Abstract
To date, expansion of bone-marrow-derived mesenchymal stem cells (MSCs) is typically carried out on two-dimensional (2D) tissue culture plastic. Since this 2D substratum is very different from the physiological situation, MSCs gradually lose their unique multipotent properties during expansion. Recently, the role of the extracellular matrix (ECM) microenvironment (“niche”) in facilitating and regulating stem cell behavior in vivo has been elucidated. As a result, investigators have shifted their efforts toward developing three-dimensional (3D) scaffolds capable of functioning like the native tissue ECM. In this study, we demonstrated that stromal-cell-derived ECM, formed within a collagen/hydroxyapatite (Col/HA) scaffold to mimic the bone marrow “niche,” promoted MSC proliferation and preserved their differentiation capacity. The ECM was synthesized by MSCs to reconstitute the tissue-specific 3D microenvironment in vitro. Following deposition of the ECM inside Col/HA scaffold, the construct was decellularized and reseeded with MSCs to study their behavior. The data showed that MSCs cultured on the ECM-Col/HA scaffolds grew significantly faster than the cells from the same batch cultured on the regular Col/HA scaffolds. In addition, MSCs cultured on the ECM-Col/HA scaffolds retained their “stemness” and osteogenic differentiation capacity better than MSCs cultured on regular Col/HA scaffolds. When ECM-Col/HA scaffolds were implanted into immunocompromised mice, with or without loading MSCs, it was found that those scaffolds formed less bone as compared with regular Col/HA scaffolds (i.e., without ECM), in both cases of with or without loading MSCs. The in vivo study further confirmed that the ECM-Col/HA scaffold was a suitable mimic of the bone marrow “niche.” This novel 3D stromal-cell-derived ECM system has the potential to be developed into a biomedical platform for regenerative medicine applications.
Introduction
To date, expansion of mesenchymal stem cells (MSCs) for therapeutic applications has been predominantly performed on standard two-dimensional (2D) substrates (e.g., cell culture flasks). Expansion of MSCs on 2D substrates is problematic as the cells spontaneously differentiate into more committed cell lineages and gradually “lose” their stem cell properties (“stemness”).1,2 Further, with extensive passaging the cells senesce due to DNA damage and can transform to become cancerous. A recent study reported spontaneous malignant transformation of nearly 50% of MSCs in long-term cultures.3 This issue becomes most critical when expanding MSCs for clinical applications, which necessitate a very large number of cells (several million per kilogram of patient's weight).4 Taken together, these issues warrant a different approach for expanding MSCs in vitro. Investigators have recognized that the loss of “stemness” arises, in part, from the fact that in vivo MSCs reside in a three-dimensional (3D) environment embedded within a tissue-specific extracellular matrix (ECM).5–8 It, therefore, logically follows that culturing MSCs in a tissue-specific 3D environment may resolve some of the issues associated with 2D expansion.
The prevalent choice for culturing cells in a 3D environment is a scaffolding biomaterial. The scaffold functions as the in vivo ECM by providing structural and functional microenvironment (i.e., “niche”) for cell growth, migration, and differentiation.9–12 Although the composition of ECM environment is unique to each tissue, the major components of ECMs are collagens, fibronectin, laminin, and various types of glycoaminoglycans and proteoglycans.13 ECM, together with growth factors, cytokines, and interactions with other cell types, renders the biological and physical cues that dictate MSC function and overall fate.14–17
Important in vitro and in vivo studies have elucidated some of the mechanisms by which bone marrow ECM dictates the fate of MSCs.18–22 For example, bone marrow stromal cells harvested from knockout mice lacking the ECM component biglycan exhibited defects in the ability to differentiate into osteoblasts.23 In two other studies, MSCs that were cultured on ECM made by bone marrow cells exhibited enhanced proliferation and restrained osteogenic differentiation while preserving their multidifferentiation capacity.24,25 These studies underscore the role of the ECM in directing the proliferation of MSCs.
