Abstract
Osteoarthritis (OA) is an irreversible pathology that causes a decrease in articular cartilage thickness, leading finally to the complete degradation of the affected joint. The low spontaneous repair capacity of cartilage prevents any restoration of the joint surface, making OA a major public health issue. Here, we developed an innovative combination of treatment conditions to improve the human chondrocyte phenotype before autologous chondrocyte implantation. First, we seeded human dedifferentiated chondrocytes into a collagen sponge as a scaffold, cultured them in hypoxia in the presence of a bone morphogenetic protein (BMP), BMP-2, and transfected them with small interfering RNAs targeting two markers overexpressed in OA dedifferentiated chondrocytes, that is, type I collagen and/or HtrA1 serine protease. This strategy significantly decreased mRNA and protein expression of type I collagen and HtrA1, and led to an improvement in the chondrocyte phenotype index of differentiation. The effectiveness of our in vitro culture process was also demonstrated in the nude mouse model in vivo after subcutaneous implantation. We, thus, provide here a new protocol able to favor human hyaline chondrocyte phenotype in primarily dedifferentiated cells, both in vitro and in vivo. Our study also offers an innovative strategy for chondrocyte redifferentiation and opens new opportunities for developing therapeutic targets.
Introduction
Osteoarthritis (OA) is a degenerative disease of articular cartilage observed in several joints, with the knee and the hip being the most affected.1 By severely limiting mobility, this pathology changes the lifestyle of 27 million Americans2; in Europe, prevalence reaches about 72% in the 65 years and older population. Nevertheless, and despite Osteoarthritis Research Society International recommendations,3 both pharmacological (nonsteroidal anti-inflammatory medicines, analgesics, and anti-arthritic medicines) and nonpharmacological treatments (exercise, weight reduction) are unable to restore a hyaline functional cartilage, even if some of them improve the patient's life. Although there are now new molecular targets,4 since 1956, in patients suffering from severe OA, the affected joint is commonly removed and replaced with an orthopedic prosthesis.5 Currently, there are some methods that can repair the affected joint, such as osteochondral allograft transplantation,6 but most of them do not restore functional hyaline cartilage and generally result in a fibrocartilage, which is more rapidly degraded.7 One of the most promising methods for repairing cartilage is based on Brittberg's procedure.8 However, chondrocyte amplification in 2D, in addition to the catabolic phenotype, induces chondrocyte dedifferentiation with inappropriate type I collagen synthesis.9 Therefore, several studies have attempted to maintain chondrocytes in a differentiated state before autologous chondrocyte implantation (ACI).10–16 Some factors are known to improve the chondrocyte phenotype, such as oxygen tension.13,17–19 At less than 5% oxygen, chondrocytes synthesize higher amounts of type II collagen and/or aggrecan,13,17 which confer the two essential biomechanical properties of cartilage: tension resistance and viscoelasticity.20 The viscoelastic properties of cartilage, due to its biphasic characteristics, are essential. Engineered chondrocyte constructs lacking a homogeneous and structural extracellular matrix (ECM) are unable to support physiological loads and are, therefore, unlikely to function successfully on implantation. Inside the joint, chondrocytes live in hypoxia with a variable oxygen percentage, from 0.5% to 5%, depending on depth.21 Furthermore, their microenvironment has a tridimensional structure. Consequently, attention has turned to the design of an efficient biomaterial to mimic natural living conditions of chondrocytes10–16 in conjunction with hypoxia (i.e., low oxygen concentrations).16–19 Nevertheless, pro-chondrogenic factors, such as bone morphogenetic proteins (BMPs) or the transforming growth factor-beta (TGF-β) family, have already shown their ability to induce markers specific to cartilage.22,23 BMP-2 is currently the best candidate among all the BMPs for improving the chondrocyte phenotype at low concentrations, although it can induce hypertrophy and osteogenesis at high concentrations.24 In a previous study using dedifferentiated chondrocytes from OA patients, we demonstrated in vitro that the chondrocytes recover their differentiated phenotype and that the index of differentiation is higher in hypoxia than in normoxia after 7 days of culture with BMP-2 (50 ng/mL) in type I collagen sponges.18 Furthermore, in OA cartilage, several proteases show increased expression, including the high temperature requirement factor A1 (HtrA1).25–27 HtrA1 mRNAs increased seven-fold in OA cartilage compared with healthy cartilage.28 This protease is particularly involved in the proteolysis of aggrecan and in the degradation of proteins from the TGF-β receptor family.29,30
In this work, we developed a combinatory cellular therapy strategy to promote a differentiated chondrocyte phenotype in human articular chondrocytes (HACs). We first cultured HACs for 7 days in hypoxia in type I collagen sponges with BMP-2, and transfected the HACs with small interfering RNAs (siRNAs). We successfully silenced type I collagen by using specific siRNAs and increased type II collagen and aggrecan, both in vitro and in vivo. We then used a siRNA targeting HtrA1 to prevent the degradation of BMP and TGF-β receptors and that of ECM proteins such as collagens and aggrecan. Taken together, our results show that this strategy helped increase cartilage-specific markers (type II collagen and aggrecan) and decrease nonspecific markers (type I collagen and HtrA1), providing recovery of chondrocyte differentiation.
Materials and Methods
Chondrocyte isolation and culture
HACs were prepared from macroscopically healthy zones of femoral heads of patients undergoing joint arthroplasty (age range: 52–83) as previously described.17,18 All patients signed an informed consent agreement form, which was approved by the Local Ethics Committee. Chondrocytes were seeded at 4×104 cells/cm2 in plastic vessels. They were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS; Invitrogen Life Technologies) and antibiotics, under 5% CO2. At confluence, and after one passage, cells were harvested by trypsinization (0.25% trypsin/1 mM EDTA; Invitrogen). For redifferentiation studies, we used sponges composed of native type I collagen (90–95%) and type III collagen (5–10%) from calf skin, manufactured by Symatèse Biomatériaux. Sponges were 2 mm thick and 5 mm in diameter. They were cross-linked using glutaraldehyde to increase their stability and sterilized with β radiation. HACs were seeded onto the sponges (10×106 cells/cm3) in 96-well culture plates and incubated at 37°C under 5% CO2 for 16 h with DMEM+2% FCS. The next day, cells were incubated in DMEM+2% FCS pre-equilibrated to 3% O2 by bubbling, with or without 50 ng/mL of BMP-2 (R&D Systems). This point established day 0, and HACs were incubated in hypoxia (3% O2) for 7 days. Hypoxic cultures were performed in a sealed chamber as previously described.17
Gene silencing experiments
After one passage (P1), HACs were harvested by trypsinization, centrifuged, and seeded onto type I collagen sponges as described earlier.18 At day zero, HACs were transfected with a mix of INTERFERin™ (Polyplus-transfection SA; 3 μL), OptiMEM (Invitrogen; 100 μL) and small interfering RNA (siRNA) for 10 min at room temperature (siRNAs were used at 5 nM for the analysis of chondrocyte phenotype and at 50 nM for kinetics and in vivo studies). Then, the INTERFERin®-siRNA complex was supplemented with 200 μL DMEM+2% FCS, pre-equilibrated to 3% O2 by bubbling, with or without 50 ng/mL of BMP-2. HACs were then cultured in hypoxia (3% O2) until day 7 or 10, and the medium was changed (DMEM+2% FCS) on day 2, 5, and 7. siRNAs specifically targeted the COL1A1 mRNA (target sequence: 5′-ACCAATCACCTGCGTACAGAA-3′; Qiagen), the HtrA1 mRNA (target sequence: 5′-CGGCCGAAGTTGCCTCTTTT-3′; Eurogentec), or a negative control (NC; target sequence: 5′-AATTCTCCGAACGTGTCACGT-3′; Qiagen).
