observed.
FIG. 10.
GM6001 blocks migration and angiogenesis. UCMSC-CM and control media were incubated with GM6001 (0–150 μM) for 1 h and the effect was assessed on endothelial cell migration (A) and capillary network formation (C) as mentioned before. Photographs showing migrated cells in the untreated respective media (a, d), 100μM GM6001 (b, e) and 150 μM GM6001 (c, f) are shown in (A). Arrows identify the cells and the scale bar represents 100 μm. (B) Chart represents the average number±SEM of cells migrated across each filter obtained from three different experiments conducted in triplicate (⋆P<0.007; ⋆⋆P<0.05). (C) Representative photographs of endothelial cell response to various treatments: untreated respective media (a, d), media treated with 100 μM GM6001 (b, e) and 150 μM GM6001 (c, f) respectively on matrigel. Scale bar represents 200 μM. Quantitative representation of the chart in (D) indicates average capillary tube length (⋆P<0.07; ⋆⋆P<0.006); (E) the number of branch points (⋆P<0.03; ⋆⋆P<0.005); and (F) number of rings (⋆P<0.04; ⋆⋆P<0.009). Each experiment was conducted in triplicate for n=3.
Capillary network formation following GM6001 treatment was assessed microscopically and a representative picture of the network formation is shown in Fig. 10C. Quantitative analysis of the pictures taken from five different areas of the wells was analyzed and the data are represented in Fig. 10D. Data obtained from control media treated with 100 μM GM6001 indicate no significant inhibition of capillary network formation (P<0.07), but significant inhibition of branch points (P<0.03) and number of rings (P<0.04) as compared with untreated control. With 150 μM treatment, a significant inhibition of network formation (P<0.005), branch points (P<0.03), and number of rings (P<0.02) as compared to untreated control was observed indicating that blocking MMP-2 with GM6001 prevents angiogenesis. However, in CM treated with 100 μM GM6001 no significant inhibition in network formation was observed, while with 150 μM, the network formation (P<0.006), branch points (P<0.005), and number of rings (P<0.009) were significantly reduced as compared to untreated CM indicating the need for a higher concentration of GM6001 required to block the excess MMP present in the CM.
Discussion
MSCs derived from various sources express basic characteristics as specified by the International Society of Stem Cell Research (ISSCR). These include the following: (1) Plastic-adherent cells; (2) Capable of tri-lineage (bone, cartilage, and fat) differentiation; (3) Phenotypically positive for CD105, CD73, and CD90; and (4) negative for CD45, CD34, CD11b, CD79, and HLA-DR [44]. Nevertheless, MSCs isolated from various tissues exhibit inherent differences [32,45,46] as well as difference in their potential to induce neovascularization [47,48].
Since the biological effects of MSCs have been attributed to its paracrine factors, we investigated the angiogenesis factors secreted by UCMSCs and studied their role on endothelial cells. Of the various angiogenic factors secreted by MSCs, VEGF is reported to play a central role in MSC-induced angiogenesis [49]. In contrast to literature showing presence of VEGF in the CM derived from MSCs obtained from bone marrow [50,51] and adipose tissue [49], we did not detect VEGF in the CM, although its molecular transcripts were expressed in the cells. Of the various angiogenic factors tested, besides MMP-2, a metalloprotease, no other was detected in significant levels.
It is known that VEGF binds and activates two tyrosine kinase receptors, VEGF-R1 (Flt-1) [52] and VEGF-R2 (KDR/Flk-1) [53]. While the expression of the former receptor is reported in monocytes, renal mesangial cells, vascular endothelial and smooth muscle cells, the latter receptor is shown in pancreatic duct cells, retinal progenitor cells, and hematopoietic cells. Mesenchymal cells are known to mobilize and migrate in response to VEGF signals induced in ischemic tissues [11,20,27], we hence studied VEGF-R expression in UCMSCs. We observed significant expression of VEGF-R (R1 and R2) genes in UCMSCs, while the only other reported study so far has shown VEGF-R1 gene expression in murine bone marrow MSCs exposed to hypoxic conditions [54].
