Abstract
We document the anti-HIV activity of nitazoxanide (NTZ), the first member of the thiazolide class of antiinfective drugs, originally effective against enteritis caused by Cryptosporidium parvum and Giardia lamblia. NTZ has been administered extensively worldwide, with no severe toxicities associated with its use. Here, we show for the first time that NTZ decreases HIV-1 replication in monocyte-derived macrophages (MDM) if present before or during HIV-1 infection. This NTZ effect is associated with downregulation of HIV-1 receptors CD4 and CCR5, and increasing gene expression of host cell anti-HIV resistance factors APOBEC3A/3G and tetherin. As NTZ is already in clinical use for other conditions, this newly described anti-HIV activity in MDM may facilitate innovative intensification strategies against HIV-1 when combined with current antiretroviral drug regimens.
Although great strides have been made in the fight against HIV/AIDS, more than 33 million people are infected with HIV-1, and infection results in the need for lifelong treatment with antiretroviral therapy.1 The two main HIV-1 cellular targets are CD4+ T lymphocytes and macrophages. Loss of CD4+ T cells by apoptosis as a result of both HIV-1 and bystander mechanisms2 is one of the hallmarks of HIV. In contrast, there is no strong evidence to support the decline of resident tissue macrophages during chronic infection.3–5 HIV-1 infection of macrophages contributes to viral pathogenesis, immune deregulation (activation), and viremia during late stage disease.3,4
Nitazoxanide (NTZ, marketed in the United States as Alinia, Romark Laboratories, L.C.) is the first of a new class of small molecules, the thiazolides, licensed in 2002 and originally developed for the treatment of diarrhea in patients with Cryptosporidium parvum and Giardia lamblia.6 NTZ has also recently shown antiviral activity against influenza,7 rotavirus,8,9 and hepatitis B virus (HBV) and hepatitis C virus (HCV)10 in cell culture and in patients.11,12 NTZ was shown to have activity against HCV and HBV infection through suppressing viral replication10 by increasing the phosphorylation of eIF2α, which plays a role in the interferon (IFN)-induced antiviral response.13 Furthermore, in a cohort of patients infected with HCV Genotype 4, NTZ was added to standard of care (SOC) with Peg-IFN-α-2a and compared against SOC. The addition of NTZ resulted in both a more rapid virologic response and higher rates of sustained virologic response (79% vs. 50%, p=0.023)11,12,14 supporting the potential for NTZ to intensify antiviral regimens. NTZ activity against HCV Genotype 1 has been more limited.15,16 Here, we tested the hypothesis that NTZ can control HIV-1 replication in monocyte-derived-macrophages infected in vitro with CCR5-using strains of virus, and we investigate the mechanisms that might facilitate the anti-HIV-1 effect.
