Abstract
Early lineage segregation in preimplantation embryos and maintenance of pluripotency in embryonic stem cells (ESCs) are both regulated by specific signaling pathways. Small molecules have been shown to modulate these signaling pathways. We examined the influence of several small molecules and growth factors on second-lineage segregation of the inner cell mass toward hypoblast and epiblast lineage during mouse embryonic preimplantation development. We found that the second-lineage segregation is influenced by activation or inhibition of the transforming growth factor (TGF)β pathway. Inhibition of the TGFβ pathway from the two-cell, four-cell, and morula stages onward up to the blastocyst stage significantly increased the epiblast cell proliferation. The epiblast formed in the embryos in which TGFβ signaling was inhibited was fully functional as demonstrated by the potential of these epiblast cells to give rise to pluripotent ESCs. Conversely, activating the TGFβ pathway reduced epiblast formation. Inhibition of the glycogen synthase kinase (GSK)3 pathway and activation of bone morphogenetic protein 4 signaling reduced the formation of both epiblast and hypoblast cells. Activation of the protein kinase A pathway and of the Janus kinase/signal transducer and activator of transcription 3 pathway did not influence the second-lineage segregation in mouse embryos. The simultaneous inhibition of three pathways—TGFβ, GSK3β, and the fibroblast growth factor (FGF)/extracellular signal-regulated kinases (Erk)—significantly enhanced the proliferation of epiblast cells than that caused by inhibition of either TGFβ pathway alone or by combined inhibition of the GSK3β and FGF/Erk pathways only.
Introduction
The first- and the second-lineage segregation in the preimplantation-stage embryo results in the formation of three different cell types: trophectoderm, epiblast, and hypoblast [1,2]. The two latter cell types originate from the inner cell mass (ICM) of the blastocyst. Several markers uniquely identify these different cell types. Cdx2 marks the trophectoderm; Nanog marks the epiblast while Gata6 and Gata4 are expressed in the hypoblast [1,3]. Later during the developmental process, the epiblast mostly forms the fetus whereas the trophectoderm and the hypoblast contribute to extraembryonic tissues [4,5]. Modulating signaling pathways using external addition of small molecules or other factors can alter cell fate decisions. In this way, relevant information of the involved molecular pathways during early development and embryonic stem cell (ESC) pluripotency can be gathered. For example, the use of three small-molecule inhibitors, namely, SU5402, PD184352, and CHIR99021, representing inhibitor of the tyrosine kinase of fibroblast growth factor (FGF) receptor, mitogen-activated protein kinase pathway, and glycogen synthase kinase (GSK)3β, respectively, supported the long-term propagation and maintenance of mouse embryonic stem cells (mESCs) in the absence of leukemia inhibitor factor (LIF) [6]. The population doubling time of these ESCs was comparable to that of ESCs maintained in LIF and serum medium. These inhibitors also allowed the derivation of mESCs from the nonpermissive CBA mouse strain [6]. Later, it was shown that the more potent inhibitor of the extracellular signal-regulated kinase (Erk) cascade PD0325901 (hereafter termed as PD) together with CHIR99021 (hereafter termed as CH) (the so-called “two inhibitors” or “2i” condition) and LIF successfully generated germ-line competent naive mESC lines from another nonpermissive mouse model, the nonobese diabetic mice [7]. Before this breakthrough, naive mESCs could only be derived from permissive strains of mice, in the presence of LIF and serum. Nowadays, mESC derivation is possible from all strains of mice with 2i.
Interestingly, when the “2i” was added to the culture media during mouse preimplantation development from the eight-cell stage onward, an increase in the number of cells in the epiblast compartment was demonstrated, coupled with a suppression of hypoblast formation [8]. Because of simultaneous inhibition of FGF and GSK3β signaling during mouse embryo development, ICM lost its capacity to form hypoblast cells, eventually resulting in the formation of only epiblast cells in blastocysts [8]. The activation of FGF signaling during embryonic development is thus important for hypoblast formation in mouse [5,8]. In contrast, an increased level of FGF signaling by exogenous supply of FGF4 blocked epiblast formation [5]. The increased number of epiblast cells and decreased number of hypoblast cells in embryos cultured in the presence of an FGF inhibitor is neither the result of selective proliferation of epiblast lineage nor the outcome of apoptosis of the hypoblast lineage but is due to selective lineage choice in the presence of these inhibitors [5]. Similarly, it was shown that 2i supplementation significantly increased the number of epiblast cells in human embryos [9]. In contrast to mice, FGF signaling appeared not to be involved in human hypoblast formation [9,10]. Another study showed that the epiblast cell number can be increased by the addition of insulin in the culture medium [11]. Insulin works via the activation of the phosphoinositide 3 kinase pathway and/or inactivation of GSK and p53 [11]. These studies emphasize the usefulness of small molecules in manipulating cell fate during lineage segregation to gain more insight in which signaling pathways are involved during early differentiation.
ESCs exhibit two different pluripotent states, being naive and primed. In mouse, naive ESCs are derived from the epiblast of the preimplantation-stage embryos [12,13], whereas primed pluripotent stem cells are derived from the epiblast of postimplantation-stage embryos [14,15]. These primed stem cells are commonly known as epiblast stem cells (EpiSCs). Human embryonic stem cells (hESCs), despite being derived from the ICM of preimplantation-stage human blastocysts [16], shared defining features with mouse primed EpiSCs in contrast to naive mESCs. In this respect, it was shown that the human ICM first undergoes a transient epiblast-like state before hESCs are established, which might explain their “primed” pluripotency status [17]. However recently, use of cocktail of small-molecule inhibitors leads to the derivation and conversion of naive hESCs without any exogenous supply of pluripotency factors [18]. Small molecules can also transform the pluripotency state of stem cells from primed to naive and vice-versa [18,19]. Naive and primed pluripotency depends on different signaling pathways and they require different growth factors for their maintenance.