RT-PCR analysis
Total RNA was extracted using TRIzol Reagent® (Invitrogen). After extraction, 1 μg of DNase I-treated total RNA was reverse transcribed at 37°C for 1 h into complementary DNA (cDNA) and processed as previously described.30 Real-time PCR amplifications were performed with SYBR Green PCR Master mix. Sequence-specific primers were designed with “Primer Express” software (Applied Biosystems).18,31 Thermal cycling parameters were as previously described,31 and amplification was carried out with an ABI Prism 7000 Sequence Detection System (Applied Biosystems). Ribosomal protein L13a (RPL13a) was used as an endogenous reference gene. The relative gene expression was calculated using the 2−ΔΔCt method or the standard curve method depending on the efficiency of the amplification of RPL13a and each target gene, and expressed as the mean of triplicate samples.32
The sequences of the primers used are as follows:
COL1A1, S: 5′- CACCAATCACCTGCGTACAGAA-3′, AS: 5′-CAGATCACGTCATCGCACAAC-3′;
COL2A1, S: 5′-GGCAATAGCAGGTTCACGTACA-3′, AS: 5′-CGATAACAGTCTTGCCCCACTT-3′;
COL9A1, S: 5′-GCCAATCCTGATCTTTGGACA-3′, AS: 5′-TGTGTGCAGTTTCCTGGAACC-3′;
COL10A1, S: 5′-AAACCAGGAGAGAGAGGACCATATG-3′, AS: 5′-CAGCCGGTCCAGGGATTC-3′;
COL11A1, S: 5′-CTCTAGTGTGAAAACGAAACGG-3′, AS: 5′-CGAGGGTTGTTACGGTGAAATC-3′;
ACAN, S: 5′-TCGAGGACAGCGAGGCC-3′, AS: 5′-TCGAGGGTGTAGCGTGTAGAGA-3′;
HtrA1, S: 5′-GGGACTGGTCGTGTTTGTGC-3′, AS: 5′-CATTGACCTTTGGGTGCTGACT-3′;
RPL13, S: 5′-GAGGTATGCTGCCCCACAAA-3′, AS: 5′-GTGGGATGCCGTCAAACAC-3′;
SOX9, S: 5′-CCC ATG TGG AAG GCA GAT-3′, AS: 5′- TTC TGA GAG GCA CAG GTG ACA-3′;
MMP-1, S: 5′-GAA GCT GCT TAC GAA TTT GCC G-3′, AS: 5′-CCA AAG GAG CTG TAG ATG TCC T-3′;
MMP-13, S: 5′-AAG GAG CAT GGC GAC TTC T-3′, AS: 5′-TGG CCC AGG AGG AAA AGC-3′;
Agrecanase-1, S: 5′-T GCC GCT TCA TCA CTG A-3′, AS: 5′-CAA TGG AGC CTC TGG TTT GTC-3′;
Agrecanase-2, S: 5′-CAG AAA CAA CGG ACG C-3′, AS: 5′-CGG AAT TAC TGT ACG GC-3′;
TIMP-1, S: 5′-GTG TCT GCG GAT ACT TCC ACA G-3′, AS: 5′-AGC TAA GCT CAG GCT GTT CCA-3′;
Cbfa1, S: 5′-GCA GCA CGC TAT TAA ATC CAA ATT-3′, AS: 5′-ACA GAT TCA TCC ATT CTG CCA CTA G-3′;
Osterix, S: 5′-TGC CTC CTC AGC TCA CCT TC-3′, AS: 5′-GCA GGT ATC AGG CAC AAG GG-3′.
Western blotting
After treatment, sponges with cells were rinsed once with ice-cold phosphate-buffered saline (PBS), crushed, and lysed in RIPA buffer to prepare cellular extracts for Western blots. The supernatants were collected by centrifugation, and the amount of protein was determined by Bradford's colorimetric procedure (Bio-Rad SA). Cellular extracts (20 μg) underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and were electrotransferred to a polyvinylidene difluoride transfer membrane (PVDF; Millipore). Primary antibodies against type I collagen (Novotec), type II collagen (Novotec), type X collagen (Sigma-Aldrich), HtrA1 (Millipore), and GAPDH (Santa-Cruz Biotechnology) were incubated with the PVDF membrane overnight at 4°C. The PVDF membrane was then washed with 1X TBS-T (Tris-buffered saline-Tween 20) solution, and secondary antibodies were added for 1 h at room temperature. Finally, proteins were revealed using a chemiluminescent detection kit (GE Healthcare Amersham™ ECL™ Western blotting detection reagent), and membranes were exposed to X-ray films.
Immunocytochemistry
After treatment, sponges with cells were rinsed once with 0.1 M phosphate buffer, pH 7.4, fixed with buffered 4% paraformaldehyde for 16 h at 4°C, and rinsed twice with 0.1 M phosphate buffer. Then, they were embedded in 5% agar (Super LM; Roth), in 0.1 M phosphate buffer. Then, 50 μm floating sections were cut with a vibratome (MICROM HM 650V) and mounted on microscope slides. They were incubated for 5 min in PBS/0.5% Triton X-100 (Sigma Aldrich), rinsed in PBS, incubated for 30 min at 37°C in PBS/0.2% hyaluronidase (Sigma Aldrich), rinsed in PBS, and treated with PBS/10% bovine serum albumin (BSA; Sigma Aldrich). Immunohistochemical staining was carried out using polyclonal-specific antibodies against type I and type II collagens diluted to 1:100 (Novotec). These primary antibodies were revealed using a goat anti-rabbit IgG conjugated to Alexa Fluor 546 diluted to 1:2000 from a 2 mg/mL solution (Invitrogen). As controls, primary antibodies were omitted. Slides were then treated with UltraCruz™ Mounting Medium and DAPI (4′,6-diamidino-2-phenylindole, dilactate; Santa Cruz Biotechnology, Inc.). The observations were made on a confocal laser-scanning microscope (Olympus FV1000). The same parameters were used for all acquisitions, and representative pictures were shown in the figures.
In vivo experiments
HACs (three groups from three OA femoral heads) were seeded in type I collagen sponges for 7 days in hypoxia, treated with or without BMP-2 and with NC siRNA, or COL1A1 siRNA, or HtrA1 siRNA, or COL1A1 and HtrA1 siRNAs as described earlier. Then, sponges were grafted in a subcutaneous location in nude mice (Athymics nude-foxn1, female, 4 weeks; Harlan France). Animal experiments were approved by the Regional Ethics Committee (N/01-10-11/18/10-14) and were performed in accordance with institutional animal guidelines. Surgical procedures were performed under general anesthesia with inhalation of 4% isoflurane. After 28 days, nude mice were euthanized under anesthesia (5% isoflurane) with CO2 inhalation. Collagen sponges were recovered, fixed with buffered 4% paraformaldehyde for 16 h at 4°C.
Immunohistochemistry
Once fixed, the neo-cartilage constructs were dehydrated in successive baths: 100% ethanol (one bath of 30 min, followed by five baths of 60 min), toluene (one bath of 30 min, followed by two baths of 60 min) and finally plunged into paraffin (one bath of 15 min, one bath of 30 min, one bath of 60 min, and one bath of 90 min). These steps were carried out by an automaton (Laboratory of Pathological Anatomy, CHU). Samples were finally placed in an embedding cassette and embedded in paraffin blocks. Then, 4 μm sections were made with a microtome and mounted on silanized slides for immunostaining. The sections were deparaffinized by successive baths of toluene (two baths of 5 min), 100% ethanol (two baths of 5 min), 90% ethanol (5 min), 70% ethanol (5 min), and, finally, distilled water. Immunostaining was initiated by a pretreatment with hyaluronidase (0.5% in PBS-BSA (3%), 30 min at room temperature) to unmask the antigenic sites. Immunohistochemistry was carried out using polyclonal-specific antibodies against type I, type II, and type X collagens and aggrecan (Novotec), diluted in PBS-BSA (3%). Slides were incubated for 1 h with type I collagen antibody (1:1000 dilution) and for 3 h with type II collagen antibody (1:250 dilution) at room temperature and overnight at 4°C with aggrecan (1:500 dilution) and type X collagen (1:2000 dilution) antibodies. For the NCs, the primary antibodies were omitted. After rinsing with PBS, the sections were permeabilized with PBS-0.2% Tween 20. The endogenous peroxidases were inhibited by incubation with 1.5% hydrogen peroxide in PBS-BSA (3%). Then, an anti-rabbit secondary antibody (undiluted, EnVision+; DAKO) was applied to each section. The peroxidase reaction was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen (DAKO). Cells were counterstained with hematoxylin (Labonord SAS), and specimens were dehydrated using an ethanol gradient starting from 70% to 100%, then toluene alone. Samples were mounted with Eukitt (DAKO) and were examined under a light microscope. Alternatively, the deparaffinized sections were also stained with hematoxylin-eosin-safran (HES) and alizarin red (2%, pH 4.1; Sigma).
Statistical analysis
For RT-PCR analysis, data are presented as box plots, representative of four experiments performed in triplicate. Box plots show the minimum value, the 25th, 50th (median), and 75th percentiles, and the maximum value. Means are shown as crosses. The Mann–Whitney U test was used to determine significant differences between two groups of treatments. No adjustment was made for multiple comparisons. Representative kinetics experiments were analyzed using Student's paired t-test to determine differences between NC siRNA and COL1A1 or HtrA1 siRNAs. p-values of less than 0.05 were considered significant: ***p<0 .001, **p<0.01, and *p<0.05.
