Abstract
We report here the novel finding that HIV-negative factor (Nef) protein is present in considerable numbers of peripheral blood mononuclear cells (PBMCs) from viremic HIV-infected patients not on antiretroviral therapy (ART) and also in patients receiving virologically suppressive ART, though to a smaller degree. Interestingly, these Nef-positive PBMCs constitute predominantly uninfected bystander cells. These results may explain systemic pathology in HIV patients, even in those receiving ART.
Negative factor (Nef) is an important HIV pathogenic factor, as demonstrated by the lack of disease progression in patients whose HIV strains are Nef deleted.1 Nef enhances virus production in vivo,2 contributes to T cell activation by enhancing the response of infected cells to exogenous stimulation, and impairs cellular immunity by down-regulating cell surface molecules such as CD4 T cell receptor and the major histocompatibility complex class I (MHC I).3–7 In transgenic mice, CD4-promoter-driven Nef expression in T cell and monocytes causes a spectrum of AIDS-like and end organ pathologies.8 On biochemical and cell biology levels, Nef is characterized as an SH3 domain binding intracellular protein. It is myristoylated, and thus a significant portion of Nef is membrane associated. The SH3 binding domain has been identified as critical for altering cell signaling in infected cells.1 This altered signaling together with myristoylation of Nef also affects membrane dynamics, leading to a number of activities including Nef-mediated induction of cell protrusions in T lymphocytes9 and transfer of Nef to other cells through conduit-like nanotubes10–12 or to secretion in exosomes.13
Conduit formation between T cells may present a novel route for HIV transmission, while Nef transfer to B cells was shown to impair antibody class switching.14 It is our hypothesis that if antiretroviral therapy (ART) reduces HIV virion production but not Nef gene expression as previously described,15 then the persistence of Nef expression might contribute to the higher risk of Nef-induced pathologies in those on ART.16 We therefore performed a preliminary investigation to assess the detection of Nef-positive circulating cells from both ART-untreated and ART-treated HIV-infected patients.
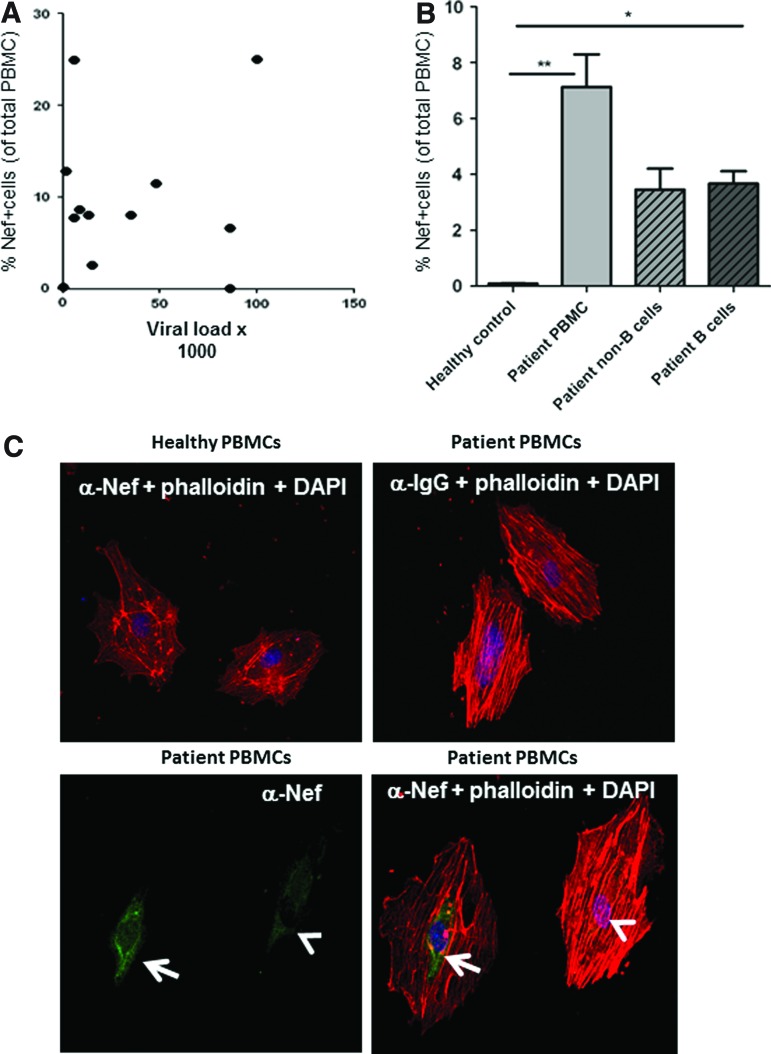
Peripheral blood mononuclear cells (PBMCs) were isolated from HIV-infected patients and uninfected controls by standard Ficoll purification. Cells were fixed with 4% paraformaldehyde and then permeabilized with 0.01% Triton X-100 prior to staining for Nef using the monoclonal anti-Nef antibody EH1 (NIH AIDS repository),17 which we have validated to be suitable for FACS analysis of Nef protein. We found an unexpectedly high mean (SD) 9. 6±8.1% number of PBMCs from HIV-infected untreated viremic patients that stained positive for Nef (Table 1 and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). We confirmed these results with five of the samples using a commercially available Nef antibody (3D12, Abcam) that recognizes a different epitope, and observed similar Nef staining (p=0.84, n=5). Of note, Nef positivity did not correlate with viral titers (r=0.017; p=0.7, Fig. 1A). Furthermore, one of the HIV patients exhibited very low Nef dissemination in PBMCs, even though viral titers were high (Table 1, patient 7). The identification of mechanisms underlying this low spreading tendency of Nef, such as the possibility of a mutation impairing Nef protein transfer, would be interesting for therapeutic targeting of Nef.