The absence of detectable VEGF in CM derived from UCMSCs, in addition to its ability to neutralize rhVEGF activity, prompted us to look for the presence of VEGF inhibitor(s) in the CM. Alternative splicing of VEGF-R1 and VEGF-R2 is reported to produce soluble receptor isoforms (sVEGF-R1 and sVEGF-R2) that can bind to and inhibit the action of VEGF [55–57]. In normal CM, we observed significant levels of sVEGF-R1 as compared with sVEGF-R2. In our study, the same number of UCMSCs exposed to increasing concentrations of VEGF could neutralize higher levels of VEGF. This ability of UCMSCs to block VEGF could be by inducing sVEGF-R(s) by an autocrine pathway. With no change in sVEGF-R1 levels either in presence or absence of increasing concentrations of VEGF, the latter's role in VEGF neutralization is not clear. However, on similar treatment, sVEGF-R2 was not detected in all conditions. In western blot analysis of the supernatants collected from untreated cells, higher levels of sVEGF-R1 were seen as compared with sVEGF-R2. In contrast, in treated conditions, the expression appears to be reversed with higher levels of sVEGF-R2 seen in comparison with sVEGF-R1. These data indicate that sVEGF-R2 might be critical in VEGF binding. The absence of sVEGF-R2 in ELISA could be due to rhVEGF binding to all the active motifs of VEGF-R2, thereby preventing its detection. To the best of our knowledge, this is the first report showing the expression of sVEGF-R in UCMSCs and demonstrating its expression in response to VEGF. Human placenta is reported to release excessive sVEGF-R1 into the mother's circulation during preeclampsia [58]. Ahmad and Ahmed compared the CM derived from normal and preeclamptic placenta and attributed the attenuated response of endothelial cell migration and in vitro tube formation to the high concentration of sVEGF-R1 in the CM of preeclamptic placenta [59]. UCMSCs are derived from the UC, which develops during pregnancy and connects the fetus and mother through the placenta. While the expression of sVEGF-R by UCMSCs might be attributed to its association with placenta, the role of these receptors in vivo following UCMSC implantation needs to be further investigated.
In view of the absence of VEGF in the CM as well the presence of sVEGF-R that have the ability to neutralize VEGF, we further explored the role of CM on various aspects of angiogenesis, including endothelial cell proliferation and migration and its assembly into capillary-like structures. While VEGF is well known to stimulate endothelial cell proliferation, sVEGF-R are known to inhibit its proliferation [60].
The absence of a significant increase in endothelial cell proliferation following rhVEGF addition to CM could be a result of the VEGF being neutralized by the sVEGF-R. The enhanced endothelial cell migration and network formation observed in the presence of CM suggest that molecules other than VEGF might be responsible for these responses. Gruber et al. had similarly observed that CM derived from bone marrow-derived MSCs stimulated endothelial cell migration and tube formation with no change in proliferation. The authors concluded that MSCs might not be responsible for the initiation of the angiogenic process, but rather would play a role in guiding the growing blood vessels to the defect site [61].
Interestingly, sVEGF-R1 and -R2 are also reported to play a crucial role in supporting endothelial cell sprouting and its migration and branching, thereby suggesting that the mechanism by which sVEGF-R1 and R2 modulate angiogenesis is more complex than a simple antiangiogenic effect [62–64]. Lorquet et al. have reported that sVEGF-R are also involved in vessel maturation through induction of mural cell recruitment [65]. Thus, the sVEGF-R secreted by UCMSCs observed by us might play an important role in the angiogenic response observed following UCMSC implantation.