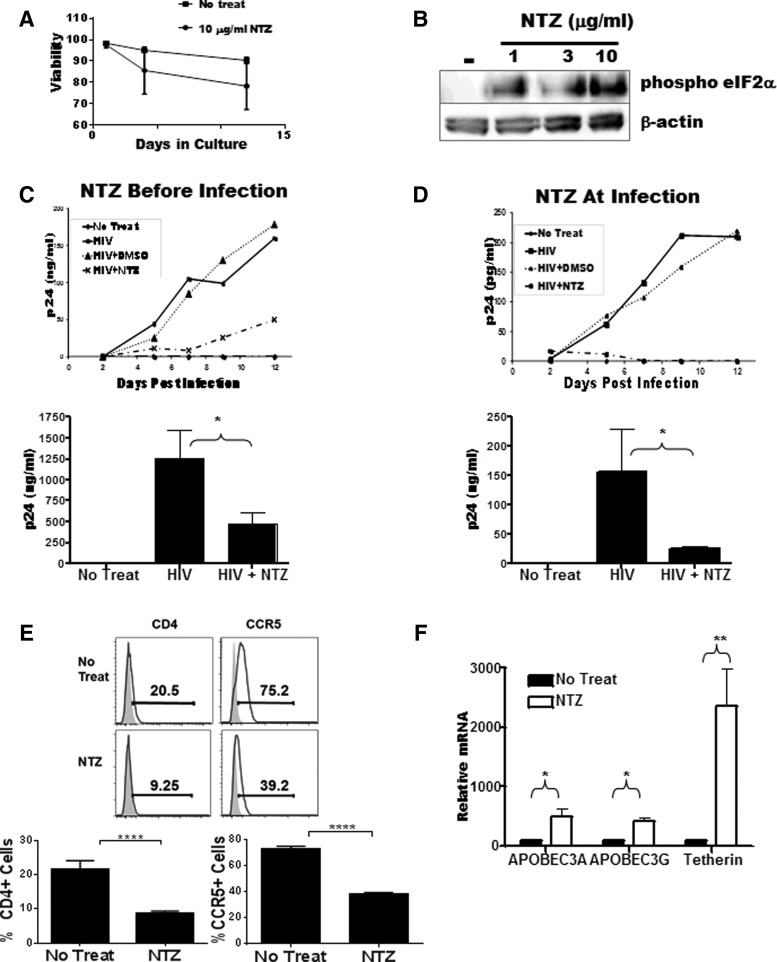
To first establish the biological activity of NTZ on monocyte-derived macrophage (MDM) viability and modulation, we incubated freshly elutriated monocytes (5×105 cells/condition obtained from the University of Pennsylvania Human Immunology Core facility) in sterile Teflon pots in complete culture medium (RPMI 1640, Sigma) supplemented with 5% human pooled sera, 10 ng/ml MCSF (Peprotech, Rocky Hill, NJ), and increasing concentrations of NTZ (0 μg/ml to 30 μg/ml) (Romark Laboratories, Tampa, FL). After 48 h, whole cell lysates were harvested and the viability and activity of NTZ were evaluated by cell dye-exclusion and Caspase 3 activation, while assaying for the expression of phosphorylated eIF2α. To first test viability, cells were harvested every 2 days for 12 days and assayed for active Caspase 3 (BD Biosciences, San Jose, CA) induction to detect apoptosis. MDM cultured in the presence of NTZ showed no significant change in survival as compared to cells cultured in the absence of NTZ (Fig. 1A). As described in cell lines but not yet in MDM,13 Western blot analysis of MDM at 48 h showed that NTZ exhibited a dose-dependent increase in phospho-eIF2α (Cell Signaling Technology, Inc., Danvers, MA) peaking at 10 μg/ml NTZ. β-Actin (Cell Signaling Technology, Inc.) was used for protein loading control (Fig. 1B).
FIG. 1.
Antiviral activity of nitazoxanide (NTZ) in HIV-infected human monocyte-derived macrophages (MDM). (A) Evidence of NTZ activity as measured by eIF2α phosphorylation in freshly isolated elutriated monocytes incubated for 48 h in the presence of 1 (μg/ml, 3 (μg/ml, and 10 (μg/ml of NTZ. (B) MDM cultured in the presence of 10 (μg/ml NTZ showed no significant decrease in cell viability compared with cells cultured in the absence of NTZ. (C) MDM incubated with 10 (μg/ml NTZ before/during or (D) after (right) viral adsorption via spinoculation with 50 ng/ml p24 equivalent of HIV-1 Bal. Virus output in both (C) and (D) on top is shown every 2 days until 12 days postinfection by p24 ELISA whereas the means±standard errors of three independent experiments at day 12 postinfection are shown at the bottom. (E) The expression of HIV-1 receptors CD4 (left) and CCR5 (right) in MDMs incubated in the absence (top) or presence (bottom) of 10 (μg/ml NTZ for 72 h. Shaded histogram represents the isotype control. Bottom panel shows a composite of CD4 and CCR5 staining from four different donors. (F) NTZ negatively regulates early and late stages in the HIV-1 lifecycle. MDMs were treated with 10 (μg/ml NTZ (filled bars) and assayed for the expression of APOBEC3A/3G and tetherin. The solid bar represents the untreated control. The bar graph represents the mean of three independent experiments±standard error. In all panels, an unpaired t-test was used to test differences with an asterisk representing a p value less than 0.05.