Though invaluable to our current understanding of the role of ECM on MSC function, the majority of these studies have been solely performed on 2D substrates.25–28 To further mimic an in vivo situation, a 3D stem cell culture system that represents the physiological architecture of the bone marrow tissue may offer new insights on MSC function. Researchers utilize a variety of scaffolds to study the behavior of MSCs in 3D cultures.29–34 Typically, these 3D scaffolds are composed of foreign materials (either natural or synthetic) and often elicit an atypical cellular response. Therefore, to study the behavior of MSCs in vitro, an ideal 3D environment should be composed of materials that mimic the native tissue. That is, a scaffold should exhibit similar mechanical, architectural, and compositional properties as exhibited in the in vivo bone marrow niche.26,29
In our previous work, the design and fabrication of a biomimetic collagen/hydroxyapatite (Col/HA) composite scaffold was described.35 The Col/HA scaffold exhibited properties similar to that of trabecular bone in terms of tissue architecture and composition. It was demonstrated that through its interconnected pore design and high (>95%) overall porosity, the Col/HA scaffold was highly biocompatible for prolonged culture of MSCs.
To better mimic the niche in this study, an additional bone marrow component was incorporated into the design of the Col/HA scaffold. More specifically, the Col/HA scaffold was remodeled and replaced with ECM secreted by bone marrow stromal cells. Following ECM deposition, the Col/HA scaffold was decellularized to create an acellular ECM-Col/HA scaffold. Following decellularization, MSCs were reseeded on the ECM-Col/HA scaffold for evaluation of their behavior. A comparison of the ECM-Col/HA scaffold with regular Col/HA scaffold without ECM (thereafter referred to as Col/HA scaffold) was used to examine the effect of the stromal-cell-derived ECM on the proliferation and stem cell properties of the MSCs. Finally, the in vivo bone formation potential of ECM-Col/HA and Col/HA scaffolds was compared through a subcutaneous implantation in immunodeficient mice.
Materials and Methods
Isolation of human MSCs
Primary bone-marrow-derived mononuclear cells (MNCs) from human donors 20–30 years of age were purchased from AllCells, LLC (Emeryville, CA). MNCs were cultured on standard culture flasks at a density of 3×105 cells/cm2 in minimum essential media alpha (α-MEM), supplemented with 15% (heat-inactivated MSC qualified) fetal bovine serum, 2 mM L-glutamine, and 1% antibiotic-antimycotic. All cell culture reagents were purchased from Life Technologies (Grand Island, NY). Once cells reached 70–80% confluence, adherent cells were detached and considered as passage-1 MSCs. For all experiments, passage 3 (P.3) MSCs were used.
Cell culture on 3D scaffolds
For 3D cultures, Col/HA scaffolds with the dimensions of 1-cm diameter and 1.5-mm thickness, made of bovine collagen followed with biomimetic mineralization, were used. The preparation and properties of the Col/HA scaffolds were described in our previous work.35 Prior to cell studies, the Col/HA discs were sterilized with ethylene oxide and subsequently prehydrated in media overnight at 37°C. Approximately 1×105 P.3 MSCs were seeded on top of the scaffolds and incubated for 1 h in a 37°C cell-culture incubator allowing cells to attach. Then, medium was added to the bottom of the wells containing the scaffolds. After an additional 2 h of incubation to allow complete cell attachment, the Col/HA constructs were transferred into six-well plates and fully submerged in culture medium.