Maintenance of naive pluripotent state is dependent on the activation of the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathway through LIF stimulation [20] or on the activation of the bone morphogenetic protein (BMP)4 signaling [21], whereas maintenance of the primed pluripotent state depends on activation of the FGF pathway and Activin signaling [15,22]. Small molecules, like Forskolin, an activator of protein kinase A (PKA) and Kenpaullone, an inhibitor of GSK pathway, can also substitute one or more of the transcription factors during the reprogramming of somatic cells to induce pluripotency [19,23]. Given these abilities of small molecules to alter the fate of pluripotent stem cells, it would be interesting to know whether they can also affect lineage segregation in embryos. In this study, we aimed to determine whether similar signaling pathways are involved in the maintenance of stem cell pluripotency as well as in the lineage segregation in embryos. To address this, we cultured mouse embryos in the presence of different small molecules and growth factors known for their ability to maintain naive or primed stem cell pluripotency.
Materials and Methods
All compounds used in this experiment were purchased from Sigma Aldrich unless otherwise stated. Experiments were carried out using diploid, parthenogenetically activated oocytes (parthenotes) unless use of naturally fertilized zygotes is mentioned.
Mice
B6D2/F1 hybrid and 129 strains of mice were purchased from Charles River Laboratories. All animal experiments were approved by the animal ethics committee of the Ghent University Hospital (ECD number 12/61).
Oocyte/zygote recovery and culture
Six- to fourteen-week-old female mice were superovulated by intraperitoneal injection of 7.5 IU equine chorionic gonadotrophin (Folligon; Intervet, Oss) followed by 7.5 IU human chorionic gonadotrophin (hCG; Chorulon, Intervet) at an interval of 46–48 h. For zygote collection, females were kept with males after the second injection. Oocytes and zygotes were recovered, from the swollen ampulla, 14 and 21 h post-hCG injection, respectively. The cumulus oocyte/zygote complexes were briefly incubated in 200 IU/mL hyaluronidase (type VIII) to free them from cumulus cells. Oocytes/zygotes were washed a few times in potassium simplex optimized medium (KSOM)–HEPES and then cultured in KSOM supplemented with 0.4% bovine serum albumin (BSA; Calbiochem). Oocytes were activated by culturing them in 10 mM SrCl2 supplemented with 2 mg/mL cytochalasin D in Ca2+-free KSOM for 4 h. Zygotes and subsequent embryos were then incubated under 37°C at 6% CO2 and 5% O2 (standard culture conditions). Embryos (both parthenotes and naturally fertilized zygotes) were cultured in KSOM (with or without small molecules) for 72 h postactivation/collection and were subsequently transferred to Cook Blastocyst® medium (with or without small molecules) and cultured up to the blastocyst stage. Blastocysts were scored at 100 h postactivation/collection and were fixed immediately for immunostaining. The current study was carried out on 2,463 parthenogenetic embryos and 124 naturally fertilized zygotes. For all experiments, oocytes/zygotes were pooled from at least two female mice.
Small molecules and growth factors used in the study
Small molecules used in this study are SB431542 (10 μM; Tocris Biosciences), A-83-01 (1 μM; Tocris Biosciences), Forskolin (10 μM), Kenpaullone (5 μM), PD0325901 (1 μM; Cayman), and CHIR99021 (3 μM; Tocris Biosciences). Besides these small molecules, the cytokine, LIF (1,000 U/mL), and TGFβ pathway proteins Activin A (50 ng/mL; R&D Biosystems) and BMP4 (50 ng/mL) were also used. As some of these molecules were dissolved in dimethyl sulfoxide (DMSO), vehicle control treatments with the appropriate concentration of DMSO were also carried out. The signaling pathways activated or inhibited by these molecules are summarized in Table 1.
Table 1.
Small Molecules/Growth Factors and the Target Pathways for Their Action
| Small molecules/growth factors | Target pathway | Activator/inhibitor |
|---|---|---|
| SB431542 | TGFβ | Inhibitor |
| A-83-01 | TGFβ | Inhibitor |
| Activin | TGFβ | Activator |
| PD0325901 | FGF/Erk | Inhibitor |
| CHIR99021 | GSK3β | Inhibitor |
| Kenpaullone | GSK | Inhibitor |
| LIF | JAK/STAT3 | Activator |
| BMP4 | BMP | Activator |
TGF, transforming growth factor; FGF, fibroblast growth factor; Erk, extracellular signal-regulated kinases; GSK, glycogen synthase kinase; JAK, janus kinase; STAT, signal transducer and activator of transcription; BMP, bone morphogenetic protein; LIF, leukemia inhibitor factor.
Immunostaining of the embryos
Embryos were fixed by incubating them in 4% paraformaldehyde for 20 min at room temperature (RT) or overnight at 4°C. Fixed embryos were kept in phosphate buffer saline (PBS) at 4°C until immunostaining was performed. For immunostaining, embryos were briefly incubated in fresh PBS and then permeabilized at RT in 0.1% and 0.5% Triton-X-100 for 10 and 20 min, respectively. After brief washing in PBS, embryos were blocked by incubating them in PBS+0.1% BSA+0.05% Tween 20 solution for 1 h at RT. Embryos were incubated overnight in primary antibodies diluted in blocking solution at 4°C. Primary antibodies were against Gata4 (1/200; Santa Cruz) and Nanog (1/100; Abcam). Next day, embryos were washed three times in PBS+0.05% Tween 20 solution, each time for 15 min at RT. Then, embryos were incubated in secondary antibodies diluted in blocking buffer for 1 h at RT. Secondary antibodies were donkey anti-goat-fluorescein isothiocyanate (1/200; Bio-Connect) and donkey anti-rabbit-Cy3 (1/2,000; Bio-Connect). Finally, embryos were washed three times before they were mounted in Vectashield containing 4′,6-diamidino-2-phenylindole (Vector Laboratories). For analysis, embryos were visualized through fluorescence microscope under 40× magnification (Zeiss) or laser scanning confocal microscope (Nikon A1R confocal microscope; Nikon Instruments) with a 60× oil immersion objective.
mESC derivation
mESCs were derived from the blastocysts obtained from parthenogenetic embryos cultured in the presence of SB431542 (SB) from morula stage, and also from control embryos. Briefly, blastocysts were allowed to hatch spontaneously and they were plated individually in gelatin-coated dishes in N2B27 medium supplemented with PD0325901 (1 μM), CHIR99021 (3 μM) and 1,000 U/mL LIF. After 3 days, the outgrowths were disaggregated into single cells with 0.25% trypsin (Invitrogen) and were plated over fresh gelatin-coated dishes. The obtained ESC colonies were passaged with 0.05% trypsin after 5 days. The subsequent passaging of ESCs was carried out every alternate day.