Having established a bioactive NTZ concentration in MDM via changes in eIF2α phosphorylation without impairing MDM viability, we next investigated whether NTZ could block HIV-1 infection and replication in MDM. We tested the ability of NTZ to regulate HIV-1 infection and replication in MDM by the addition of NTZ before/during (pre) or after (post) HIV-1 adsorption. Five×106 cells/ml elutriated monocytes were differentiated into MDM for 4 days in 24-well plates using media as above. At day 5 of culture, 10 μg/ml NTZ was added and the cells were preincubated for an additional 3 days (72 h) before the addition of HIV-1. HIV-1Bal (equivalent to 50 ng p24, University of Pennsylvania Center for AIDS Research), a CCR5 using laboratory-adapted strain of HIV-1, was added in the presence of NTZ and the cells were spinoculated at 1,800 rpm for 3 h to maximize viral binding. The virus-containing supernatant was removed and the virus exposed cells incubated for an additional 12 days with NTZ replenished every 2 days. A sample of the culture supernatant was harvested on day 0 and every 2 days thereafter at the time of media change. To test the effects of NTZ if added after (post) adsorption, MDMs were cultured and spinoculated as above with HIV exposure, to be immediately followed by three warm 1×phosphate-buffered saline (PBS) washes prior to the addition of 10 μg/ml NTZ and subsequent culture as above. Using HIV p24 antigen levels as a measure of viral replication (ELISAs measured at the University of Pennsylvania CFAR) in supernatant, we observed that NTZ treatment initiated before/during (pre) or after (post) infection resulted in a 2- to 10-fold decrease in viral replication when compared to infected untreated control MDMs. These observations were reproduced in three independent experiments (Fig. 1C and D).
We next investigated the mechanism of action by which NTZ may be reducing HIV replication in MDM. First, to determine whether NTZ affects HIV-1 binding or entry, we analyzed the modulation of cell-surface HIV-1 receptor and coreceptor (CD4 and CCR5, respectively) expression on MDMs after a 72-h exposure to 10 μg/ml NTZ. Cells incubated in the presence of NTZ showed a significant decrease in cell surface expression of both CD4 and CCR5 (Fig. 1E), which is consistent with a lower infection and subsequent viral replication outcome as shown above.
Second, we tested whether regulation of interferon-stimulated genes associated with inhibition of HIV-117–20 was induced with NTZ as thiazolides have been shown to interfere with viral infection through activation of type I interferon-associated mechanisms.13,21,22 We analyzed the modulation of two known type I interferon-induced negative regulators of viral replication (ABOBEC 3G and tetherin23–28) following 48-h incubation of MDM with 10 μg/ml NTZ. We utilized the following real-time reverse transcriptase polymerase chain reaction (RT-PCR) primers to characterize the expression of IFN-response genes following NTZ exposure, i.e., tetherin, primer sequence: 5′-AAG AAA GTG GAG GAG CTT GAG G-3′ (forward); 5′-CCT GGT TTT CTC TTC TCA GTC G-3′ (reverse), APOBEC3A, primer sequence: 5′-TTC TTT GCA GTT GGA CCC GG-3′ (forward); 5′-CTC ATC TAG TCC ATC CCA GG-3′ (reverse), and APOBEC3G, primer sequence: 5′-TTA CCT GCT TCA CCT CCT GG-3′ (forward); 5′-TCA TCT AGT CCA TCC CAG GG-3′ (reverse). β-Actin was used as an internal control; primer sequence: 5′-TTC CTG GGG ATG GAG TC-3′ (forward); 5′-CAG GTC TTT GCG GAT GTC-3′ (reverse). Our data showed that both APOBEC3A/G, and tetherin, potent anti-HIV host factors,17,24–26 were significantly upregulated in MDMs treated exogenously with NTZ (Fig. 1F), supporting the belief that NTZ may be acting by limiting infection at early and late stages of the viral life cycle. As NTZ is metabolized in vivo to tizoxanide,29 we independently confirmed that the latter metabolite (having better bioavailability in the blood) if added in vitro, as described for NTZ above, resulted in similar suppressive activity against HIV-1 replication via the upregulation of tetherin and APOBEC 3A/G (data not shown).