Results
BMP-2, hypoxia, and collagen sponges improve the chondrocyte phenotype
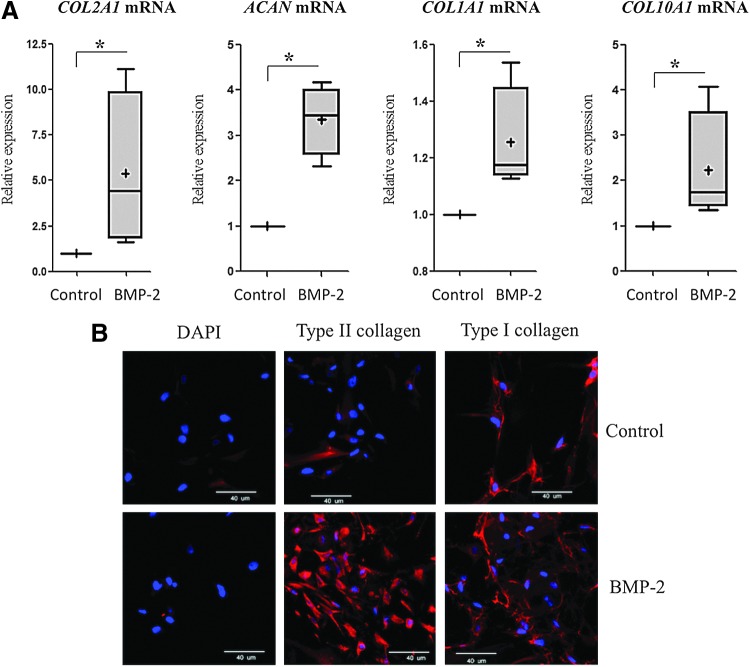
We first focused on the redifferentiation of dedifferentiated OA chondrocytes to improve their phenotype before ACI. This is an absolute necessary step to ensure the best composition and structure of healthy hyaline cartilage. In a previous study, we obtained a good differentiated phenotype when chondrocytes were cultured in 3D collagen sponges rather than in a 2D monolayer culture. In sponges, chondrocytes expressed more type II collagen and aggrecan and less type I collagen when cultured in hypoxia (3% O2) rather than in normoxia (21% O2).18 Moreover, our study suggested that BMP-2 helps increase the cartilage-specific markers. Similarly, in this study, the combination of BMP-2, hypoxia, and collagen sponges increased the expression of specific markers of healthy cartilage (type II collagen, aggrecan) after 7 days of culture (5.5- and 3.5-fold, respectively), and enhanced types I and X collagens to a lesser extent (1.3- and 2.4-fold respectively) (Fig. 1A). However, immunohistochemistry analysis revealed that although BMP-2 increased type II collagen expression, type I collagen expression persisted (Fig. 1B and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). Thus, to overcome type I collagen expression, an siRNA specifically targeting COL1A1 mRNA was added to our culture model. Moreover, to enhance the action of BMP-2 and reduce cartilage degradation, an siRNA targeting the HtrA1 mRNA was also added to our 3D culture model in hypoxia.
FIG. 1.
Bone morphogenetic protein (BMP)-2 increased the expression of hyaline cartilage-specific markers in chondrocytes cultured in collagen sponges and in hypoxia. Human articular chondrocytes (HACs) were cultured in type I collagen sponges for 7 days in hypoxia, in the absence (control), or in the presence of 50 ng/mL BMP-2. (A) The steady-state mRNA levels of type II collagen, aggrecan, type I, and type X collagens were measured using real-time RT-PCR with primers specific to type II collagen (COL2A1 mRNA), aggrecan (ACAN mRNA), type I collagen (COL1A1 mRNA), or type X collagen (COL10A1 mRNA). All results were normalized to RPL13a mRNA, compared with untreated sponge-cultured cells, and are presented as the relative expression of each gene. Box plots represent four independent experiments in triplicate. Statistically significant differences between the untreated cells and BMP-2-treated cells were determined using the Mann–Whitney U test (*p<0.05). (B) Immunostaining was carried out to detect type I and type II collagens (red). The nuclei were counterstained with DAPI (blue). Images shown are representative of the experimental results. Magnification ×40. Scale bar: 40 μm. Color images available online at www.liebertpub.com/tec
Improvement of the chondrocyte phenotype with COL1A1 and/or HtrA1 siRNAs
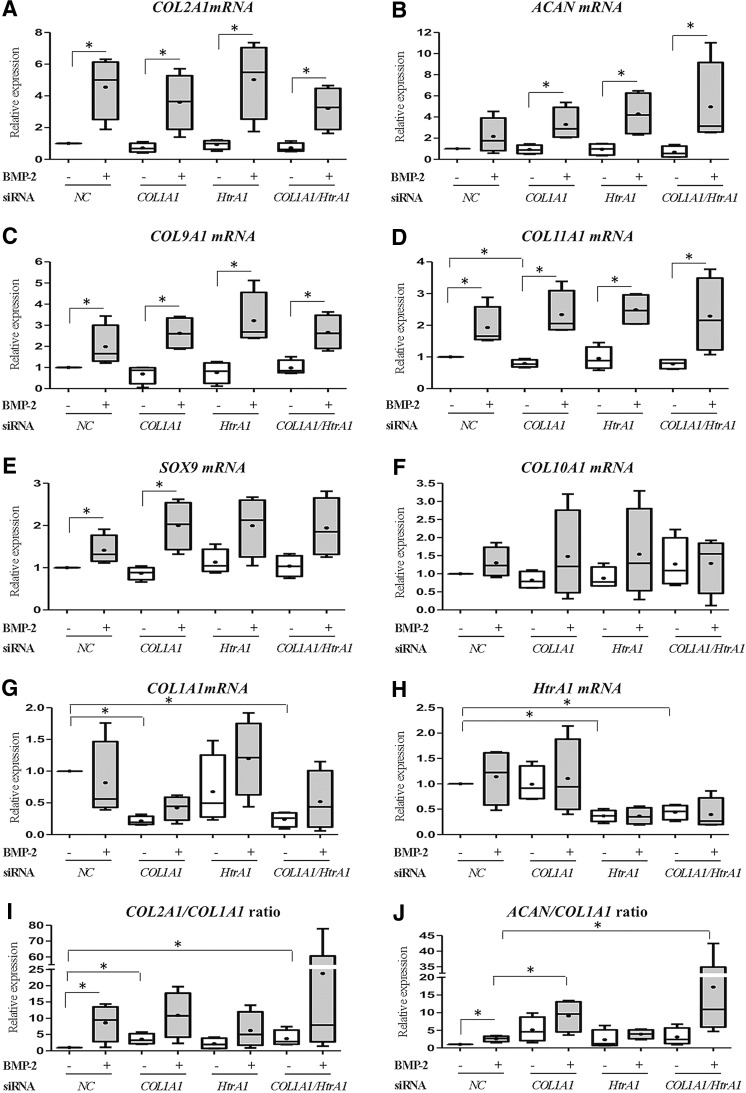
HACs, seeded in type I collagen sponges, were transfected with an NC siRNA, with COL1A1 siRNA, with HtrA1 siRNA, or with COL1A1 and HtrA1 siRNAs and then cultured for 7 days in hypoxia with or without BMP-2. In addition to the analyses of COL1A and HtrA1 mRNA levels, the phenotypic profile of chondrocytes were determined by analyses of mRNA levels of two well-known chondrocyte phenotype markers, type II collagen and aggrecan, SOX9, which positively regulates their expression, associated markers (type IX and XI collagens), and type X collagen, an indicator of chondrocyte hypertrophy (Fig. 2). At day 7, treatment with BMP-2 increased the steady-state mRNA levels of type II collagen (four-fold in the NC siRNA treatment, Fig. 2A) and, to a lesser extent, aggrecan (Fig. 2B). BMP-2 also increased the mRNA levels of type IX and XI collagens (two-fold in the NC siRNA treatment, Fig. 2C, D), and those of SOX9 (1.5-fold in the NC siRNA treatment, Fig. 2E), and had no significant effect on type X collagen mRNA (Fig. 2F). COL1A1 and HtrA1 mRNA levels did not increase with the BMP-2 treatment (Fig. 2G, H). COL1A1 and HtrA1 siRNAs inhibited their specific target after 7 days of culture with or without BMP-2. We observed 80% inhibition of COL1A1 mRNA and 60% inhibition of HtrA1 mRNA (Fig. 2G, H). The combination of both siRNAs was also effective with or without BMP-2. We noticed that neither siRNA species nor the combination of both siRNAs influenced the BMP-2-induced expression of type II, type IX, or type XI collagens, aggrecan, or SOX9 mRNA levels. The expression of type X collagen was likewise not influenced by siRNA treatments (Fig. 2F), reflecting the specific response of siRNA treatments. To better apprehend the chondrocyte phenotype, we also calculated two differentiation indexes corresponding to the ratio of COL2A1 mRNA to COL1A1 mRNA (Fig. 2I) and of ACAN mRNA to COL1A1 mRNA (Fig. 2J). COL1A1 siRNA increased COL2A1:COL1A1 ratio by 3.5-fold and ACAN:COL1A1 ratio by five-fold compared with the NC siRNA treatment. In the presence of BMP-2, COL1A1 siRNA did not have a significant effect on COL2A1:COL1A1 ratio but increased that of ACAN:COL1A1 (3.5-fold compared with NC siRNA with BMP-2). HtrA1 siRNA had no effect on COL2A1:COL1A1 or ACAN:COL1A1 ratio with or without BMP-2 (NC siRNA compared with HtrA1 siRNA and NC siRNA with BMP-2 compared with HtrA1 siRNA with BMP-2). The combination of both siRNAs had no more effect than COL1A1 siRNA alone.