To confirm cell growth on the Col/HA scaffolds, qualitative and quantitative assays were performed. For qualitative assessment, the cells were stained with a fluorescent live/dead kit (Life Technologies). In this live/dead assay, the cytoplasm of viable cells is stained green (Ex/Em: 495 nm/515 nm) whereas the nucleus of dead cells is stained orange (Ex/Em: 528 nm/617 nm). For quantitative assessment of proliferation, a Quanti-iT PicoGreen dsDNA kit (Life Technologies) was used. In this assay, cell quantification is achieved through the measurement of double-stranded DNA stained by a fluorescent dye. To release the DNA from the cells for quantification, the scaffolds were dissolved in a lysis buffer comprised of 50 mM Tris, 100 mM EDTA, 100 mM NaCl, 1% sodium dodecyl sulfate (SDS), and 1 mg/mL proteinase K. The scaffolds were incubated in the lysis buffer for 48 h in a water bath at 56°C. Following the dissolution of the scaffolds, 100 μL of the cell lysate was added to 100 μL of the PicoGreen working reagent according to the manufacturer's instructions. The amount of DNA in the cell lysate was calculated based on the florescence measurement and pre-established calibration curve between DNA quantities versus fluorescence intensity. During calibration, it was also established that a single MSC contains ∼9.13 pg of DNA; this number is very similar to that found in literature.33 Thus, once the amount of DNA was calculated, a close approximation of the number of cells was derived.
Stromal-cell-derived ECM formation and evaluation
To synthesize the ECM on the 3D Col/HA scaffolds, MSCs were cultured on the scaffolds for 21 days. Following cell culture, histology and environmental scanning electron microscopy (ESEM) techniques were used to evaluate the deposition of the ECM within the constructs.
For histological evaluation of cell and ECM distribution, the ECM-Col/HA constructs were fixed in 10% formalin followed by overnight decalcification with Cal-Ex II (Thermo Fisher Scientific, Waltham, MA). Following decalcification, samples were washed with water and placed in 70% ethanol until processing. For hematoxylin and eosin staining, samples were embedded in paraffin and cut into 10-μm-thick sections.
A Zeiss EVO50-EPS ESEM was used to examine the ECM deposition on the constructs. To accomplish this, the constructs were fixed with 2.5% glutaraldehyde at the end of the culture period and subsequently dehydrated through increasing concentrations of ethanol with a final overnight dehydration step using hexamethyldisilazane. Following complete dehydration, the constructs were sputter coated with gold and imaged at an acceleration voltage of 20 kV.
Decellularization of the ECM-Col/HA scaffold
Following cell culture, the ECM-Col/HA constructs were washed with phosphate-buffered saline (PBS) and subsequently treated with 1% SDS for 1 h at room temperature. Remaining nucleic fragments were removed by treating the constructs with a combination of 0.5 mg/mL DNase I and 0.05 mg/mL of RNase A (both from bovine pancreas) in PBS containing 50 mM Tris-HCL and 10 mM MgCl2 using mild agitation at 37°C for an additional 2 h. To ensure that all cell debris has been eliminated, the Col/HA discs were washed overnight in PBS containing antibiotics followed by an additional overnight wash with α-MEM. After treatment, the ECM-Col/HA discs were stored in media until use.
Qualitative and quantitative fluorescent analyses were used to verify that the decellularization technique was successful and that all the nuclear materials were eliminated. Qualitative analysis was performed using a Hoechst 33342 stain, which emits a blue fluorescent light at a wavelength range of 460–490 nm upon bindings to minor DNA grooves. Therefore, remaining nuclear material will fluoresce blue if present. Quantitative analysis was performed using the Quanti-iT PicoGreen assay as previously described.
Immunohistochemistry (IHC) was used to detect the major ECM proteins synthesized on the Col/HA constructs as well as the integrity of the ECM following the decellularization process. The paraffin-embedded sections were first deparaffinized with xylene followed by an antigen-retrieval process in citrate buffer at 97°C for 15 min. After quenching of endogenous peroxidases and blocking with 5% goat serum, the samples were incubated with primary antibodies (1:100) for 1 h. Four different primary antibodies (goat against human) for type-1 collagen, fibronectin, biglycan, and laminin (Santa Cruz Technology, Santa Cruz, CA) were used in this study to detect the major ECM proteins synthesized on the Col/HA scaffolds. Following primary antibody incubation, the samples were further incubated with biotin-conjugated secondary antibody to perform immunoperoxidase staining using an ABC staining system.