Taken together, these data indicate that NTZ can negatively regulate early and late stages in the infection cycle including (1) decreased binding and fusion via downregulation of CD4 and CCR5, (2) impairment of particle infectivity by competing against viral DNA synthesis through an APOBEC-dependent mechanism,23,28,30 and (3) inhibition of viral particle release via upregulation of tetherin.27,31 Our data do not exclude a role for added interferon-mediated antiviral mechanisms associated with interferon signaling, e.g., MX2.19,32,33
NTZ anti-HIV activity has also been suggested by a recent report by Tan et al.34 that describes a novel drug screening method used to identify strongly efficacious and synergistic drug pairs, with the overall goal of expanding current antiretroviral therapy (ART) repertoires. This study found that in a T cell line (Sup T1), NTZ synergizes with known HIV drugs (integrase inhibitors, nucleoside and nonnucleoside reverse transcription inhibitors), exerting its antiviral effect post HIV-1 entry, before or at reverse transcription. Although the SupT1 data support an antiviral activity for NTZ in T cells, future experiments will need to directly establish NTZ's anti-HIV activity in primary CD4 T cells.
The recent demonstration that type-I interferons, when added to ART, can mediate HIV-1 suppression in the subsequent absence of ART and result in a decrease of total HIV DNA, raises the priority for strategies that may induce similar effects.35,36 Our study supports the premise that NTZ may provide an interferon-like effect on monocyte-derived macrophages, thereby acting to decrease the HIV load within this cell subset in vivo. Taken together, our present data support the hypothesis that NTZ could intensify ART-based strategies by activation of anti-HIV host mechanisms in MDM otherwise not targeted by current ART regimens. Future experiments will need to quantify the HIV cellular infection rate or expression in tissue macrophages of subjects receiving NTZ therapy in conjunction with ART to explore this hypothesis further.
Acknowledgments
This work was supported by National Institutes of Health Grant A1047760 and a Wistar Cancer Center Support grant (National Cancer Institute grant P30 CA010815).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS JUNPoHA: UNAIDS Report on the Global AIDS Epidemic, 2012
- 2.Badley AD, Pilon AA, Landay A, and Lynch DH: Mechanisms of HIV-associated lymphocyte apoptosis. Blood 2000;96:2951–2964 [PubMed] [Google Scholar]
- 3.Orenstein JM: The macrophage in HIV infection. Immunobiology 2001;204:598–602 [DOI] [PubMed] [Google Scholar]
- 4.Orenstein JM, Fox C, and Wahl SM: Macrophages as a source of HIV during opportunistic infections. Science 1997;276:1857–1861 [DOI] [PubMed] [Google Scholar]
- 5.Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, et al. : Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis 2013;209:739–748 [DOI] [PubMed] [Google Scholar]
- 6.Keeffe EB: Advances in hepatology: Current developments in the treatment of hepatitis and hepatobiliary disease. Gastroenterol Hepatol (NY) 2009;5:620–622 [PMC free article] [PubMed] [Google Scholar]
- 7.Rossignol JF, La Frazia S, Chiappa L, Ciucci A, and Santoro MG: Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem 2009;284:29798–29808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Frazia S, Ciucci A, Arnoldi F, Coira M, Gianferretti P, Angelini M, et al. : Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J Virol 2013;87:11096–11106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossignol JF, Abu-Zekry M, Hussein A, and Santoro MG: Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: Randomised double-blind placebo-controlled trial. Lancet 2006;368:124–129 [DOI] [PubMed] [Google Scholar]
- 10.Korba BE, Montero AB, Farrar K, Gaye K, Mukerjee S, Ayers MS, and Rossignol JF: Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res 2008;77:56–63 [DOI] [PubMed] [Google Scholar]
- 11.Rossignol JF, Elfert A, El-Gohary Y, and Keeffe EB: Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology 2009;136:856–862 [DOI] [PubMed] [Google Scholar]
- 12.Rossignol JF, Elfert A, and Keeffe EB: Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol 2010;44:504–509 [DOI] [PubMed] [Google Scholar]
- 13.Elazar M, Liu M, McKenna SA, Liu P, Gehrig EA, Puglisi JD, et al. : The anti-hepatitis C agent nitazoxanide induces phosphorylation of eukaryotic initiation factor 2alpha via protein kinase activated by double-stranded RNA activation. Gastroenterology 2009;137:1827–1835 [DOI] [PubMed] [Google Scholar]