FIG. 2.
Effects of COL1A1 and HtrA1 siRNAs on mRNA expression of specific and nonspecific cartilage markers. HACs seeded onto collagen sponges were transfected with an INTERFERin®-siRNA complex (5 nM negative control (NC) siRNA, or 5 nM COL1A1 siRNA and/or 5 nM HtrA1 siRNA) as described in the “Materials and Methods” section. HACs were then cultured in 3% O2 for 7 days and treated with or without BMP-2 (50 ng/mL). Relative mRNA expression of type II collagen (A), Aggrecan (B), type IX collagen (C), type XI collagen (D), SOX9 (E), type X collagen (F), type I collagen (G), and HtrA1 (H) was obtained as described in Figure 1. All results were normalized to RPL13a mRNA, compared with untreated NC, and presented as the relative expression of each gene. We also determined the COL2A1:COL1A1 mRNA ratio (I) and the ACAN:COL1A1 mRNA ratio (J). Box plots represent four independent experiments performed in triplicate. Statistically significant differences between untreated cells and BMP-2-treated cells, NC siRNA-untreated cells, and other siRNA untreated cells, and between NC siRNA BMP-2-treated cells and other siRNA BMP-2-treated cells were determined using the Mann–Whitney U test (*p<0.05).
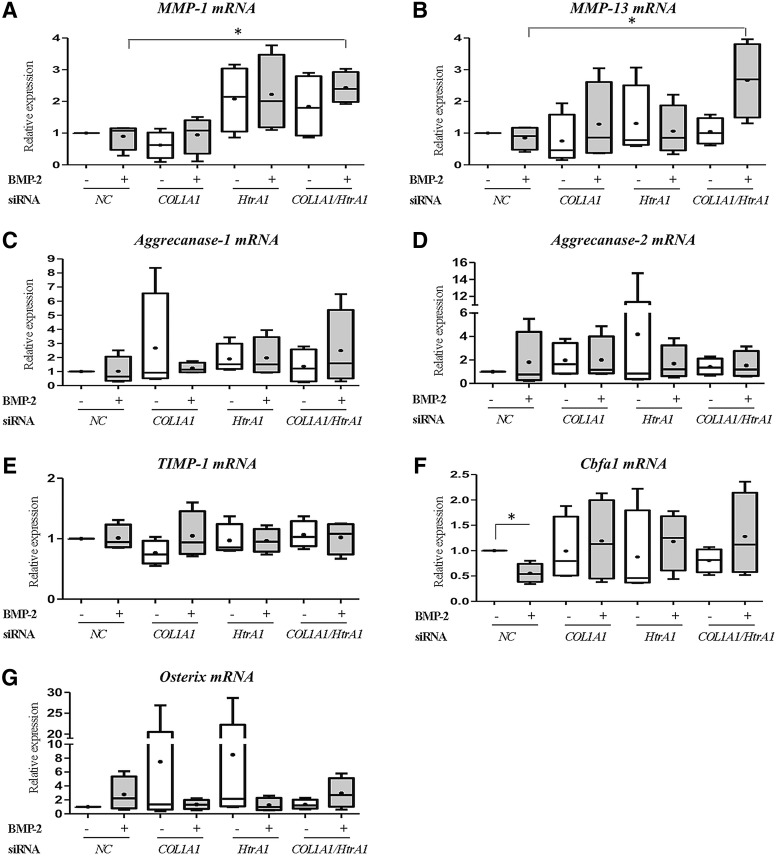
Finally, the phenotypic profile of HACs was further determined by the analysis of some proteases involved in the OA process (MMP-1 and -13, Aggrecanase-1 and -2), of their inhibitor (TIMP-1) and of some transcription factors implicated in hypertrophy (Cbfa1) or in osteogenesis (Osterix) (Fig. 3). BMP-2 did not affect the steady-state mRNA levels of catabolic markers such as MMP-1, MMP-13, Aggrecanase-1, and Aggrecanase-2, compared with the NC siRNA treatment. Moreover, a slight induction of MMP-1 was observed when chondrocytes were transfected with HtrA1 siRNA (Fig. 3A). The combination of both siRNAs significantly increased MMP-1 and MMP-13 mRNA levels in the presence of BMP-2 (2.5-fold compared with NC siRNA with BMP-2; Fig. 3A, B), whereas the mRNA amounts of Aggrecanase-1, Aggrecanase-2, and TIMP-1 were not modified by any treatment (Fig. 3C–E). While BMP-2 decreased Cbfa1 mRNA amounts (two-fold compared with NC siRNA), it tended to increase Osterix mRNA, but these effects were lost with the siRNA treatments (Fig. 3F, G). The effects of the different treatments on Osteocalcin mRNA levels were not measured, because we previously showed that in our culture conditions, BMP-2 does not induce Osteocalcin mRNA (Fig. 1B and Supplementary Fig. S2).
FIG. 3.
Effects of COL1A1 and HtrA1 siRNAs on the mRNA expression of catabolic, hypertrophic, and osteogenic markers. HACs seeded onto collagen sponges were transfected with an INTERFERin-siRNA complex (5 nM NC siRNA, or 5 nM COL1A1 siRNA and/or 5 nM HtrA1 siRNA) as described in the “Materials and Methods” section. HACs were then cultured in 3% O2 for 7 days and treated with or without BMP-2 (50 ng/mL). Relative mRNA expression of MMP-1 (A), MMP-13 (B), Aggrecanase-1 (C), Aggrecanase-2 (D), TIMP-1 (E), Cbfa1 (F), and Osterix (G) was obtained as described in Figure 1. Statistically significant differences between untreated cells and BMP-2-treated cells, NC siRNA-untreated cells, and other siRNA untreated cells, and between NC siRNA BMP-2-treated cells and other siRNA BMP-2-treated cells were determined using the Mann–Whitney U test (*p<0.05).
At the transcriptional level, the addition of COL1A1 siRNA to our culture model seemed to be the best way to improve the chondrocyte phenotype without affecting the catabolic pathway.
COL1A1 and HtrA1 siRNAs inhibit type I collagen expression
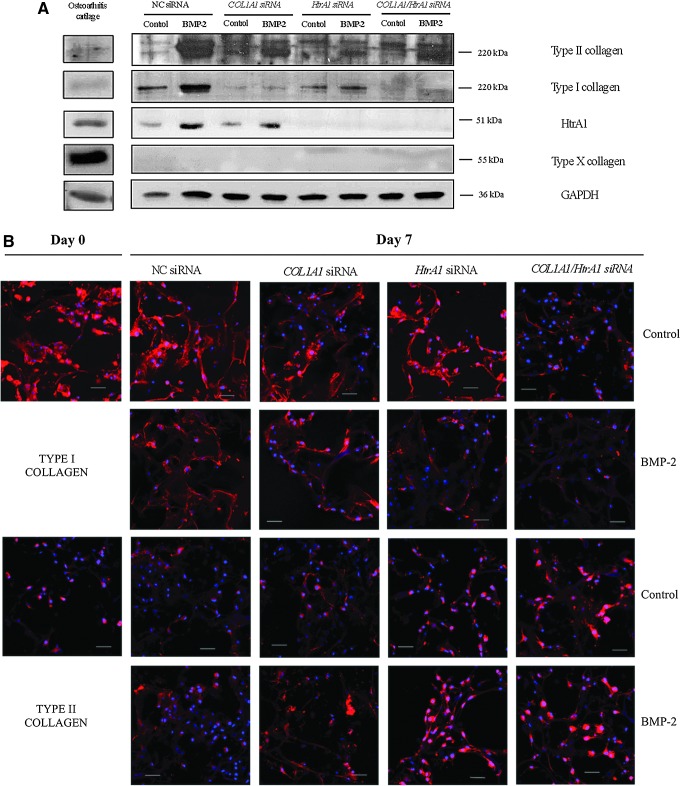
Western blots (Fig. 4A) and their quantification (Fig. 1B and Supplementary Fig. S3) showed that BMP-2 increased mainly type II collagen synthesis and, to a lesser extent, HtrA1 and type I collagen. The COL1A1 siRNA significantly decreased the amount of type I collagen (85% in the absence or presence of BMP-2) without affecting the BMP-2-induced type II collagen synthesis. COL1A1 siRNA had no further effect on HtrA1 synthesis. The HtrA1 siRNA also downregulated type I collagen synthesis but to a lesser extent compared with COL1A1 siRNA (50% in the absence and 25% in the presence of BMP-2), without noticeably affecting the BMP-2-induced type II collagen synthesis. HtrA1 siRNA obviously had a much greater effect on the synthesis of HtrA1 (knockdown >80% in the absence or presence of BMP-2). The combination of both siRNAs did not further affect the type I and II collagen synthesis compared with COL1A1 siRNA alone. Type X collagen synthesis was almost undetectable in our culture model, regardless of the conditions employed. OA cartilage, used as a control, revealed a high amount of HtrA1 and type X collagen proteins.