Evaluation of the proliferation and differentiation capacity of cells maintained on ECM-Col/HA scaffold
The effect of the deposited ECM was investigated by comparing the proliferation and phenotype of MSCs cultured on Col/HA and ECM-Col/HA scaffolds. Cell proliferation was measured on days 7, 14, and 21 using the PicoGreen assay. To ensure accurate analysis, scaffolds were washed with PBS and centrifuged to dislodge any remaining dead cells; subsequently, cell/scaffold pellets were stored at −80°C until analysis.
A colony-forming unit fibroblast (CFU-F) assay was used to determine the number of MSCs following 14 days of culture on the ECM-Col/HA and Col/HA 3D scaffolds, respectively. To harvest the cells from the 3D cultures, the constructs were washed with PBS and treated with 0.25% trypsin-EDTA at 37°C for 10 min. The constructs were then further washed with culture media to dislodge any remaining cells and neutralize the trypsin-EDTA. For CFU assay, the cells obtained were seeded in a six-well plate at a density of 300 cells per well. A half-media change was performed every 5 days. After 10 days of culture, the colonies were fixed with 100% methanol and stained with 0.5% crystal violet and enumerated.
For the comparison of osteogenic differentiation capacity between the cells cultured on ECM-Col/HA and Col/HA scaffolds, bone marrow stromal cells (P.3) were first cultured on the two different scaffolds (n=10 per group) for 14 days in growth media. Then, half of the constructs (n=5 per group) were switched to osteogenic induction media, comprised of α-MEM supplemented with 10 nM dexamethasone, 100 μM ascorbic acid, and 10 mM β-glycerophosphate. The osteogenic induction of the two groups was carried out for an additional 21 days (a total of 35 days in culture). Gene expression of ECM proteins (fibronectin, collagen type I, biglycan, and laminin), osteogenic markers (alkaline phosphatase, RUNX2, bone sialoprotein, and osteopontin), and stem cell marker (Nanog) was measured before (i.e., day 14) and after the addition of osteogenic media (i.e., day 35) using qRT-PCR. The fold change in gene expression was compared between the two groups to assess MSC differentiation capacity.
To examine gene expression via qRT-PCR, the constructs were dissected first. Total RNA was extracted from the cells on the constructs using Ultraspec (Biotecx Laboratories, Houston, TX) and reverse transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The transcripts of interest were amplified from cDNA using TaqMan Universal PCR Master Mix and all the primers were purchased from Applied Biosystems. Amplification and detection were carried out with an ABI 7500 Real-Time PCR System (Applied Biosystems). Gene expression was quantified by subtracting the GAPDH threshold cycle (Ct) value from the Ct value of the gene of interest and expressed as 2−ΔCt.
In vivo implantation study
To evaluate and compare the in vivo bone formation of the Col/HA versus ECM-Col/HA scaffolds, four groups were tested: (1) Col/HA, (2) Col/HA+MSCs, (3) ECM-Col/HA, and (4) ECM-Col/HA+MSCs. For the groups containing the cells, the scaffolds were loaded with 2.25×105 (P.3) MSCs and incubated for 3 h allowing cells to attach to the scaffolds. Then, the ECM-Col/HA and Col/HA scaffolds, both with or without carrying cells, were implanted subcutaneously into the dorsal surface of 6–7-week-old immunodeficient mice (n=3 per group). All surgeries were conducted according to the protocols approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee. The constructs were harvested in 8 weeks and immediately fixed in chilled 10% formalin. Postimplantation analysis was performed on the four groups using a Skyscan 1172 micro-computed tomography (Micro-CT) operating at a voltage of 40 kV and a resolution of 6 μm. The amount of HA present on the Col/HA scaffold prior to implantation was treated as the background. Following Micro-CT analysis, the retrieved implants were decalcified with 10% EDTA for histological analysis.