Table 1.
Nef Is Detectable in Peripheral Blood Mononuclear Cells from HIV Patients With/Without Antiretroviral Therapy
| CD4 counts (cells/μl) | Viral load RNA (copies/ml) | Nef % of total PBMCs | ART duration (weeks) | |
|---|---|---|---|---|
| Pooled uninfected controls | N/A | N/A | 0.09 | N/A |
| Patient 1 | 356 | 5,700 | 7.78 | 0 |
| Patient 2* | 797 | 8,400 | 8.66 | 0 |
| Patient 3* | 413 | 1,490 | 12.84 | 0 |
| Patient 4* | 913 | 47,800 | 11.46 | 0 |
| Patient 5* | 315 | 13,000 | 8.04 | 0 |
| Patient 6 | 840 | <50 | 0.20 | 0 |
| Patient 7 | 321 | 85,800 | 0.02 | 0 |
| Patient 8 | 503 | 35,000 | 8.02 | 0 |
| Patient 9* | 593 | 100,000 | 25.02 | 0 |
| Patient 10 | 321 | 5,600 | 24.9 | 0 |
| Patient 11 | 395 | 86,000 | 6.59 | 0 |
| Patient 12 | 978 | 15,000 | 2.94 | 0 |
| Patient 13 | 855 | <50 | 1.05 | 811 |
| Patient 14 | 234 | <50 | 1.09 | 32 |
| Patient 15 | 254 | <50 | 1.41 | 48 |
| Patient 16 | 205 | <50 | 1.23 | 16 |
| Patient 17 | 855 | <50 | 0.95 | 780 |
Percentage of Nef+ peripheral blood mononuclear cells (PBMCs) (stained with Eh1 antibody and analyzed by FACS, see also Supplementary Fig. S1) with clinical parameters in patients without and with antiretroviral therapy (ART) (indicated as ART duration). Patients who had Nef staining confirmed with the Abcam 3D12 antibody are marked with an asterisk (*).
FIG. 1.
Nef staining does not correlate with viral load, is detectable in B cells and can be transferred to human endothelial cells. (A) Pearson correlation between viral load and Nef distribution in peripheral blood mononuclear cells (PBMCs) (r=−0.2274, R2=0.05170, n.s.). (B) Comparison of Nef-positive B cell and non-B cell populations in percent of total PBMCs after immune-magnetobead-based separation of CD19-positive B cells. Data represent the mean±SD from three patients in whom measurements were made in triplicate. (C) Nef staining of HUVEC after 24 h in contact with PBMCs from HIV patients shown as Nef staining only (green, left lower panel) or as overlay with red phalloidin staining depicting endothelial-like cytoskeletal actin staining (red and green, right lower panel). Color images available online at www.liebertpub.com/aid
The unexpectedly high levels of Nef-positive PBMCs cannot be explained by direct HIV infection of cells. In fact, Nef antibody staining was significantly more prominent in uninfected HIV-p24-negative cells than in p24-positive cells (Supplementary Fig. S2). Furthermore, the strongest Nef signal was detected in p24-positive cells, which is in line with our hypothesis that Nef protein from infected cells is transferred to bystander cells and thus diluted. Moreover, when we isolated B cells and non-B cells from patient PBMCs using the Miltenyi anti-PE multisort kit and CD19 antibody and stained them for Nef, almost 50% of Nef-positive cells appear to be B cells (Fig. 1B). These data are in line with previous in vitro studies showing that Nef can be transferred from HIV-infected monocytes to B cells.14
Nef is one of the three immediate early HIV genes, which are still transcribed in HIV-infected cells even in those receiving ART.15 Interestingly, we also found significant levels of Nef-positive PBMCs [mean (SD), 1.15±0.178%, p=0.001 compared to noninfected controls] in ART-treated patients with HIV RNA viral loads <50 copies/ml (Table 1). This finding could be explained by transfer of Nef from infected cells located in lymphatic tissues, a major HIV reservoir.18,19 High endothelial venules enable lymphocyte circulation between blood and lymph nodes20 and are most likely in prolonged direct contact with Nef-containing mononuclear cells.21 To test if Nef from lymphatic or blood-derived mononuclear cells could also transfer to venous endothelial cells, we cocultured human umbilical cord vein endothelial cells (HUVEC) with PBMCs from viremic untreated HIV-infected patients for 24 h.