Statistical analysis
Instat-3 from Graph-Pad software was used for all statistical analyses. Blastocyst-formation rate was calculated by analyzing a contingency table using two-sided Fisher's exact test. The ICM cell numbers were compared using one-way analysis of variance followed by Tukey post-test. P<0.05 was considered significant.
Results
Inhibition of TGFβ signaling during mouse embryonic development affects epiblast formation without affecting its functionality
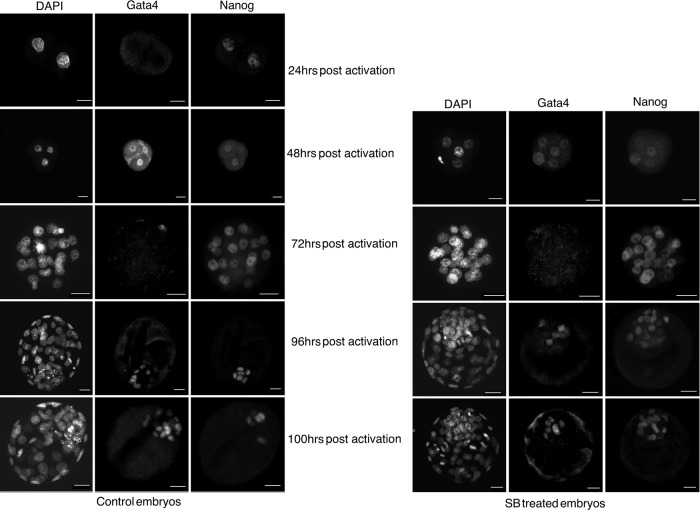
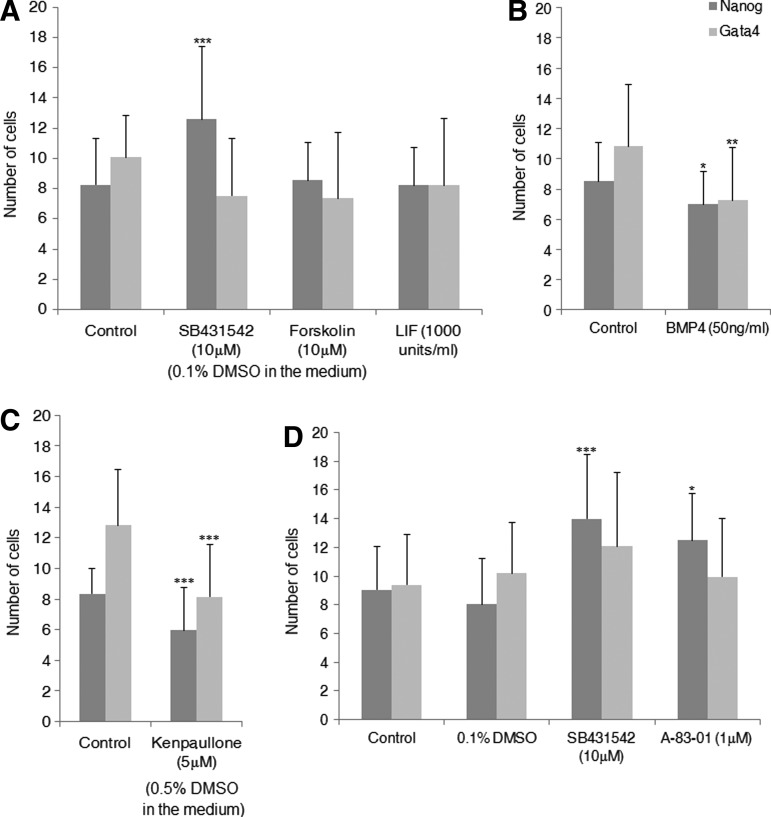
Different small molecules targeting modulation of different signaling pathways were used to identify the pathways involved in epiblast and hypoblast segregation in mouse. SB, LIF, and Forskolin were added separately to the embryo culture medium from the two-cell (24 h postactivation) up to the blastocyst stage (100 h postactivation), after which embryos were immunostained for Nanog (epiblast marker) and Gata4 (hypoblast marker) (representative image of blastocyst immunostaining, Fig. 1). Similar rates of blastocyst were observed in all groups (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). Compared with embryos treated with Forskolin or LIF, as well as compared with the control, the number of Nanog-positive cells was significantly higher in SB-treated embryos (Supplementary Table S1). No significant difference was seen in the number of Gata4-positive cells between the control and the other groups (Fig. 2A).
FIG. 1.
Expression of Nanog and Gata4 in preimplantation mouse embryos (in control and in SB431542 [SB]-treated embryos). Scale bars represent 22 μm.
FIG. 2.
Number of Nanog- and Gata4-positive cells in blastocysts cultured in the presence of different small molecules. (A) Inhibitor of TGFβ signaling, SB431542; activator of PKA pathway, Forskolin; and activator of JAK/STAT3 pathway, LIF. (B) Activator of BMP signaling, BMP4. (C) Inhibitor of GSK signaling, Kenpaullone. (D) Inhibitors of TGFβ signaling, SB431542 and A-83-01. P<0.05 is considered significant. ***P<0.001, **P<0.01, and *P<0.05, when compared with control.
In a separate set of experiments, the roles of BMP4 and Kenpaullone during second-lineage segregation were also examined. In the presence of both BMP4 and Kenpaullone, significant decrease in the number of Nanog- and Gata4-positive cells (Fig. 2B, C) was observed. To confirm that inhibition of TGFβ signaling can increase the epiblast proliferation, mouse embryos were cultured in the presence of another inhibitor of TGFβ signaling, A-83-01 [24], from morula stage (72 h postactivation) up to the blastocyst stage (100 h postactivation). As in the embryos cultured in SB, significant increase in the number of epiblast cells were observed in the embryos treated with A-83-01. Consistent with the results obtained from SB, no change was observed in the number of hypoblast cells when TGFβ signaling was inhibited with A-83-01 (Fig. 2D and Supplementary Table S1).
In summary, our results indicate that active TGFβ signaling in the early embryo has a suppressive effect on epiblast formation. Activation of BMP signaling with BMP4 and of GSK3 signaling with Kenpaullone impaired both epiblast and hypoblast formation, while activation of other pathways, like JAK/STAT pathway and PKA pathway, did not seem to differentially affect epiblast and hypoblast formation.