FIG. 4.
Effects of COL1A1 and/or HtrA1 siRNA on collagen synthesis. (A) The combination of hypoxia, BMP-2, COL1A1, and HtrA1 siRNAs promotes redifferentiation of chondrocyte at the protein level. HACs seeded onto collagen sponges were transfected with an INTERFERin-siRNA complex (5 nM NC siRNA, or 5 nM COL1A1 siRNA and/or 5 nM HtrA1 siRNA) as described in the “Materials and Methods” section. HACs were then cultured in 3% O2 for 7 days and treated with or without BMP-2 (50 ng/mL). Protein extracts were analyzed by Western blotting for type I, II, and X collagens, and HtrA1 versus GAPDH. Representative blots are shown. (B) Immunostaining of type I collagen and type II collagen reveals effective redifferentiation of HACs with siRNAs. HACs were treated as described earlier but with 50 nM of each siRNA. Immunostaining was carried out to detect type I and type II collagens (red). The nuclei were counterstained with DAPI (blue). Images shown are representative of the experimental results. Magnification ×40. Scale bar: 40 μm. Color images available online at www.liebertpub.com/tec
In conclusion, the Western blot analyses revealed that the COL1A1 and HtrA1 siRNAs significantly and specifically decreased type I collagen synthesis, and favored type II collagen synthesis.
Immunofluorescence experiments were then carried out to visualize collagen synthesis in the sponge cultures before (day 0), and after siRNA transfections (day 7) (Fig. 4B). The quantification of fluorescence intensity is presented in Fig. 1B and Supplementary Figure S4. As expected, dedifferentiated chondrocytes (day 0) strongly expressed type I collagen after their 2D amplification; whereas type II collagen expression was very weak. In contrast, HACs cultured for 7 days in hypoxia with BMP-2, and COL1A1 and/or HtrA1 siRNAs expressed higher levels of type II collagen and lower levels of type I collagen, suggesting initiation of the redifferentiation process. Moreover, COL1A1 siRNA alone had a poor effect on the levels of type I collagen, whereas HtrA1 siRNA alone had the ability to inhibit type I collagen protein and also to increase that of type II collagen induced by BMP-2. However, the combination of both siRNAs synergistically decreased type I collagen levels and increased type II collagen levels, but masked the effects of BMP-2.
Thus, immunofluorescence experiments confirmed that HtrA1 siRNA can decrease the level of type I collagen synthesis.
siRNAs are still efficient after 10 days of culture
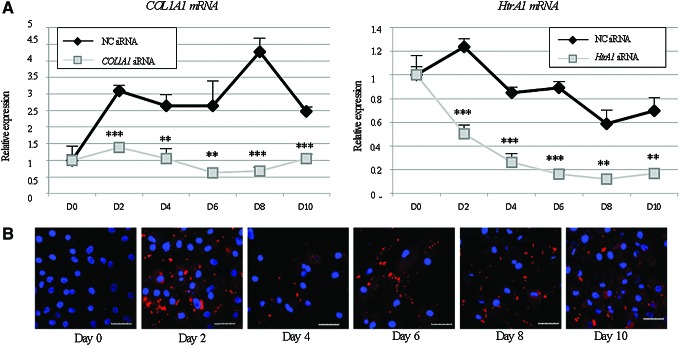
A short kinetic experiment was then performed to assess the effect of siRNAs over time before starting the in vivo experiments (Fig. 5). As expected, siRNAs targeting COL1A1 and HtrA1 transcripts significantly inhibited their respective mRNA expression (Fig. 5A). This extinction was still observed after 10 days of culture in collagen sponges and for approximately 14 days in case of the inhibition of COL1A1 mRNA (Fig. 1B and Supplementary Fig. S5) whereas the HtrA1 siRNA knockdown effect persisted, although weakly, for approximately 21 and 28 days. When HACs were transfected with fluorescent siRNA (Alexa Fluor 546), the siRNA remained present in the cells after 10 days (Fig. 5B). Moreover, optical microscopy showed that siRNA aggregates were well distributed throughout the collagen sponges.
FIG. 5.
Short kinetic study of siRNAs. (A) Inhibition of type I collagen and HtrA1 expressions at the mRNA level. HACs seeded onto collagen sponges were transfected with an INTERFERin-siRNA complex (50 nM NC siRNA, or 50 nM COL1A1 siRNA and/or 50 nM HtrA1 siRNA) as described in the “Materials and Methods” section. HACs were then cultured in hypoxia (3% O2) with culture arrest on day 0, 2, 4, 6, 8, or 10. Relative mRNA expression of type I collagen and HtrA1 was obtained as described in Figure 1. Results of one representative experiment performed in triplicate were normalized to RPL13a mRNA and are presented as the relative expression of each gene. Statistically significant differences between NC siRNA and COL1A1 or HtrA1 siRNAs were determined at each time point by Student's paired t-test ***p<0.001, **p<0.01, and *p<0.05. (B) Monitoring a fluorescent NC siRNA for 10 days of culture. HACs seeded onto collagen sponges were transfected with an INTERFERin-siRNA complex (alexa fluor 546, 50 nM). HACs were then cultured in 3% O2 with a medium change or culture arrest on day 0, 2, 4, 6, 8, or 10. The pictures were taken on a confocal laser-scanning microscope. Scale bar: 20 μm. Color images available online at www.liebertpub.com/tec
The sustained effects of siRNAs guarantee the effective inhibition of OA-induced proteins and help maintain a functional chondrocyte phenotype for a long period of time during culture for further in vivo experiments.
Neo-cartilage is formed in vivo
To evaluate the behavior of our collagen scaffold in vivo, the sponge constructs were implanted subcutaneously in the back of nude mice for 28 days and then analyzed by histology and immunohistochemistry (Figs. 6 and 7).
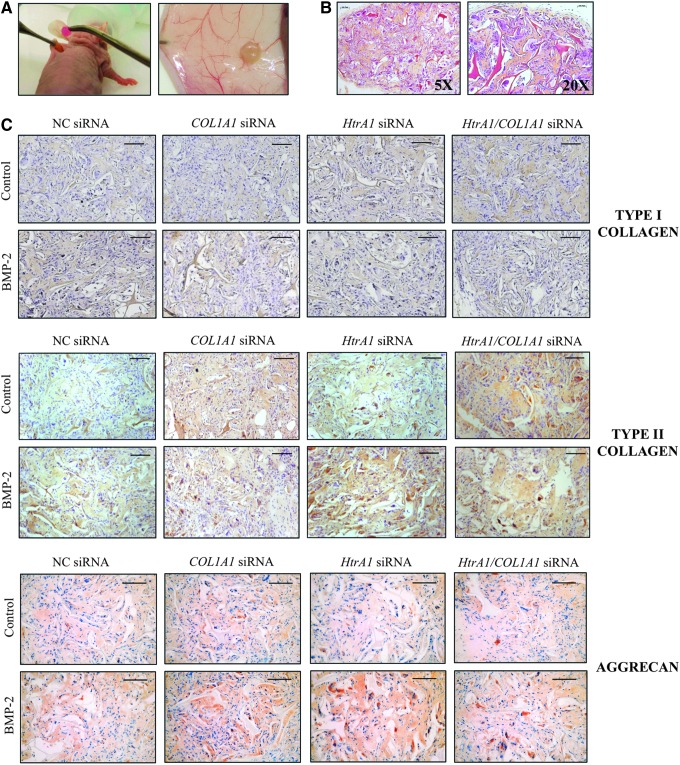
FIG. 6.