- 14.Esmat G, El Raziky M, El Kassas M, Hassany M, and Gamil ME: The future for the treatment of genotype 4 chronic hepatitis C. Liver Int 2012;32(Suppl 1):146–150 [DOI] [PubMed] [Google Scholar]
- 15.Amorosa VK, Luetkemeyer A, Kang M, Johnson VA, Umbleja T, Haas DW, et al. : Addition of nitazoxanide to PEG-IFN and ribavirin to improve HCV treatment response in HIV-1 and HCV genotype 1 coinfected persons naive to HCV therapy: Results of the ACTG A5269 trial. HIV Clin Trials 2013;14:274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laufer N, Abusamra L, Bolcic F, Gun A, Rolon MJ, Perez H, et al. : No reduction of HCV viral load in HIV patients co-infected with HCV genotype 1 during a 30 days course of nitazoxanide monotherapy. Antiviral Res 2011;92:497–499 [DOI] [PubMed] [Google Scholar]
- 17.Bitzegeio J, Sampias M, Bieniasz PD, and Hatziioannou T: Adaptation to the interferon-induced antiviral state by human and simian immunodeficiency viruses. J Virol 2013;87:3549–3560 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 18.Bowie AG. and Unterholzner L: Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 2008;8:911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, et al. : MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 2013;502:563–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khabar KS. and Young HA: Post-transcriptional control of the interferon system. Biochimie 2007;89:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clerici M, Trabattoni D, Pacei M, Biasin M, and Rossignol J-F: The anti-infective nitazoxanide shows strong immunomodulating effects. J Immunol 2011;186:155 [Google Scholar]
- 22.Wang C ZX, Osinusi A, Masur H, Fisbein D, and Kottilil S: Augmentation of interferon signaling pathway by nitazoxanide: A novel therapeutic strategy for relapsers to peg-interferon and ribavirin therapy. Hepatology 2010;52(Suppl):1213A [Google Scholar]
- 23.Aguiar RS. and Peterlin BM: APOBEC3 proteins and reverse transcription. Virus Res 2008;134:74–85 [DOI] [PubMed] [Google Scholar]
- 24.Franca R, Spadari S, and Maga G: APOBEC deaminases as cellular antiviral factors: A novel natural host defense mechanism. Med Sci Monit 2006;12:RA92–98 [PubMed] [Google Scholar]
- 25.Izumi T, Shirakawa K, and Takaori-Kondo A: Cytidine deaminases as a weapon against retroviruses and a new target for antiviral therapy. Mini Rev Med Chem 2008;8:231–238 [DOI] [PubMed] [Google Scholar]
- 26.Neil SJ: The antiviral activities of tetherin. Curr Top Microbiol Immunol 2012;371:67–104 [DOI] [PubMed] [Google Scholar]
- 27.Neil SJ, Zang T, and Bieniasz PD: Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008;451:425–430 [DOI] [PubMed] [Google Scholar]
- 28.Romani B, Engelbrecht S, and Glashoff RH: Antiviral roles of APOBEC proteins against HIV-1 and suppression by Vif. Arch Virol 2009;154:1579–1588 [DOI] [PubMed] [Google Scholar]
- 29.Stockis A, Deroubaix X, Lins R, Jeanbaptiste B, Calderon P, and Rossignol JF: Pharmacokinetics of nitazoxanide after single oral dose administration in 6 healthy volunteers. Int J Clin Pharmacol Ther 1996;34:349–351 [PubMed] [Google Scholar]
- 30.Argyris EG, Acheampong E, Wang F, Huang J, Chen K, Mukhtar M, and Zhang H: The interferon-induced expression of APOBEC3G in human blood-brain barrier exerts a potent intrinsic immunity to block HIV-1 entry to central nervous system. Virology 2007;367:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, and Bieniasz PD: Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 2009;139:499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, et al. : Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 2013;502:559–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Pan Q, Ding S, Qian J, Xu F, Zhou J, et al. : The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 2013;14:398–410 [DOI] [PubMed] [Google Scholar]
- 34.Tan X, Hu L, Luquette LJ, 3rd, Gao G, Liu Y, Qu H, et al. : Systematic identification of synergistic drug pairs targeting HIV. Nat Biotechnol 2012;30:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, et al. : Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis 2013;207:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun H, Buzon MJ, Shaw A, Berg RK, Yu XG, Ferrando-Martinez S, et al. : Hepatitis C therapy with interferon-alpha and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis 2014;209:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]