Owing to the fact that the inhibition of TGFβ signaling increases the epiblast proliferation, our next goal was to investigate whether these epiblast cells in embryos cultured in the presence of SB were functional or not. Therefore, blastocysts obtained from the embryos cultured in SB were plated for stem cell derivation. Sixteen (eight from control embryos and eight from SB-cultured embryos) mESC lines were successfully established from these blastocysts in 2i culture conditions. Since the blastocysts cultured in SB had higher number of epiblast cells, we hypothesized that the epiblast formed in the SB-treated embryos can give more ESC colonies than the epiblast formed in the control embryos. For this purpose, we counted the number of ESC colonies obtained after the trypsinization of ICM outgrowth from both control and SB-treated embryos. As expected, the number of ESC colonies from ICM outgrowths derived from SB-treated embryos (17.87±19.28) was higher (although not significant) than those derived from control embryos (11.25±13.05). Hence, we can conclude that inhibition of TGFβ signaling during embryo development increases the epiblast formation without compromising its function.
SB does not influence the kinetics of expression of Nanog and Gata4 in preimplantation mouse embryos
Our next goal was to determine whether the influences of inhibition of TGFβ signaling in the lineage segregation in mouse embryos were actually the consequence of the changes in the kinetics of the expression of Nanog and Gata4. Therefore, we collected parthenogenetic embryos after 24 h (two-cell stage), 48 h (four-cell), 72 h (morula), 96 h (early blastocysts), and 100 h (expanded blastocysts) postactivation from both control and SB-treated groups for immunostaining. We observed that very low level of Nanog proteins is expressed at the two-cell stage. However, Gata4 was completely absent at this point. At four-cell stage, heterogeneous expression of both Nanog and Gata4 was observed. In some embryos (n=3), both Nanog and Gata4 were expressed in all four blastomeres and in some cases (n=2) both were absent in all of them. There were still other embryos (n=10) in which either one or both markers were randomly expressed in <4 blastomeres. Interestingly, at morula stage, embryos showed distinct expression of Nanog, but Gata4 was completely absent. In early and expanded blastocysts both of these molecules were found to be localized in the ICM (Fig. 1).
Inhibition of TGFβ signaling with low-to-high SB doses increases epiblast proliferation while hypoblast proliferation is affected by high doses
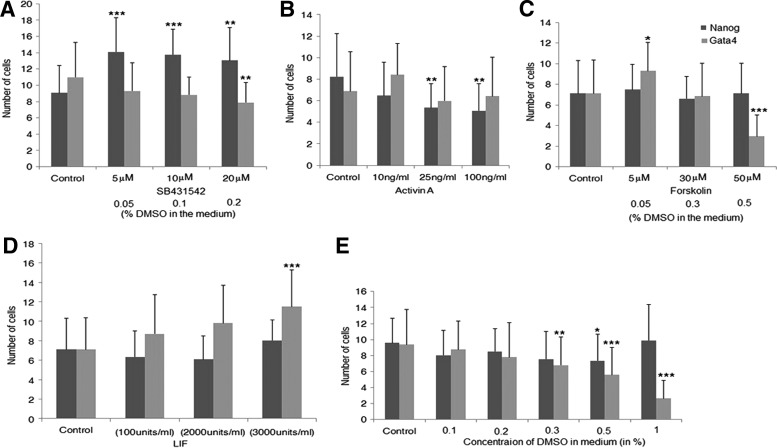
Next, the effect of different concentrations of SB, added from the two-cell stage up to the blastocyst stage, was studied. Blastocyst-formation rate was similar across all concentrations (Supplementary Table S2). In line with our first results, the number of Nanog-positive cells was always significantly higher in the SB-treated embryos in all concentrations compared with controls (Supplementary Table S2). Interestingly, the number of Gata4-positive cells was significantly reduced in the 20 μM-SB-treated group, compared with untreated controls (Fig. 3A, E and Supplementary Table S2). Hence, these experiments indicate that TGFβ signaling exerts a positive effect on hypoblast proliferation, but this effect is only apparent when a higher concentration of SB is used.
FIG. 3.
Number of Nanog- and Gata4-positive cells for different doses of small molecules. (A) SB431542, (B) ACTIVIN A, (C) Forskolin, (D) leukemia inhibitor factor (LIF), and (E) dimethyl sulfoxide (DMSO). P<0.05 is considered significant. ***P<0.001, **P<0.01, and *P<0.05, when compared with control.
Since inhibition of the TGFβ pathway showed increased epiblast formation, we next aimed to examine the effect of also activating this pathway. Mouse embryos were cultured in different concentrations of Activin A, an activator of TGFβ signaling. Similar blastocyst-formation rates were observed across all doses of Activin A (Fig. 3B and Supplementary Table S2). At concentrations of 25 and 100 ng/mL Activin A, a significant reduction in the number of Nanog-positive cells was observed compared with controls. However, no significant difference was observed in the number of Gata4-positive cells (Fig. 3B).
Since, no effect of LIF and Forskolin on epiblast and hypoblast formation was observed in our previous experiment with the particular dose used, a dose-dependent response was evaluated (Supplementary Table S2). Treatment of mouse embryos with Forskolin or LIF from the two-cell stage onward did not affect the number of Nanog-positive cells significantly at any dose. However, a significant increase in the number of Gata4-positive cells was observed when embryos were cultured in the presence of 3,000 U/mL LIF compared with controls (Fig. 3D and Supplementary Table S2). In addition, the number of Gata4-positive cells was strongly reduced in the embryos treated with 50 μM Forskolin compared with the control (Fig. 3C and Supplementary Table S2). However, the latter could be attributed to the toxic effect of a high DMSO concentration, as the presence of more than 0.2% of DMSO in the culture medium detrimentally affects embryonic developmental potential as well as the hypoblast formation (Fig. 3E).