Statistical analysis
Other than the CFU assay that was performed in triplicate, all in vitro cell experiments were performed in quintets (n=5). Results are presented as means±standard deviations. All statistical tests were performed with the aid of GraphPad Prism version 5.00. A two-tailed, unpaired Student's t-test was used to compare between two groups. To compare more than two groups, an analysis of variance was used with a Bonferroni multiple-comparison post-test. A p-value of <0.05 was considered statistically significant.
Results
Formation of bone marrow stromal-cell-derived ECM on Col/HA scaffolds
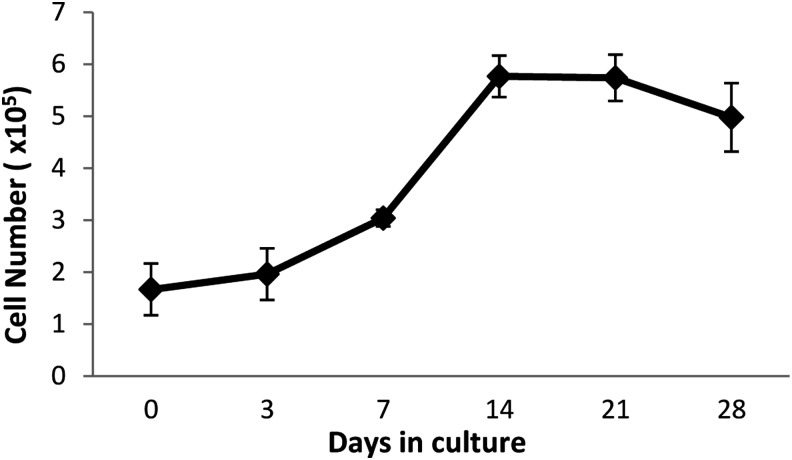
The proliferation kinetics of MSCs cultured on the Col/HA scaffolds were monitored for a period of 28 days (Fig. 1). During the first 7 days of culture, the cells are in lag phase. From day 7 to 14, the MSCs are in log phase. By day 14, cell growth has ceased and maintained a plateau up until day 21. From day 21 onward, some cell death can be seen. Before the plateau phase, very little cell death was observed using the live/dead kit (data not shown). This data demonstrates the excellent biocompatibility of the Col/HA scaffold.
FIG. 1.
Cell expansion kinetics of MSCs cultured on a Col/HA scaffold for 28 days. From day 0 to 7, a steady yet slow increase in cell number can be seen (i.e., “lag phase”). From day 7 to 14, a sharp increase in proliferation is evident (“log phase”), which maintains a plateau to day 21. From day 21 to 28, a decrease in cell number (“cell death”) was seen (n=5). Col/HA, collagen/hydroxyapatite; MSCs, mesenchymal stem cells.
Following the determination of MSC expansion on the Col/HA scaffolds, ECM deposition by MSCs was observed by histological analysis. As shown in Figure 2, MSCs progressively remodeled the original bovine collagen (shown in light red) and replaced it with a network of ECM (shown in light purple and indicated by black arrows). By day 21, most of the original scaffold was replaced by stromal-cell-derived ECM.
FIG. 2.
Hematoxylin-and-eosin-stained images of MSCs cultured on the Col/HA scaffolds at days 3, 7, 14, and 21; scale bars are 500 μm. Note the progressive remodeling of the native Col/HA scaffold with the formation of MSC-derived ECM (indicated by black arrows). ECM, extracellular matrix. Color images available online at www.liebertpub.com/tec
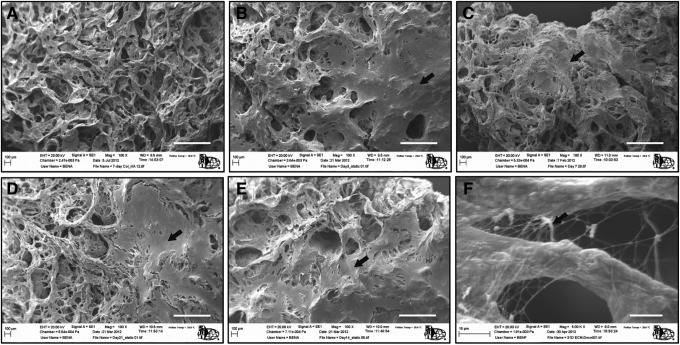
Images obtained from ESEM, shown in Figure 3, further confirmed the deposition of ECM with time. Extensive ECM layer was already formed on the construct after 3 days of culture; yet, the porosity of the constructs was only minimally reduced. This fact was further corroborated by measuring overall construct porosity using helium pycnometry before and after ECM deposition.35 It was shown that without ECM the overall porosity of the Co/HA scaffold was 96.7% whereas after ECM synthesis (i.e., day 21) the porosity was only slightly reduced to 94.1%.