MMPs, a family of zinc binding, Ca2+-dependent endopeptidases are known to act in concert with other enzymes to degrade most components of the ECM during capillary morphogenesis [66,67]. During angiogenesis, MMPs breakdown the capillary basement membrane and allow the migration of endothelial cells into the surrounding matrix. MSCs are reported to secrete active MMP-2 and also its inhibitors, tissue inhibitors of metalloproteinases (TIMP1 & TIMP2) [68]. Following UCMSC implantation, the reduced fibrosis observed in the bleomycin-induced lung injury model in mice has been attributed to the upregulation of MMP-2 [69]. In contrast, the downregulation of MMP-2 observed in a similar model using bone marrow-derived MSCs [70] points to source-dependent differences in MSC response. We have seen a strong expression of MMP-2 in UCMSCs as compared with MMP-9. MMP-2 upregulation has been reported to play an important role in VEGF-mediated angiogenesis in vitro [71]. Recently, Ebrahem et al. had reported the upstream and downstream role of MMPs in VEGF signaling [72]. In our study, the CM in combination with VEGF did not significantly improve angiogenesis indicating that VEGF might not play a major role in CM-induced angiogenesis. Nevertheless, UCMSCs respond to VEGF treatment by increasing MMP-2 expression in a dose-dependent manner, indicating that VEGF might influence UCMSC response to angiogenesis.
With the metalloprotease activity being strongly implicated in endothelial cell migration and ECM degradation, our observed increase in endothelial cell migration and tube formation by CM might be due to MMP-2. To confirm this, we compared the response of endothelial cells to control media or CM in the presence of GM6001, a potent inhibitor of MMPs. By virtue of its MMP blocking property, GM6001 is reported to suppress migration of human dermal microvascular endothelial cells in a dose-dependent manner [73]. We observed suppression of endothelial cell migration with GM6001 at 100 μM in control media. While migration of endothelial cells in response to CM treated with 100 μM GM6001was not effected, a significant inhibition in cell migration was observed at 150 μM, indicating the need for a higher concentration of GM6001 that is required to block the excess MMP-2 present in CM. Similarly, the ability of a higher concentration of GM6001 to block CM-induced capillary network formation as compared to the control media suggests that MMP-2 present in the CM plays an important role in CM-induced angiogenesis. The role of MMP-2 in inducing endothelial network formation reported in this study is supported by Glaeser et al. who had reported that pro-MMP-2 secreted by MSCs is further activated by endothelial cells and the total MMP-2 plays a pivotal role in endothelial network formation [74].
Conclusion
While the potential of MSCs to induce angiogenesis thereby leading to regeneration has been well documented in preclinical and clinical studies, the potential of paracrine factors secreted by these MSCs is currently under investigation. Many of the studies investigating the paracrine factors secreted from MSCs derived from various sources have reported the presence of VEGF and have implicated its importance in angiogenesis. In this study, we investigated the paracrine profile of MSCs derived from UC. Contrary to published reports, we did not detect VEGF in the CM, although transcripts of VEGF were detected in the cells. The nondetection of exogenous rhVEGF added to the CM, in addition to the presence of sVEGF-R in the CM, indicated that a VEGF-independent pathway could be involved in the biological response observed with the CM. The proangiogenic response observed with endothelial cells exposed to the CM indicates that factors other than VEGF play a role in the CM-induced angiogenesis. Besides the significant levels of MMP-2, the presence of sVEGF-R in the CM could also play an important role in angiogenesis.
Acknowledgments
The authors acknowledge the encouragement and support of Reliance Life Sciences Pvt. Ltd. to carry out the research work (www.rellife.com). The authors thank Ms. Khushnuma Cooper and members of the regenerative medicine group and therapeutic protein group for their support.
Author Disclosure Statement
The authors indicate no conflicts of interest.