Inhibition of the TGFβ pathway with SB strongly increases epiblast formation using concentration of 5–20 μM, while hypoblast formation is reduced only from concentrations higher than 20 μM. In contrast, activation of same pathway with Activin A at concentrations of 25 and 100 ng/mL decreases the epiblast formation, while there was no effect on the hypoblast. The fact that epiblast proliferation is already affected with low doses of SB and hypoblast proliferation only at a high SB concentration and also the absence of any effect on hypoblast proliferation with exogenous activation of TGFβ signaling possibly reflects the existence of different signaling thresholds. While hypoblast formation seems to be stimulated by saturating levels of TGFβ signaling, limiting levels of TGFβ signaling seem to control epiblast formation; LIF and Forskolin, on the other hand, do not seem to influence second-lineage segregation at any dose applied.
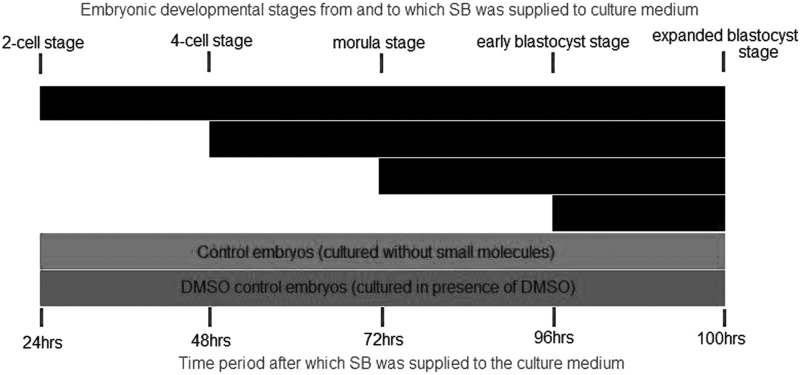
Mouse embryos are sensitive to TGFβ signaling from two-cell stage to morula stage
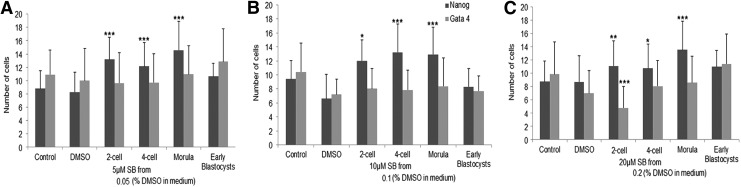
Our next goal was to determine to what extent supplementation of SB at different stages of preimplantation development has an effect on second-lineage segregation. Different experimental conditions were set up as shown in Fig. 4 depending on the time point at which the TGFβ pathway inhibitor SB was added to the culture medium. Experiments were carried out with three different concentrations of SB: 5, 10, and 20 μM. Blastocyst-formation rate and the number of Nanog- and Gata4-positive cells are shown in Supplementary Table S3. In all concentrations of SB, the number of Nanog-positive cells was significantly higher when SB was added at any stage of development except when it was added at early blastocyst stage, compared with controls. No significant effect of addition of small molecules at any developmental stage was seen on the number of Gata4-positive cells with 5 or 10 μM (Fig. 5A, B and Supplementary Table S3). Confirming our previous results, addition of SB at the concentration of 20 μM from the two-cell stage onward up to the blastocyst stage resulted in a significant decrease in the number of Gata4-positive cells compared with controls (Fig. 5C and Supplementary Table S3). No expansion of Gata4-positive cells was seen at the other stages of SB addition. Overall, these results show that inhibiting TGFβ signaling at any stage, from two-cell stage to morula, can affect epiblast formation in mouse. Since addition of SB at morula stage showed highest number of cells in epiblast lineage with 5 and 20 μM SB, it seems to be the stage when the receptors for this signal are most sensitive. However, hypoblast lineage is affected only when SB is used in concentration higher than 20 μM and used for longer duration starting as early as two-cell stage up to blastocyst stage.
FIG. 4.
Culture conditions for stage-specific inhibition of transforming growth factor (TGF)β pathway with SB431542.
FIG. 5.
Number of Nanog- and Gata4-positive cells for stage-specific inhibition of the TGFβ pathway with different doses of SB431542. (A) 5 μM SB431542, (B) 10 μM SB431542, and (C) 20 μM SB431542. ***P<0.001, **P<0.01, and *P<0.05, when compared with control.
Inhibition of TGFβ, FGF, and GSK3β signaling applies synergistic effect in the proliferation of epiblast
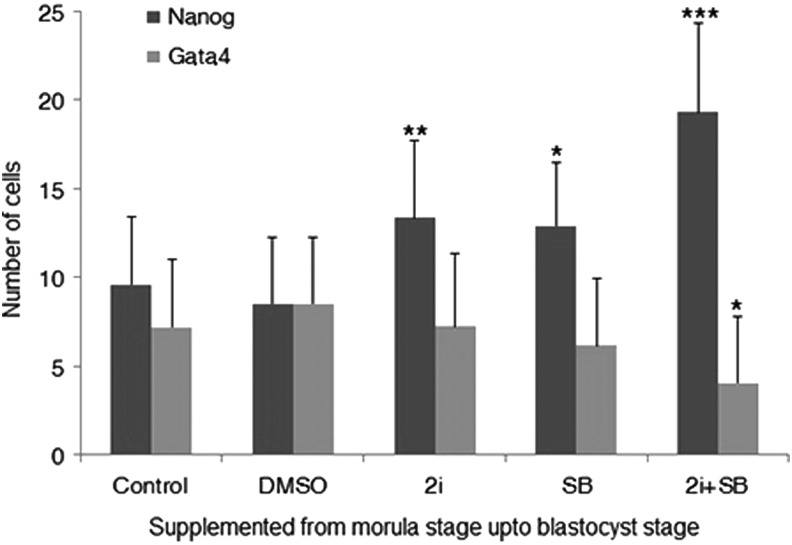
Our early experiments clearly demonstrated the involvement of TGFβ signaling in epiblast proliferation and a previous study has shown similar effect with inhibition of GSK3β signaling [8]. Strong regulation of hypoblast lineage by FGF signaling in mouse has also been proven [5,8]. Hence, the synergy between these three signaling pathways was examined. Therefore, mouse embryos were cultured in different conditions consisting of 2i and/or SB from the morula stage up to the blastocyst stage (Supplementary Table S4). We found that culturing embryos in either 2i or SB or combination of both significantly increased the proliferation of Nanog-positive cells when compared with controls (Fig. 6 and Supplementary Table S4). The number of Nanog-positive cells was significantly higher in the 2i+SB group when compared with the groups treated with either 2i only or with SB alone. However, compared with the control group, Gata4 was reduced significantly only when the embryos were cultured in 2i+SB but not at any other condition (Supplementary Table S4 and Fig. 6). Our results suggest that 2i and SB work in synergy to increase the epiblast proliferation and decrease the hypoblast formation.