FIG. 3.
Electron micrographs of the Col/HA scaffolds before cell culture (A) and 3 (B), 7 (C), 14 (D), and 21 (E) days after culture. At day 3, MSC-derived ECM (indicated by black arrows) was already evident on the Col/HA scaffold with a considerable ECM network formed by day 21. A high-resolution image (F) showing ECM synthesis at day 21; scale bars are 500 μm in (A–E) and 10 μm in (F).
Decellularization of Col/HA constructs
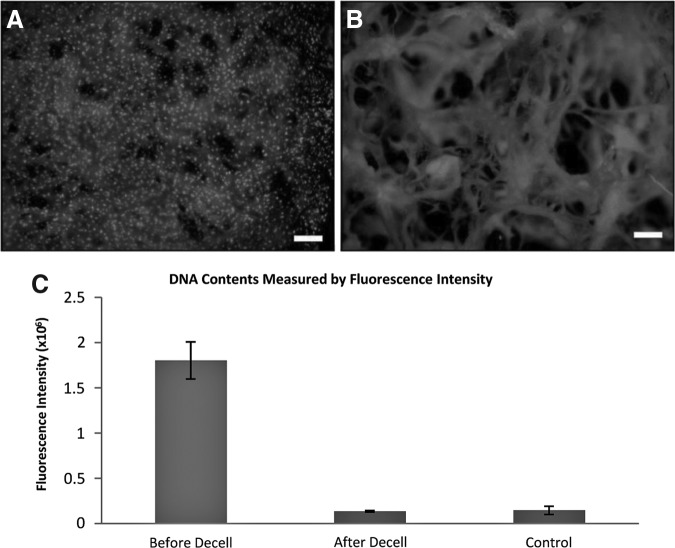
To evaluate that all the cellular phases have been removed, the cells on the Col/HA were fluorescently stained with Hoechst and PicoGreen. Qualitative analysis using Hoechst demonstrated that before the decellularization process (i.e., day 21), a plethora of cells (shown as bright dots in Fig. 4A) can be seen on the Col/HA construct. In contrast, after decellularization, only the material of the Col/HA scaffold was seen in the background with no visible cells on the scaffold (Fig. 4B). To validate these results, quantitative analysis compared the nuclear content on the construct before and after decellularization and on Col/HA scaffold without seeding cells (i.e., control), which demonstrated that all the nuclear materials were indeed removed (Fig. 4C).
FIG. 4.
Qualitative (A, B) and quantitative (C) analyses of decellularization. Before decellularization a large number of cells is evident within the Col/HA scaffold (A); after decellularization only the background Col/HA is visible (B); scale bars in (A, B) are 100 μm. The quantitative analysis (C) shows the fluorescence intensity before and after cell removal. The intensity of decellularized scaffold is similar to that of the control group, indicating the cells on the scaffold have been removed (n=5). Control group in (C) is Col/HA scaffolds without cells.