Persistence of the chondrocyte phenotype in vivo. HACs seeded onto collagen sponges were transfected with an INTERFERin-siRNA complex (50 nM NC) siRNA, or 50 nM COL1A1 siRNA and/or 50 nM HtrA1 siRNA). HACs were then cultured in hypoxia (3% O2) with or without BMP-2 (50 ng/mL) until day 7. They were next implanted subcutaneously in nude mice for 28 days as described in the “Materials and Methods” section. (A) Implantation of type I collagen sponges containing HACs under the skin of nude mice (left). Sponges were harvested 28 days later (right). (B) HES staining of the constructs at 28 days after implantation in mice. (C) Immunostaining of type I and II collagens and aggrecan in the neo-cartilage constructs at 28 days after implantation in mice. Images shown are representative of the experimental results of three independent experiments. Scale bar: 100 μm.
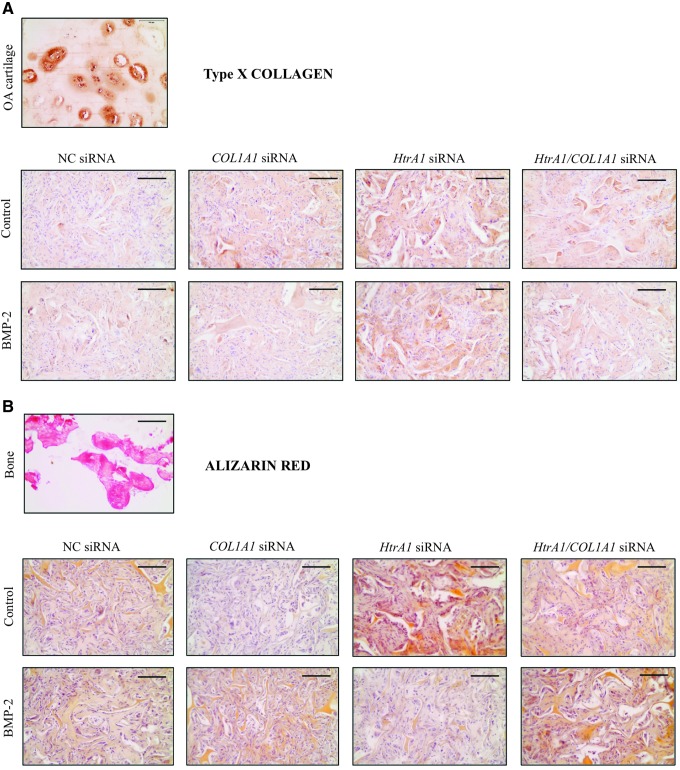
FIG. 7.
No induction of hypertrophic or osteogenic phenotypes in vivo. HACs seeded onto collagen sponges were transfected with an INTERFERin-siRNA complex (50 nM NC siRNA, or 50 nM COL1A1 siRNA and/or 50 nM HtrA1 siRNA). HACs were then cultured in hypoxia (3% O2) with or without BMP-2 (50 ng/mL) until day 7. They were then implanted subcutaneously in nude mice for 28 days as described in the “Materials and Methods” section. (A) Immunostaining of type X collagen in an osteoarthritis cartilage (top) and in the neo-cartilage constructs at 28 days after implantation in mice (bottom). (B) Alizarin red staining in a bone (top) and in the constructs at 28 days after implantation in mice (bottom). Images shown are representative of the experimental results of three independent experiments. Scale bar: 100 μm.
Macroscopic observations of sponges containing HACs showed a very strong difference between the day of implantation and the day of harvest (Fig. 6A). After 28 days, sponges looked similar to hyaline cartilage with a pearlescent appearance. Moreover, HES staining showed strong collagen production, with a homogeneous distribution of cells between the collagen network of the sponge (Fig. 6B). Interestingly, no degradation of the biomaterial occurred after 1 month under the skin of nude mice. In parallel, we also grafted sponges that did not contain cells and the collagen network was still present at 28 days later (data not shown).
Immunohistological analysis showed that, in the presence of NC siRNA, BMP-2 slightly induced type I collagen; whereas it increased type II collagen and aggrecan to a greater extent (Fig. 6C). In the presence of BMP-2, the levels of type I collagen were lower than those of the NC, with COL1A1 siRNA alone or in combination with HtrA1 siRNA. HtrA1 siRNA decreased the expression of type I collagen slightly in the presence of BMP-2. HACs embedded in collagen scaffolds, cultured in hypoxia+BMP-2, and transfected with siRNA against COL1A1 and HtrA1 showed high production of type II collagen as well as of aggrecan. It should be noted that HtrA1 siRNA seemed to be the best inducer of type II collagen and aggrecan in the presence of BMP-2. The ECM appeared much more compact and denser with COL1A1 and/or HtrA1 siRNAs compared with the one observed with NC siRNA.
Finally, hypertrophy and the osteogenesis phenotype were evaluated with type X collagen immunohistochemistry analyses and with alizarin red staining, respectively (Fig. 7). We did not find any signs of hypertrophy. Low levels of type X collagen appeared to be present in chondrocytes compared with the high levels found in OA cartilage, and BMP-2 did not induce type X collagen (Fig. 7A). However, HtrA1 siRNA seemed to favor type X collagen synthesis. Similarly, alizarin red staining did not reveal any OA induction in chondrocytes cultured in sponges (light brown staining) compared with bone tissue (red) (Fig. 7B).
Altogether, these results confirmed the effectiveness of our culture model in producing a cartilage-like matrix for long-term in vivo implantation.
Discussion
During OA, chondrocytes lose their characteristic phenotype. This process, called dedifferentiation, features the induction of type I collagen expression instead of type II collagen, and also occurs during the first expansion step in ACI. The success of cartilage regenerative medicine is based on the phenotypic status of HACs and on the presence of the essential components of its ECM, such as type II collagen. Type II collagen expression should be enhanced without inducing that of type I collagen. For this reason, recent research has focused on the role of biomaterials or the roles played by hypoxia and chondrogenic factors to induce the redifferentiation of dedifferentiated chondrocytes.19,33,34 However, a few cellular therapy approaches to inhibit type I collagen have shown great efficiency. We previously showed that dedifferentiated chondrocytes cultured in type I collagen sponges under hypoxia (3% O2) express more type II collagen and aggrecan than in normoxia (21% O2) and that BMP-2 favors this process.18 The chondrocyte phenotype is further enhanced in collagen sponge scaffolds by using COL1A1 siRNA with BMP-2 under hypoxia. In this previous study, we nucleofected HAC with COL1A1 siRNA and HACs were then seeded onto type I collagen sponges for 24–48 h of culture to establish the proof of concept. Here, we aimed at improving this process with chondrocytes seeded onto sponges and incubated with a transfecting reagent. HACs were also grown longer (7 days) to obtain the best phenotype in type I collagen sponges and in hypoxia.18 In addition, we extensively analyzed the chondrocyte phenotype and tested whether this phenotype can be maintained in vivo.
In the presence of NC siRNA, BMP-2 increased the expression of a marker specific to cartilage, type II collagen, and its associated collagens (type IX and XI collagens) and, to a lesser extent, aggrecan. This was associated with an improvement of the chondrocyte phenotype index evaluated using the COL2A1:COL1A1 mRNA ratio and the ACAN:COL1A1 mRNA ratio, without any signs of hypertrophy (no induction of type X collagen or Cbfa1) or induction of osteogenesis (no induction of Osterix or calcification). Our analyses at the mRNA and/or protein levels confirmed the efficiency of our culture conditions in inducing cartilage-specific ECM synthesis in the collagen scaffolds. The immunohistochemical study of the collagen sponges obtained in vitro and grafted in vivo in nude mice were in agreement with Western blot analyses. Other studies have reported a beneficial effect of 3D culture, hypoxia, chondrogenic factors, or the combination on the chondrocyte phenotype.19,23,35 Most of these investigations evaluated mRNA and/or protein expression of markers specific to articular cartilage, but a few have analyzed the synthesis of nonspecific markers, a critical and essential point for assessing the functionality of the neo-formed cartilage, to discriminate between hyaline ECM and fibrocartilaginous ECM.