FIG. 6.
Number of NANOG- and GATA 4-positive cells in 2i (1 μM PD032591 and 3 μM Chir99021) plus 10 μM SB431542 conditions. ***P<0.001, **P<0.01, and *P<0.05, when compared with control.
2i exhibits a similar effect on lineage segregation in both parthenotes and naturally fertilized zygotes
In contrast to previous results reported by Nichols et al. [8] and Yamanaka et al. [5], the inhibition of FGF signaling did not prevent hypoblast formation, despite it was suppressed to some extent in our setting. Initially, we suspected that this discrepancy might have originated from the difference in nature of the embryos used, since we worked with parthenotes and previous studies were carried out in naturally fertilized zygotes. To address this, we compared the inhibition of FGF signaling in both parthenotes and naturally fertilized zygotes. We exposed embryos obtained by parthenogenetic activation and by natural mating to 2i condition (PD and CH) at eight-cell stage and cultured until blastocyst stage. Blastocyst-formation rate from 2i-treated embryos in both naturally fertilized zygotes and parthenotes was similar to that in control embryos (Supplementary Table S5). The number of Nanog-positive cells appeared higher in treated embryos in both cases compared with controls (Supplementary Table S5), although the increase did not reach statistical significance. A reduced number of Gata4-positive cells was seen in both parthenotes and naturally fertilized zygotes (Supplementary Table S5); however, the statistical significance was observed only in the parthenotes. Together, these data indicate that 2i has similar effects both on parthenotes and naturally fertilized embryos. In addition, inhibition of hypoblast formation was not observed even when naturally fertilized embryos were exposed to PD.
Embryos collected on E0.5 and E2.5 days postinsemination show similar response to 2i during second-lineage segregation
To mimic more closely the study by Nichols et al. [8], embryos from E2.5 days postinsemination (dpi) were collected. As a control, we also collected zygotes on E0.5, cultured them to eight-cell stage, and subsequently exposed them to 2i. When embryos collected on E0.5 and E2.5 dpi at eight-cell stage were exposed to 2i, neither of them showed complete inhibition of hypoblast formation (Supplementary Table S6). However, we could observe a significant decrease in the number of Gata4-positive cells and a significant increase in the number of Nanog-positive cells in blastocysts obtained from E0.5 B6D2/F1 embryos compared with controls. However, in all cases, embryos showed the clear presence of Gata4-positive cells. These results indicate that except FGF signaling hypoblast formation might be dependent on other signaling pathways and other mechanisms also; therefore, inhibition of FGF signaling always does not necessarily inhibit hypoblast formation.
2i affects the second-lineage segregation only in late blastocysts
To verify whether the stage of blastocyst fixation can influence epiblast and hypoblast allocation, we fixed blastocysts at different time points during blastocyst development. We collected naturally fertilized zygotes on E0.5 and exposed them to 2i from eight-cell stage onward until the time they were fixed. Some early cavitation-stage embryos (before second-lineage segregation) were fixed at 75 h post zygote collection on day 4, some at 90 h, and the rest (fully expanded blastocysts in which second-lineage segregation had already occurred) at 100 h post zygote collection on day 5 (counting the day of collection of zygotes as day 1). Embryos fixed at 75 h and 90 h are referred to as early blastocysts while those fixed at 100 h are referred to as late blastocysts. Similar blastocyst-formation rates were observed in both control and 2i-treated embryos (Supplementary Table S7). In the blastocysts that were fixed at 75 h postcollection, cavitation just started at the time of fixation. Both control and 2i-treated embryos had similar number of Nanog-positive cells at this stage. However, at this stage embryos had not gone through second-lineage segregation yet, as indicated by the lack of Gata4-positive cells in both cases (Supplementary Table S7). The number of Nanog- and Gata4-positive cells was similar in both experimental and control embryos that were fixed at 90 h postcollection. Most embryos at this stage were fully expanded. We observed an increased number of Nanog (24.16±9.51)–positive and a decreased number of Gata4 (48.0±7.58)–positive cells in 2i-treated embryos and compared with controls (Nanog: 17.42±9.71 and Gata4: 13.85±9.31) (Supplementary Table S7) and in embryos fixed 100 h postcollection. However, neither increase in Nanog-positive cells nor decrease in Gata4-positive cells was significant statistically.
Discussion
Small molecules have earlier been shown to influence the lineage segregation in preimplantation embryos [5,8]. Nichols et al. [8] and Yamanaka et al. [5] showed that inhibition of the FGF signaling pathway blocks the hypoblast formation. Moreover, Nichols et al. [8] also showed that simultaneous inhibition of FGF with GSK3β pathway not only inhibits hypoblast formation but also stimulates the proliferation of epiblast cells. Here, we demonstrate that apart from these two pathways, the TGFβ pathway is also involved in the early lineage-segregation events in mouse.
LIF is essential for maintaining mESC pluripotency [25,26] as removal of LIF from culture media results in the rapid differentiation of mESCs. Despite its importance in maintaining pluripotency in mESCs, our results indicate that LIF is unable to modulate early lineage segregation in mouse embryos. Exposure of preimplantation embryos to LIF neither impaired nor enhanced the epiblast or hypoblast lineage in mouse in our setting. Under serum-free culture conditions, signaling through the BMP pathway is essential for the maintenance of mESC pluripotency. BMP signaling mediates mESC pluripotency through induction of the inhibitors of differentiation (Id) genes [21]. The combination of LIF and BMP4 suppresses differentiation of mESCs [21]. Considering the importance of BMP4 signaling in mESCs, it was expected to have positive impact on second-lineage segregation toward epiblast lineage. However, both Nanog- and Gata4-positive cell numbers decreased in the presence of BMP4. The results from LIF and BMP4 indicate that the molecular pathways involved in maintenance of mESC pluripotency might not be involved in proliferation of epiblast cells during mouse embryo development.