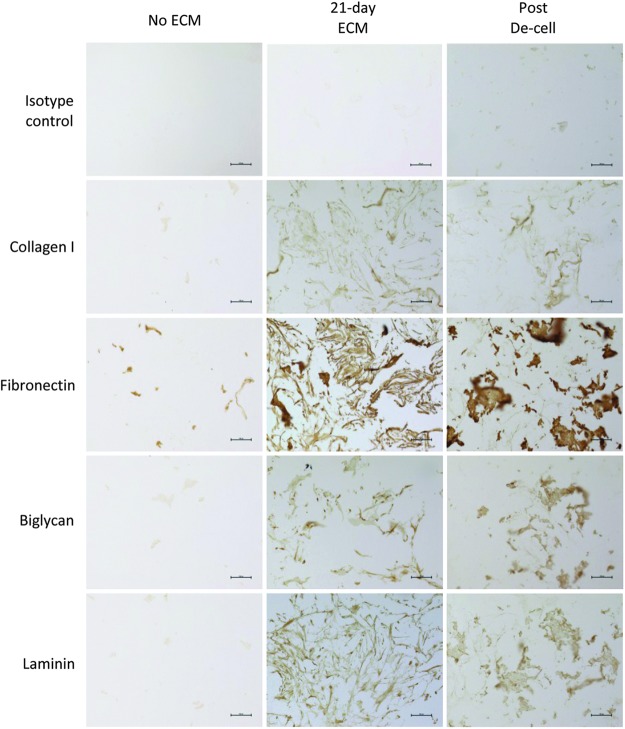
The composition of the ECM-Col/HA scaffold was identified via IHC focusing on four major human ECM proteins, namely, collagen type I, fibronectin, laminin, and biglycan. Figure 5 shows minimal differences in protein contents on the ECM-Col/HA scaffolds before and after cell removal, indicating that the decellularization process had minimal deleterious effect on the four major ECM components and the overall integrity of the 3D ECM network was preserved.
FIG. 5.
Immunohistochemistry images illustrating the effect of the decellularization process on four major ECM proteins, namely, collagen I, fibronectin, biglycan, and laminin. The images show similar amounts of the proteins before and after decellularization, indicating that the process had no adverse effects on ECM integrity. Isotype controls are shown on the first row of the figure. Scale bars are 100 μm. Color images available online at www.liebertpub.com/tec
Cell proliferation and differentiation capacity on decellularized ECM-Col/HA scaffold
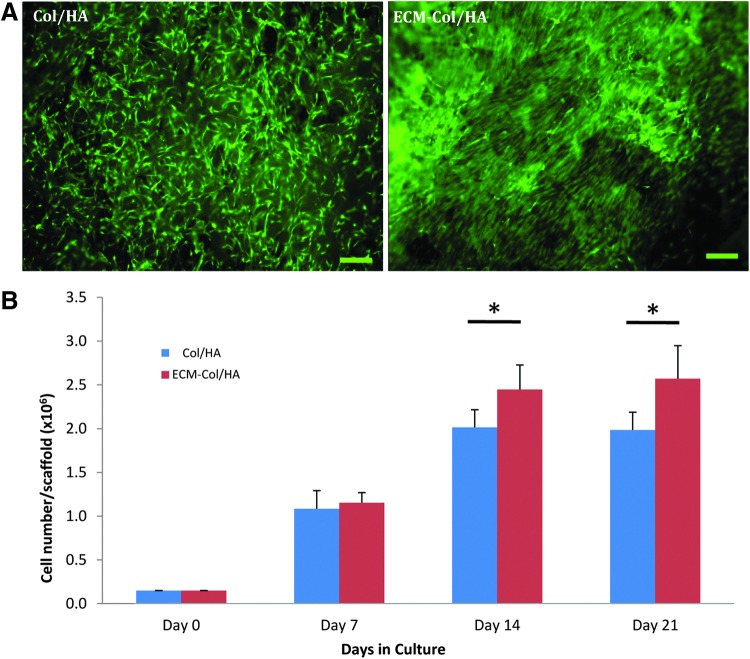
After verification of the decellularization process, the proliferation of MSCs cultured on Col/HA and ECM-Col/HA scaffolds was compared (Fig. 6). Already at day 1 postculture, marked differences could be observed between the groups in terms of cell alignment; specifically, in the ECM-Col/HA scaffold, the MSCs were attached and elongated according to the arrangement of the ECM proteins, whereas in the Col/HA scaffold the MSCs were sporadically attached with random directionality (Fig. 6A). Quantitative analysis comparing the two groups revealed that MSCs cultured on ECM-Col/HA scaffolds proliferated significantly faster than the cells cultured on the Col/HA scaffolds on days 14 and 21 (Fig. 6B).