References
- 1.Epstein SE, Fuchs S, Zhou YF, Baffour R. and Kornowski R. (2001). Therapeutic interventions for enhancing collateral development by administration of growth factors: basic principles, early results and potential hazards. Cardiovasc Res 49:532–542 [DOI] [PubMed] [Google Scholar]
- 2.Pepper MS, Ferrara N, Orci L. and Montesanoet R. (1991). Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun 181:902–906 [DOI] [PubMed] [Google Scholar]
- 3.Leung DW, Cachianes G, Kuang WJ, Goeddel DV. and Ferrara N. (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309 [DOI] [PubMed] [Google Scholar]
- 4.Wilting J, Christ B. and Weich HA. (1992). The effects of growth factors on the day 13 chorioallantoic membrane (CAM): a study of VEGF165 and PDGF-BB. Anat Embryol 186:251–257 [DOI] [PubMed] [Google Scholar]
- 5.Laham RJ, Leimbach M, Chronos NA, Vansant JP, Pearlman JD, Pettigrew R, Guler HP, Whitehouse MJ, Hung D, et al. (1999). Intracoronary administration of recombinant fibroblast growth factor-2 in patients with severe coronary artery disease: results of phase I. J Am Coll Cardiol 33:383A. [DOI] [PubMed] [Google Scholar]
- 6.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, et al. (1998). Results of intracoronary recombinant human vascular endothelial growth factor (rhVEGF) administration trial. J Am Coll Cardiol 31(Suppl) 65A [Google Scholar]
- 7.Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY. and Quyyumi AA. (2000). Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J Am Coll Cardiol 36:1239–1244 [DOI] [PubMed] [Google Scholar]
- 8.Karp JM. and Leng Teo GS. (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216 [DOI] [PubMed] [Google Scholar]
- 9.Spaeth E, Klopp A, Dembinski J, Andreeff M. and Marini F. (2008). Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther 15:730–738 [DOI] [PubMed] [Google Scholar]
- 10.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M. and Marini FC. (2009). Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27:2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M. and Isner JM. (1999). VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasala GP. and Minguell JJ. (2011). Vascular disease and stem cell therapies. Br Med Bull 98:187–197 [DOI] [PubMed] [Google Scholar]
- 13.Fisher SA, Dorée C, Brunskill SJ, Mathur A. and Martin-Rendon E. (2013). Bone marrow stem cell treatment for ischemic heart disease in patients with no option of revascularization: a systematic review and meta-analysis. PLoS One 8:e64669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva Meirelles L, Caplan AI. and Nardi NB. (2008). In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299 [DOI] [PubMed] [Google Scholar]
- 15.Barry FP. and Murphy JM. (2004). Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584 [DOI] [PubMed] [Google Scholar]
- 16.Erices A, Conget P. and Minguell JJ. (2000). Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109:235–242 [DOI] [PubMed] [Google Scholar]
- 17.Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, Yang S, Liu P, Xu J, et al. (2009). Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of antiinflammation and angiogenesis. Cell Physiol Biochem 24:307–316 [DOI] [PubMed] [Google Scholar]
- 18.Ishikane S, Ohnishi S, Yamahara K, Sada M, Harada K, Mishima K, Iwasaki K, Fujiwara M, Kitamura S, Nagaya N. and Ikeda T. (2008). Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells 26:2625–2633 [DOI] [PubMed] [Google Scholar]
- 19.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M. and Werner C. (2004). Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22:377–384 [DOI] [PubMed] [Google Scholar]
- 20.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, et al. (2005). Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111:150–156 [DOI] [PubMed] [Google Scholar]
- 21.Laflamme MA, Zbinden S, Epstein SE. and Murry CE. (2007). Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol 2:307–339 [DOI] [PubMed] [Google Scholar]
- 22.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K. and Kitamura S. (2005). Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 66:543–551 [DOI] [PubMed] [Google Scholar]
- 23.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS. and Dzau VJ. (2003). Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9:1195–1201 [DOI] [PubMed] [Google Scholar]
- 24.Boomsma RA. and Geenen DL. (2012). Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One 7:e35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boomsma RA, Swaminathan PD. and Geenen DL. (2007). Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol 122:17–28 [DOI] [PubMed] [Google Scholar]