Small molecules have been reported to have the capacity to replace one or more of the transcription factors during the reprogramming process. Murine fibroblasts were reprogrammed to induced pluripotent stem cells (iPSCs) by replacing Klf4 with the chemical compound Kenpaullone [23]. Similarly, another small molecule, Forskolin, an activator of PKA, was found to regulate the expression of Klf4/Klf2 and also to be able to substitute these during the reprogramming to iPSCs [19]. Importantly, Forskolin together with LIF, PD, and CH was able to convert the primed hESCs to naive hESCs [19]. Since Kenpaullone and Forskolin support the naive pluripotent state, we expected them to increase epiblast formation. However, kenpaullone was suppressive for both epiblast and hypoblast proliferation whereas forskolin did not affect either of them. Hence, exogenous activation of PKA signaling also does not influence the early lineage commitment decisions in mice.
The TGFβ pathway plays a key role in the maintenance of the primed state of pluripotency [14,15]. Studies in human have shown that the TGFβ/Activin A pathway plays an important role in the self-renewal of hESCs [27,28]. Activation of TGFβ pathway stimulates SMAD2/3 that maintains pluripotency in hESCs by controlling the expression of Nanog [29,30]. Activin A supports the derivation and maintenance of mEpiSCs [14,15]. Culturing naive mESCs in Activin A converts the cells to primed state of pluripotency [31]. Inhibition of the TGFβ pathway by SB together with inhibition of FGF signaling by PD was shown to yield mESCs with 100% efficiency [32]. All these studies show the importance of TGFβ signaling in maintenance, regulation, and establishment of pluripotency in stem cells. Our data show for the first time that the TGFβ signaling is also important for regulating early lineage segregation in mouse.
We found that inhibition of this pathway with SB or A83 increases the proliferation of epiblast cells, similar to the “2i” condition reported by Nichols and Yamanaka et al. [5,8]. Inhibition of the TGFβ pathway in mouse embryos does not compromise the function of the epiblast development, as these epiblast cells are capable of giving rise to ESCs. Since the number of first stem cell colonies obtained after trypsinization of the ICM outgrowth was higher in SB-treated embryos than in control embryos, we speculate that SB treatment might increase the ESC derivation efficiency from the single epiblast cell as well. The inhibition of TGFβ signaling does not function by altering the time of expression of either Nanog or Gata4, as both SB-treated and nontreated embryos showed similar kinetics of expression of these markers.
Our results further demonstrate that inhibition of this pathway also influences the hypoblast formation but only in a dose-dependent manner. Low doses of SB do not influence hypoblast cells when added at any stage of embryonic development while higher doses reduce the hypoblast formation when the small molecule is added as early as at the two-cell stage. However, addition of SB from four-cell stage does not aid to the hypoblast reduction. Our time sequential analysis showed that Gata4 is completely absent in two-cell mouse embryos (collected after 24 h postactivation), but it is expressed in four-cell embryos (collected after 48 h postactivation). It is lost completely at morula stage and re-expression of Gata4 is observed at blastocyst stage. Since four-cell embryos already have some expression of Gata4, addition of SB from four-cell or morula stage does not affect the hypoblast formation. Our results indicate that a low concentration of SB acts only on the epiblast lineage, but a higher concentration affects both the epiblast and the hypoblast lineages if cells are exposed for a longer time period.
Interestingly, no change in the number of ICM cells was observed after addition of the SB inhibitor, implying that SB may work by shifting the hypoblast lineage toward epiblast. When comparing stage-specific addition of SB, addition of SB from the morula stage onward showed the highest number of Nanog-positive cells in mouse blastocysts when compared with other groups. This provides insight that the right timing for modulating TGFβ signaling by use of small molecules during embryonic development starts from the morula stage onward rather than at earlier stages of preimplantation development. Still, irrespective of the time point of addition of SB in developing mouse embryos, the presence of SB results in a significant increase in epiblast proliferation.
SB is an inhibitor of activin receptor-like kinase (ALK) receptors—ALK4, ALK5, and ALK7—and it represses the phosphorylation of SMAD2 [33]. In mESCs, SB treatment, along with suppression of SMAD2, increases the level of Id (Id1 and Id3) genes [34]. Id1 maintains the self-renewal of mESCs by upregulation of Nanog [35]. Similar mechanisms might be controlling the increased Nanog expression in embryos treated with SB. Moreover, activation of the TGFβ pathway with Activin A reduced the number of Nanog-positive cells. Since Activin A in combination with FGF is capable of converting mESCs to mEpiSCs [31], this compound was not expected to aid to the proliferation of epiblast. Activin and SB play antagonistic roles during lineage segregation as well. Hence from our results, it can be concluded that like in mESCs, the TGFβ pathway is involved in second-lineage segregation during mouse embryonic development as well.
Inhibition of the three pathways FGF/Erk, GSK3β, and TGFβ works in synergy to increase the proliferation of epiblast cells as the combination of 2i+SB significantly increased cells in the epiblast lineage than only 2i or SB. However, unlike previous studies, inhibition of FGF signaling could not inhibit the hypoblast formation completely in our setting as was previously reported [5,8]. Even under seemingly identical conditions, we still observed hypoblast cells in embryos although their number was significantly reduced compared with controls. Our results are in line with what was reported in human embryos cultured in 2i [9]. Therefore, we propose that additional mechanisms governing hypoblast formation events do exist besides FGF signaling, which need to be further investigated. Our results also demonstrate that inhibition of FGF and GSKβ signaling does not affect the number of Nanog- and Gata4-positive cells in early cavitating and expanded blastocysts. The effect is only observed in late blastocysts, which have already gone through second-lineage segregation. Therefore, the time interval when embryos are evaluated should be revealed in detail. Our results also demonstrate that the 2i+SB combination also reduces the proliferation of the hypoblast lineage. Hence, these combined molecules induce the proliferation of epiblast cells while reducing the proliferation of hypoblast cells.
Activation of the JAK/STAT pathway or PKA or BMP4 signaling during mouse embryonic development is not involved in the second-lineage segregation fate in mouse embryos. Activation of the TGFβ pathway with Activin A and inhibition of the GSK 3β pathway by Kenpaullone reduces the number of cells in epiblast lineage. Second-lineage segregation in mouse preimplantation embryos is profoundly affected by the inhibition of the TGFβ pathway by SB. The use of SB (5–20 μM) during mouse embryonic development results in the allocation of an increased number of cells to the epiblast lineage. Within this range of concentrations, as the concentration of the inhibitor of the TGFβ pathway increases, there is subsequent reduction in the number of cells in the hypoblast lineage. The combined inhibition of the three pathways FGF/Erk, GSK3β, and TGFβ has a stronger effect on lineage segregation than 2i or SB alone. We conclude that the morula stage might be the right time for the addition of small molecules rather than other stages of embryonic development in mice.