As shown in Fig. 1C (left lower panel), this experiment resulted in strongly (arrow) and less strongly (arrowhead) Nef-positive endothelial cells, which is likely due to different levels of Nef transfer. Of note, these endothelial cells are not leukocytes based on characteristic cytoskeletal morphology as determined by staining with phalloidin-Cy5 (Fig. 1C, right lower panel). Together, our findings suggest that Nef protein may be widely transferred from HIV-infected cells to uninfected blood cells and bystander tissue cells, thus providing a means of pathogenic Nef activity even when virus replication is controlled.
In conclusion, the novel findings of this report are the detection of HIV Nef protein in circulating uninfected bystander PBMCs from HIV-infected patients not on ART and the establishment that Nef is still detectable, albeit at significantly lower levels, in patients receiving virologically suppressive ART. Although beyond the scope of this report, it will be necessary to show that these remaining Nef-positive PBMCs exhibit an altered phenotype; if so, these findings might explain why HIV patients are still at increased risk for HIV complications even in the absence of detectable viral loads in the blood. Given that the power of our study was low and the distribution of Nef in PBMCs demands further characterization by FACS including FACS sorting, additional studies need to address whether determining levels of Nef dissemination in PBMCs could potentially be utilized as a predictor for vascular or other HIV-related end organ diseases.
Supplementary Material
Acknowledgments
This study was supported by the NIH-NHLBI, contract Grants R01 HL095149 (M.C. and S.G.), R01 HL083491 (S.C.F.), T32HL079995 (L.G.), R21 HL120390 (M.C.), and AHA 13PRE14780025 (T.W.). The project described was also supported by the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative and by the Indiana Clinical and Translational Sciences Institute funded in part by Grant TR000006 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 Nef monoclonal antibody (EH1) from Dr. James Hoxie. Ting Wang and Linden A. Green contributed equally to this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Foster JL. and Garcia JV: HIV-1 Nef: At the crossroads. Retrovirology 2008;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kestler HW 3rd, et al. : Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 1991;65:651–662 [DOI] [PubMed] [Google Scholar]
- 3.Lundquist CA, Tobiume M, Zhou J, et al. : Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J Virol 2002;76:4625–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangasarian A, Piguet V, Wang JK, et al. : Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol 1999;73:1964–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz O, Marechal V, Le Gall S, et al. : Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 1996;2:338–342 [DOI] [PubMed] [Google Scholar]
- 6.Garcia JV. and Miller AD: Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 1991;350:508–511 [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry A, et al. : The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J Immunol 2005;175:4566–4574 [DOI] [PubMed] [Google Scholar]
- 8.Weng X, et al. : CD4+T cells from CD4C/HIVNef transgenic mice show enhanced activation in vivo with impaired proliferation in vitro but are dispensable for the development of a severe AIDS-like organ disease. J Virol 2004;78:5244–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobile C, et al. : HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. J Virol 2010;84:2282–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudnicka D, et al. : Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol 2009;83:6234–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudnicka D. and Schwartz O: Intrusive HIV-1-infected cells. Nat Immunol 2009;10:933–934 [DOI] [PubMed] [Google Scholar]
- 12.Xu W, et al. : HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol 2009;10:1008–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenassi M, et al. : HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+T cells. Traffic 2010;11:110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao X, et al. : Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol 2006;7:302–310 [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, et al. : Biphasic decay kinetics suggest progressive slowing in turnover of latently HIV-1 infected cells during antiretroviral therapy. Retrovirology 2008;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friis-Moller N. and Worm SW: Can the risk of cardiovascular disease in HIV-infected patients be estimated from conventional risk prediction tools? Clin Infect Dis 2007;45:1082–1084 [DOI] [PubMed] [Google Scholar]
- 17.Chang AH, Hoxie JA, Cassol S, et al. : Construction of single-chain antibodies that bind an overlapping epitope of HIV-1 Nef. FEBS Lett 1998;441:307–312 [DOI] [PubMed] [Google Scholar]
- 18.Chiueh CC, Andoh T, and Chock PB: Roles of thioredoxin in nitric oxide-dependent preconditioning-induced tolerance against MPTP neurotoxin. Toxicol Appl Pharmacol 2005;207:96–102 [DOI] [PubMed] [Google Scholar]
- 19.Hunt PW: Th17, gut, and HIV: Therapeutic implications. Curr Opin HIV AIDS 2010;5:189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay CR, Marston WL, and Dudler L: Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med 1990;171:801–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stolp B, et al. : HIV-1 Nef interferes with T-lymphocyte circulation through confined environments in vivo. Proc Natl Acad Sci USA 2012;109:18541–18546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.