- 26.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM. and Silberstein LE. (2006). Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24:1030–1041 [DOI] [PubMed] [Google Scholar]
- 27.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S. and Epstein SE. (2004). Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 94:678–685 [DOI] [PubMed] [Google Scholar]
- 28.Ghajar CM, Kachgal S, Kniazeva E, Mori H, Costes SV, George SC. and Putnam AJ. (2010). Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316:813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Tredget EE, Wu PY. and Wu Y. (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I. and Kalka C. (2009). Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One 4:e5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, et al. (2008). VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 99:622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prockop DJ. (2009). Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17:939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K. and Zhou C. (2008). Comparative analysis of mesenchymal stem cells from bone marrow, cartilage and adipose tissue. Stem Cells Dev 17:761–773 [DOI] [PubMed] [Google Scholar]
- 34.Hass R, Kasper C, Böhm S. and Jacobs R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty P, Thakur AM, Ravindran G. and Viswanathan C. (2011). Directed therapeutic angiogenesis by mesenchymal stem cells from umbilical cord matrix in preclinical model of ischemic limb disease. Stem Cell Studies 1:97–104 [Google Scholar]
- 36.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK. and Kang KS. (2006). Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells 24:1620–1626 [DOI] [PubMed] [Google Scholar]
- 37.Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX, Wang YQ, Cao XF, Lv J, et al. (2012). Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev 21:3289–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nascimento DS, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, Araújo AF, Valente M, Almeida J, et al. (2014). Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling following myocardial infarction by pro-angiogenic, anti-apoptotic and endogenous cell activation mechanisms. Stem Cell Res Ther 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper K. and Viswanathan C. (2011). Establishment of a mesenchymal stem cell bank. Stem Cells Int 2011:905621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shetty P, Cooper K. and Viswanathan C. (2010). Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci 4:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall J. (2011). Transwell(®) invasion assays. Methods Mol Biol 769:97–110 [DOI] [PubMed] [Google Scholar]
- 42.Wu FT, Stefanini MO, Mac Gabhann F, Kontos CD, Annex BH. and Popel AS. (2010). A systems biology perspective on sVEGFR1: its biological function, pathogenic role & therapeutic use. J Cell Mol Med 14:528–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X. and Kerbel RS. (2004). A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res 2:315–326 [PubMed] [Google Scholar]
- 44.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ. and Horwitz E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- 45.Zhu SF, Zhong ZN, Fu XF, Peng DX, Lu GH, Li WH, Xu HY, Hu HB, He JM, Su WY. and He YL. (2013). Comparison of cell proliferation, apoptosis, cellular morphology and ultrastructure between human umbilical cord and placenta-derived mesenchymal stem cells. Neurosci Lett 541:77–82 [DOI] [PubMed] [Google Scholar]
- 46.Secco M, Moreira YB, Zucconi E, Vieira NM, Jazedje T, Muotri AR, Okamoto OK, Verjovski-Almeida S. and Zatz M. (2009). Gene expression profile of mesenchymal stem cells from paired umbilical cord units: cord is different from blood. Stem Cell Rev 5:387–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y, Kim H, Cho H, Bae Y, Suh K. and Jung J. (2007). Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemi. Cell Physiol Biochem 20:867–876 [DOI] [PubMed] [Google Scholar]
- 48.Hsiao ST, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY. and Dilley RJ. (2012). Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 21:2189–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oskowitz A, McFerrin H, Gutschow M, Carter ML. and Pochampally R. (2011). Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res 6:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoch AI, Binder BY, Genetos DC. and Leach JK. (2012). Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One 7:e35579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hung SC, Pochampally RR, Chen SC, Hsu SC. and Prockop DJ. (2007). Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 25:2363–2370 [DOI] [PubMed] [Google Scholar]