Supplementary Material
Acknowledgments
This research was funded by a doctoral grant provided by the Special Research Funds (Bijzonder Onderzoeksfonds, BOF, University Ghent) awarded to S.G. (grant number 01D04212). P.D.S. is holder of a fundamental clinical research mandate by the Research Foundation—Flanders (FWO). The authors would like to thank Ferring Pharmaceuticals (Aalst, Belgium) for an unrestricted educational grant.
Author Disclosure Statement
Authors declare no conflicts of interest.
References
- 1.Marikawa Y. and Alarcon VB. (2009). Establishment of trophectoderm and inner cell mass lineages in the mouse embryo. Mol Reprod Dev 76:1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossant J. and Tam PPL. (2009). Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136:701–713 [DOI] [PubMed] [Google Scholar]
- 3.Nichols J. and Smith A. (2011). The origin and identity of embryonic stem cells. Development 138:3–8 [DOI] [PubMed] [Google Scholar]
- 4.Rossant J. (2004). Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol 15:573–581 [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka Y, Lanner F. and Rossant J. (2010). FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137:715–724 [DOI] [PubMed] [Google Scholar]
- 6.Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P. and Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453:519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols J, Jones K, Phillips JM, Newland SA, Roode M, Mansfield W, Smith A. and Cooke A. (2009). Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med 15:814–818 [DOI] [PubMed] [Google Scholar]
- 8.Nichols J, Silva J, Roode M. and Smith A. (2009). Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136:3215–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Jeught M, O'Leary T, Ghimire S, Lierman S, Duggal G, Versieren K, Deforce D, Lopes SCdS, Heindryckx B. and De Sutter P. (2013). The combination of inhibitors of FGF/MEK/Erk and GSK3 beta signaling increases the number of OCT3/4-and NANOG-positive cells in the human inner cell mass, but does not improve stem cell derivation. Stem Cells Dev 22:296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuijk EW, van Tol LTA, Van de Velde H, Wubbolts R, Welling M, Geijsen N. and Roelen BAJ. (2012). The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development 139:871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JM, Nottle MB, Vassiliev I, Mitchell M. and Lane M. (2012). Insulin increases epiblast cell number of in vitro cultured mouse embryos via the PI3K/GSK3/p53 pathway. Stem Cells Dev 21:2430–2441 [DOI] [PubMed] [Google Scholar]
- 12.Evans MJ. and Kaufman MH. (1981). Establishment in culture of pluripotent cells from mouse embryos. Nature 292:154–156 [DOI] [PubMed] [Google Scholar]
- 13.Martin GR. (1981). Isolation of a pluripotent cell-line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem-cells. Proc Natl Acad Sci U S A 78:7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, de Sousa Lopes SMC, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA. and Vallier L. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195 [DOI] [PubMed] [Google Scholar]
- 15.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL. and McKay RDG. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199 [DOI] [PubMed] [Google Scholar]
- 16.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS. and Jones JM. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 17.O'Leary T, Heindryckx B, Lierman S, van Bruggen D, Goeman JJ, Vandewoestyne M, Deforce D, Lopes SMCdS. and De Sutter P. (2012). Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat Biotechnol 30:278–282 [DOI] [PubMed] [Google Scholar]
- 18.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, et al. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature 504:282–286 [DOI] [PubMed] [Google Scholar]
- 19.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW. and Jaenisch R. (2010). Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A 107:9222–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niwa H, Burdon T, Chambers I. and Smith A. (1998). Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 12:2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying QL, Nichols J, Chambers I. and Smith A. (2003). BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–292 [DOI] [PubMed] [Google Scholar]
- 22.Vallier L, Alexander M. and Pedersen RA. (2005). Activin/nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 118:4495–4509 [DOI] [PubMed] [Google Scholar]
- 23.Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, Lairson LL, Charette BD, et al. (2009). Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A 106:8912–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M. and Imamura T. (2005). The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci 96:791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M. and Rogers D. (1988). Inhibition of pluripotential embryonic stem-cell differentiation by purified polypetides. Nature 336:688–690 [DOI] [PubMed] [Google Scholar]
- 26.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA. and Gough NM. (1988). Myeloid-leukemia inhibitory factor maintains the developmental potential of embryonic stem-cells. Nature 336:684–687 [DOI] [PubMed] [Google Scholar]
- 27.Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC. and Hayek A. (2005). Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 23:489–495 [DOI] [PubMed] [Google Scholar]
- 28.Xiao L, Yuan X. and Sharkis SJ. (2006). Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells 24:1476–1486 [DOI] [PubMed] [Google Scholar]
- 29.Xu R-H, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R. and Thomson JA. (2008). NANOG is a direct target of TGF beta/Activin-mediated SMAD signaling in human ESCs. Cell Stem Cell 3:196–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MWB, Cho CHH, Martinez A, et al. (2009). Activin/nodal signalling maintains pluripotency by controlling Nanog expression. Development 136:1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W. and Smith A. (2009). Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136:1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassani S-N, Totonchi M, Farrokhi A, Taei A, Larijani MR, Gourabi H. and Baharvand H. (2012). Simultaneous suppression of TGF-beta and ERK Signaling contributes to the highly efficient and reproducible generation of mouse embryonic stem cells from previously considered refractory and non-permissive strains. Stem Cell Rev 8:472–481 [DOI] [PubMed] [Google Scholar]
- 33.Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ. and Hill CS. (2002). SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74 [DOI] [PubMed] [Google Scholar]
- 34.Du J, Wu Y, Ai Z, Shi X, Chen L. and Guo Z. (2014). Mechanism of SB431542 in inhibiting mouse embryonic stem cell differentiation. Cell Signal 26:2107–2116 [DOI] [PubMed] [Google Scholar]
- 35.Romero-Lanman EE, Pavlovic S, Amlani B, Chin Y. and Benezra R. (2012). Id1 maintains embryonic stem cell self-renewal by up-regulation of Nanog and repression of brachyury expression. Stem Cells Dev 21:384–393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.