The association of HACs, BMP-2, hypoxia, and collagen sponges fulfilled the goal of reconstructing functional cartilage but a non-negligible background of type I collagen synthesis remained. We successfully employed an siRNA strategy directly targeting COL1A1 mRNA to reduce type I collagen synthesis. Thus, the association of COL1A1 siRNA and BMP-2 improved the chondrocyte differentiation index when HACs were cultured in hypoxia. A recent study used a lentiviral vector encoding TGF-β3 and shRNA directed against type I collagen to mediate chondrocyte redifferentiation in a 3D chondrocyte culture.36 However, the use of a viral vector and shRNA is known to induce stable knockdown, whereas siRNAs induce a transient knockdown.37,38 Our experimental strategy was developed so as to avoid a gene therapy approach, and favor a cell therapy approach to cartilage repair, thereby avoiding the ethical problems behind genome modification. In addition, our study aimed first and foremost at decreasing the expression of type I collagen transiently to initiate the redifferentiation of dedifferentiated chondrocytes. Moreover, we transiently decreased the expression of two major genes (COL1A1 and HtrA1) whose expression is enhanced during OA. Our study generates the hypothesis that dedifferentiated HAC can recover active metabolism with a differentiated phenotype when cultured in a 3D scaffold, in hypoxic conditions with BMP-2 and COL1A1 siRNA. The present data also highlight that the recovered HAC phenotype can be further enhanced by adding HtrA1 siRNA. Inhibition of metalloproteinases and aggrecanases by using si/shRNAs has emerged in recent years. For example, shRNAs against ADAMTS-5 and ADAMTS-9 increase matrix deposition in a 3D chondrocyte culture.39 It has been recently shown that an intra-articular injection of lentivirus-mediated ADAMTS-5 siRNA prevents the degradation of rat articular cartilage by inhibiting the production of ADAMTS-5.40
In our study, low concentrations of siRNAs (5 nM) were able to decrease type I collagen and HtrA1 expression. Higher concentrations of siRNAs (50 nM) were used for in vivo studies to ensure a durable effect, validated in a kinetic study (Fig. 5 and Fig. 1B and Supplementary Fig. S5). We think that type I collagen sponges can retain siRNAs by acting as a tank to release some siRNAs when the medium is changed. Our goal is to further decrease the concentration of siRNAs employed to eliminate any off-target effects. The use of very low concentrations of siRNA results in less off-target effects on several genes such as the other collagen isotypes.41 These off-target effects may partly explain the potential nonspecific effects of HtrA1 siRNA on type I collagen expression in vivo and in immunocytochemistry experiments where it was used at 50 nM. The real time RT-PCR analysis revealed no off-target effects on any collagen gene when it was used at 5 nM. Another explanation is that HtrA1 inhibition indirectly favors other proteases involved in type I collagen degradation such as MMP-1. Indeed, we found that HtrA1 siRNA increased MMP-1 mRNA with or without BMP-2 (Fig. 3A) and increased MMP-13 mRNA (only with BMP-2 and with the combination of both siRNAs; Fig. 3B), whereas aggrecanase-1 and -2 and the inhibitor of MMPs (TIMP-1) were not induced (Fig. 3C–E). Nevertheless, as in healthy cartilage, HACs seeded onto sponges can alternate between synthesis and degradation to promote new matrix deposition. The indirect decrease in type I collagen in the presence of HtrA1 siRNA may be offset by that of type II collagen and aggrecan as observed here in vivo. Moreover, the inhibition of HtrA1, involved in the degradation of the TGF-β receptor family and in the proteolysis of aggrecan,29,30 may also result in the increase of the aggrecan expression observed in vivo and in the persistent BMP-2 effects.
Type I collagen sponges are already used for grafting human reconstructed skin. Recombinant human BMP-2 is also used in bone repair in human clinical therapy42,43 and a recent study employing BMP-2 fused to a collagen binding domain within a 3D collagen sponge showed that BMP-2 enhances its effect in bone remodeling.44 Our process for obtaining neo-cartilage is, thus, ready to enter clinical trials in animals (e.g., horse), and, ultimately, can be tested in preclinical trials for future use in humans.
Several studies have evaluated the quality of the ECM produced during redifferentiation of chondrocytes.19,23,35 A recent work has established a relationship between scaffold fiber size and cell morphology,45 suggesting that the most effective biomaterial would present a loose network of small fibers. Our model allows chondrocytes to fill the biomaterial properly and uniformly, offering a three-dimensional organization of chondrocytes similar to that observed in situ in healthy articular cartilage. Our collagen sponges have a pore size of 100 μm, and electronic microscopy shows that HACs are homogeneously distributed from the surface to the deeper layers of the scaffold.18 We plan to use electron microscopy to monitor our culture model over time and to determine whether it has a regular and complex molecular organization of collagen fibers46 before and after ACI. Another promising approach consists of differentiating autologous mesenchymal stem cells of different sources into chondrocytes.47
Our objective regarding cell therapy of articular cartilage was to improve the chondrocyte phenotype before their autologous implantation in the dysfunctional joint. This study is among the first to decrease type I collagen significantly in chondrocytes while increasing cartilage-specific markers significantly. Here, we reported two mechanisms by which the chondrocyte phenotype could be enhanced using a combination of type I collagen sponges, BMP-2, hypoxia, and two siRNAs. The COL1A1 siRNA directly inhibited type I collagen synthesis. The HtrA1 siRNA indirectly inhibited type I collagen synthesis at the protein level, via an unknown mechanism, and directly inhibited the HtrA1 protease that is overexpressed in OA. As a result, in both cases, type II collagen and aggrecan syntheses were favored. These two approaches are not mutually exclusive and can be used together. siRNAs are powerful tools for decreasing specific gene expression and are used in many types of cell therapy, such as cancer therapy.48 The success of cartilage regenerative medicine is based on the phenotypic status of HACs and one of the essential components of cartilage: type II collagen. Under our experimental conditions, the expression of the two essential cartilage markers (type II collagen and aggrecan) increased in a very significant manner and was associated with an improvement in the chondrocyte phenotype index. The risk of obtaining fibrocartilage is minimized, but should be confirmed by in vivo studies in ACIs in larger mammals. Our original reconstructed neo-cartilage with an active metabolism of phenotypically stabilized HACs can likely be improved, for example, by increasing the rate of reconstruction using a bioreactor. Furthermore, the mechanical resistance of the artificial cartilage should be assessed.
In conclusion, in this study, we developed a method for redifferentiating human dedifferentiated chondrocytes in vitro, combining relevant physicochemical factors (hypoxic conditions, 3D collagen scaffolds), a chondrogenic factor (BMP-2), and RNA interference targeting two markers that are overexpressed in OA (type I collagen and HtrA1). This strategy is an attempt to obtain redifferentiated chondrocytes and should be improved for human clinical use. Redifferentiation of dedifferentiated chondrocytes was estimated by extensive gene and protein analyses to determine the phenotypic profile of chondrocytes. In these culture conditions, chondrocytes synthesized a cartilage hyaline-like matrix characterized by a substantial expression of type II collagen without any signs of fibrotic, hypertrophic, or osteogenic phenotypes. Our culture process improves the recovery and a better stabilization of the human hyaline chondrocyte phenotype in primarily dedifferentiated cells, both in vitro and in vivo. This work, therefore, provides new insights into the molecular mechanisms of stability and/or induction of the chondrocyte phenotype and a new clinical possibility for ACI. However, developing a new method for ACI in humans requires the proof of concept of such a process in clinical trials in animals, after long-term implantation in cartilage lesions.
Supplementary Material
Acknowledgments
This work was supported by the French National Research Agency (ANR) and by the Regional Council of Lower-Normandy on the ANR Tecsan PROMOCART program, by a FEDER (“Fonds structurels européens”) grant (HIPPOCART 1 n°2897/33535), by a Regional Council of Lower-Normandy grant (HIPPOCART N° 2013-AGRI-236/n°13P07492), and partially by the Lions Club of Normandy (France).
The authors are thankful to Pr J-J. Parienti from the Unité de Biostatistiques et de Recherche Clinique (Délégation à la Recherche Clinique et à l'Innovation (DRCI), Pôle de Recherche et d'Epidémiologie Clinique, CHU de Caen) for his helpful and critical comments in the biostatistic analyses.
F.L.: Post-doctoral fellow supported by a PROMOCART grant (Health Technology) from the French National Agency of Research (ANR).
D.O.: Fellowship from the French Ministry of Research and Technology.
Disclosure Statement
No conflict of interest is declared by all the authors.