- 52.Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, Matsushime H. and Sato M. (1990). Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5:519–524 [PubMed] [Google Scholar]
- 53.Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG. and Lemischka IR. (1991). A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci U S A 88:9026–9030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okuyama H, Krishnamachary B, Zhou YF, Nagasawa H, Bosch-Marce M. and Semenza GL. (2006). Expression of vascular endothelial growth factor receptor 1 in bone marrow-derived mesenchymal cells is dependent on hypoxia-inducible factor 1. J Biol Chem 281:15554–15563 [DOI] [PubMed] [Google Scholar]
- 55.Kendall RL. and Thomas KA. (1993). Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 90:10705–10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsatsaris V, Goffin F. and Foidart JM. (2004). Circulating angiogenic factors and preeclampsia. N Engl J Med 350:2003–2004 [DOI] [PubMed] [Google Scholar]
- 57.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, et al. (2006). VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290:H560–H576 [DOI] [PubMed] [Google Scholar]
- 58.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R. and Charnock-Jones DS. (1998). A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation of preeclampsia. Biol Reprod 59:1540–1548 [DOI] [PubMed] [Google Scholar]
- 59.Ahmad S. and Ahmed A. (2004). Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95:884–891 [DOI] [PubMed] [Google Scholar]
- 60.Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G. and Marmé D. (2001). Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis 4:143–154 [DOI] [PubMed] [Google Scholar]
- 61.Gruber R, Kandler B, Holzmann P, Vögele-Kadletz M, Losert U, Fischer MB. and Watzek G. (2005). Bone marrow stromal cells can provide a local environment that favors migration and formation of tubular structures of endothelial cells. Tissue Eng 11:896–903 [DOI] [PubMed] [Google Scholar]
- 62.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW. and Bautch VL. (2004). The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood 103:4527–4535 [DOI] [PubMed] [Google Scholar]
- 63.Kappas NC, Zeng G, Chappell JC, JB Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH. and Bautch VL. (2008). The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol 181:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chappell JC, Taylor SM, Ferrara N. and Bautch VL. (2009). Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell 17:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lorquet S, Berndt S, Blacher S, Gengoux E, Peulen O, Maquoi E, Noël A, Foidart JM, Munaut C. and Péqueux C. (2010). Soluble forms of VEGF receptor-1 and -2 promote vascular maturation via mural cell recruitment. FASEB J 24:3782–3795 [DOI] [PubMed] [Google Scholar]
- 66.Matrisian LM. (1992). The matrix-degrading metalloproteinases. Bioessays 14:455–463 [DOI] [PubMed] [Google Scholar]
- 67.Nagase H. and Woessner JF., Jr. (1999). Matrix metalloproteinases. J Biol Chem 274:21491–21494 [DOI] [PubMed] [Google Scholar]
- 68.Lozito TP. and Tuan RS. (2011). Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol 226:385–396 [DOI] [PubMed] [Google Scholar]
- 69.Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R. and Trounson A. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 175:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N. and Phinney DG. (2003). Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 100:8407–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamoreaux WJ, Fitzgerald ME, Reiner A, Hasty KA. and Charles ST. (1998). Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res 55:29–42 [DOI] [PubMed] [Google Scholar]
- 72.Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A, Asosingh K, Erzurum S. and Anand-Apte B. (2010). Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol 176:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akahane T, Akahane M, Shah A, Connor CM. and Thorgeirsson UP. (2004). TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res 301:158–167 [DOI] [PubMed] [Google Scholar]
- 74.Glaeser JD, Geissler S, Ode A, Schipp CJ, Matziolis G, Taylor WR, Knaus P, Perka C, Duda GN. and Kasper G. (2010). Modulation of matrix metalloprotease-2 levels by mechanical loading of three-dimensional mesenchymal stem cell constructs: impact on in vitro tube formation. Tissue Eng Part A 16:3139–3148 [DOI] [PubMed] [Google Scholar]