References
- 1.Dawson J., Linsell L., Zondervan K., Rose P., Randall T., Carr A., and Fitzpatrick R.Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology (Oxford) 43,497, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ludar D., White P.H., Callahan L.F., Chang R.W., Helmick C.G., Lappin D.R., Melnick A., Moskowitz R.W., Odom E., Sacks J., Toal S.B., and Waterman M.B.A National Public Health Agenda for Osteoarthritis 2010. Semin Arthritis Rheum 39,323, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Zhang W., Moskowotz R.W., Nuki G., Abramson S., Altman R.D., Arden N., Bierma-Zeinstra S., Brandt K.D., Croft P., Doherty M., Dougados M., Hochberg M., Hunter D.J., Kwoh K., Lohmander L.S., and Tugwell P.OARSI recommandations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16,137, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Alcaraz M.J., Megias J., Garcia-Arnandis I., Clérigues V., and Guillen M.I.New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol 80,13, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Trueta J.Osteo-arthritis: an approach to surgical treatment. Lancet 270,585, 1956 [DOI] [PubMed] [Google Scholar]
- 6.Bedi A., Feeley B.T., and Williams R.J.Management of articular cartilage defects of the Knee. J Bone Joint Surg Am 92,994, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Roberts S., Menage J., Sandell L.J., Evans E.H., and Richardson J.B.Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 16,398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., and Peterson L.Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331,889, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre V., Peeters-joris C., and Vaes G.Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim Biophys Acta 1051,266, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Sams A.E., and Nixon A.J.Chondrocyte-laden collagen scaffolds for resurfacing extensive articular cartilage defects. Osteoarthritis Cartilage 3,47, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Ohno T., Tanisaka K., Hiraoka Y., Ushida T., Tamaki T., and Tateishi T.Effect of type I and type II collagen sponges as 3D scaffolds for hyaline cartilage-like tissue regeneration on phenotypic control of seeded chondrocytes in vitro. Mater Sci Eng 24,407, 2004 [Google Scholar]
- 12.Pulkkinen H., Tiitu V., Lammentausta E., Laasanen M.S., Hämäläinen E.R., Kiviranta I., and Lammi M.J.Cellulose sponge as a scaffold for cartilage tissue engineering. Biomed Mater Eng 16,287, 2006 [PubMed] [Google Scholar]
- 13.Murphy C.L., and Sambanis A.Effect of oxygen tension and alginate encapsulation of the differentiated phenotype of passaged chondrocytes. Tissue Eng 7,791, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Schlegel W., Nürnberger S., Hombauer M., Albrecht C., Vécsei V., and Marlovits S.Scaffold-dependent differentiation of human articular chondrocytes. Int J Mol Med 22,691, 2008 [PubMed] [Google Scholar]
- 15.Bonaventure J., Kadhom N., Cohen-Solal L., Ng K.H., Bourguignon J., Lasselin C., and Freisinger P.Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res 212,97, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Freyria A.M., Ronzière M.C., Cortial D., Galois L., Hartmann D., Herbage D., and Mallein-Gerin F.Comparative phenotypic analysis of articular chondrocytes cultured within type I or type II collagen scaffolds. Tissue Eng Part A 15,1233, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Duval E., Leclercq S., Elissalde J.M., Demoor M., Galéra P., and Boumédiene K.Hypoxia-inducible factor 1alpha inhibits the fibroblast-like markers type I and type III collagen during hypoxia-induced chondrocyte redifferentiation: hypoxia not only induces type II collagen and aggrecan, but it also inhibits type I and type III collagen in the hypoxia-inducible factor 1alpha-dependent redifferentiation of chondrocytes. Arthritis Rheum 60,3038, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Legendre F., Ollitrault D., Hervieu M., Baugé C., Maneix L., Goux D., Chajra H., Mallein-Gerin F., Boumediene K., Galera P., and Demoor M.Enhanced hyaline cartilage matrix synthesis in collagen sponge scaffolds by using siRNA to stabilize chondrocytes phenotype cultured with BMP-2 under hypoxia. Tissue Eng Part C 19,550, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Foldager C.B., Nielsen A.B., Munir S., Ulrich-Vinther M., Søballe K., Bünger C., and Lind M.Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta Orthop 82,234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafont J.E.Lack of oxygen in articular cartilage: consequences for chondrocyte biology. Int J Exp Pathol 91,302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund-Olesen K.Oxygen tension in synovial fluids. Arthritis Rheum 13,769, 1970 [DOI] [PubMed] [Google Scholar]
- 22.Claus S., Aubert-Foucher E., Demoor M., Camuzeaux B., Paumier A., Piperno M., Damour O., Duterque-Coquillaud M., Galéra P., and Mallein-Gerin F.Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer culture. J Cell Biochem 111,1642, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Gouttenoire J., Bougault C., Aubert-Foucher E., Perrier E., Ronzière M.C., Sandell L., Lundgren-Akerlund E., and Mallein-Gerin F.BMP-2 and TGF-beta1 differentially control expression of type II procollagen and alpha 10 and 11 integrins in mouse chondrocytes. Eur J Cell Biol 89,307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valcourt U., Ronzière M.C., Winkler P., Rosen V., Herbage D., and Mallein-Gerin F.Different effects of bone morphogenetic proteins 2, 4, 12, and 13 on the expression of cartilage and bone markers in the MC615 chondrocyte cell line. Exp Cell Res 251,264, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya A., Yano M., Tocharus J., Kojima H., Fukumoto M., Kawaichi M., and Oka C.Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone 37,323, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Polur I., Lee P.L., Servais J.M., Xu L., and Li Y.Role of HtrA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol 25,599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clausen T., Kaiser M., Huber R., and Ehrmann M.HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol 12,152, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Hu S.I., Carozza M., Klein M., Nantermet P., Luk D., and Crowl R.M.Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J Biol Chem 273,34406, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Chamberland A., Wang E., Jones A.R., Collins-Racie L.A., LaVallie E.R., Huang Y., Liu L., Morris E.A., Flannery C.R., and Yang Z.Identification of a novel HtrA1-susceptible cleavage site in human aggrecan: evidence for the involvement of HtrA1 in aggrecan proteolysis in vivo. J Biol Chem 284,27352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka C., Tsujimoto R., Kajikawa M., Koshiba-Takeuchi K., Ina J., Yano M., Tsuchiya A., Ueta Y., Soma A., Kanda H., Matsumoto M., and Kawaichi M.HtrA1 serine protease inhibits signaling mediated by TGF family proteins. Development 131,1041, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Legendre F., Bogdanowicz P., Boumédiene K., and Pujol J.-P.Role of interleukin 6 (IL-6)/IL-6R-induced signal tranducers and activators of transcription and mitogen-activated protein kinase/extracellular. J Rheumatol 32,1307, 2005 [PubMed] [Google Scholar]
- 32.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Claassen H., Schicht M., and Paulsen F.Impact of sex hormones, insulin, growth factors and peptides on cartilage health and disease. Prog Histochem Cytochem 45,239, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen L.H., Kudva A.K., Guckert N.L., Linse K.D., and Roy K.Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 17,2465, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Schrobback K., Klein T.J., Crawford R., Upton Z., Malda J., and Leavesley D.I.Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res 347,649, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Yao Y., Zhang F., Pang P.X., Su K., Zhou R., Wang Y., and Wang D.A.In vitro study of chondrocyte redifferentiation with lentiviral vector-mediated transgenic TGF-β3 and shRNA suppressing type I collagen in three-dimensional culture. J Tissue Eng Regen Med 5,e219, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Moore C.B., Guthrie E.H., Huang M.T., and Taxman D.J.Short hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown. Methods Mol Biol 629,141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sliva K., and Schnierle B.S.Selective gene silencing by viral delivery of short hairpin RNA. Virol J 21,248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coughlan T.C., Crawford A., Goldring M.B., Hatton P.V., and Barker M.D.Lentiviral shRNA knock-down of ADAMTS-5 and -9 restores matrix deposition in 3D chondrocyte culture. J Tissue Eng Regen Med 4,611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu X., You H., Yuan X., Zhao W., Li W., and Guo X.Protective effect of lentivirus-mediated siRNA targeting ADAMTS-5 on cartilage degradation in a rat model of osteoarthritis. Int J Mol Med 31,1222, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Caffrey D.R., Zhao J., Song Z., Schaffer M.E., Haney S.A., Subramanian R.R., Seymour A.B., and Hughes J.D.siRNA off-target effects can be reduced at concentrations that match their individual potency. PLoS One 6,e21503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sellers R.S., Peluso D., and Morris E.A.The Effect of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) on the Healing of Full-Thickness Defects of Articular Cartilage. J Bone Joint Surg Am 79,1452, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Zhou N., Wang J., Liu C., Fan C., Zhang Q., Xue F., and Zhang J.Clinical application of bioactive CPC loading rhBMP-2 in repairing bone defects. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23,257, 2009 [PubMed] [Google Scholar]
- 44.Visser R., Arrabal P.M., Becerra J., Rinas U., and Cifuentes M.The effect of an rhBMP-2 absorbable collagen sponge-targeted system on bone formation in vivo. Biomaterials 30,2032, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Nuernberger S., Cyran N., Albrecht C., Redl H., Vécsei V., and Marlovits S.The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 32,1032, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Becerra J., Andrades J.A., Guerado E., Zamora-Navas P., López-Puertas J.M., and Reddi A.H.Articular cartilage: structure and regeneration. Tissue Eng Part B Rev 16,617, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Rasmusson I.Immune modulation by mesenchymal stem cells. Exp Cell Res 312,2169, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Yang X.Z., Dou S., Sun T.M., Mao C.Q., Wang H.X., and Wang J.Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J Control Release 156,203, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.