FIG. 6.
Qualitative (A) and quantitative (B) comparison of MSC proliferation on ECM-Col/HA and Col/HA scaffolds. After 1 day in culture (A), MSCs grown on the ECM-Col/HA scaffold showed remarkable alignment in the direction of the ECM proteins, compared with the random orientation of the cells when cultured on the regular Col/HA scaffold; in panel (A), live MSCs are stained green and scale bars are 100 μm. Growth kinetics of MSCs on the two types of scaffolds on days 0, 7, 14, and 21 (B) revealed significantly more cells on the ECM-Col/HA scaffold after 14 and 21 days in culture (n=5; *p=0.023 and 0.015, respectively). Color images available online at www.liebertpub.com/tec
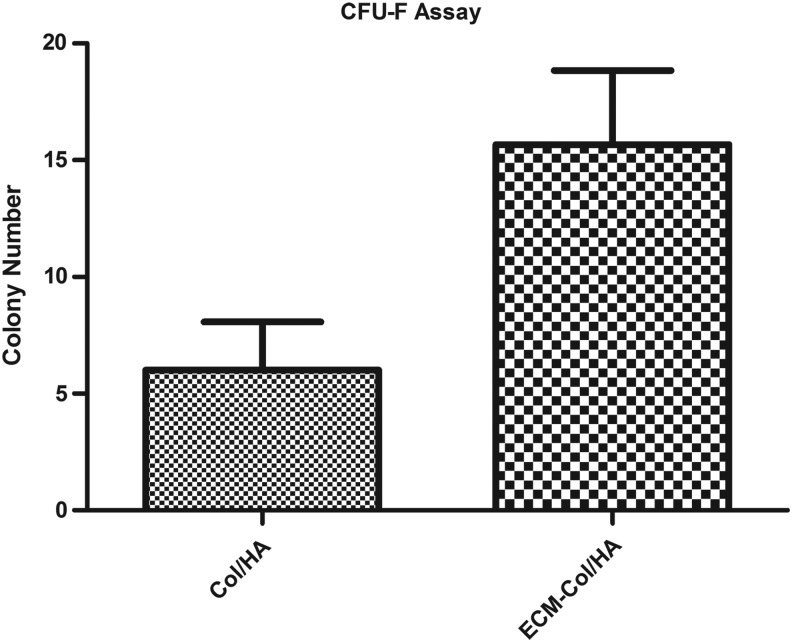
The clonogenic ability of MSCs (CFU-F assay) was also assessed (Fig. 7) on the two types of scaffolds and complemented the cell proliferation data. More specifically, the MSCs that were cultured on the ECM-Col/HA scaffolds exhibited a larger number of colonies, which correlates to higher numbers of primitive MSCs,14,36 than MSCs cultured on the Col/HA scaffolds. Taken together, these sets of data indicate that the stromal-cell-derived ECM promotes MSC proliferation and facilitates the retention of primitive MSCs on the ECM-Col/HA scaffold.
FIG. 7.
Comparison of colony numbers obtained from MSCs cultured for 14 days on Col/HA and ECM-Col/HA scaffolds revealed that more colonies (n=3; p=0.06) were obtained from cells cultured on the ECM-Col/HA scaffolds, indicating that the ECM-Col/HA scaffolds facilitated the retention of higher number of MSCs. CFU-F, colony-forming unit fibroblast.
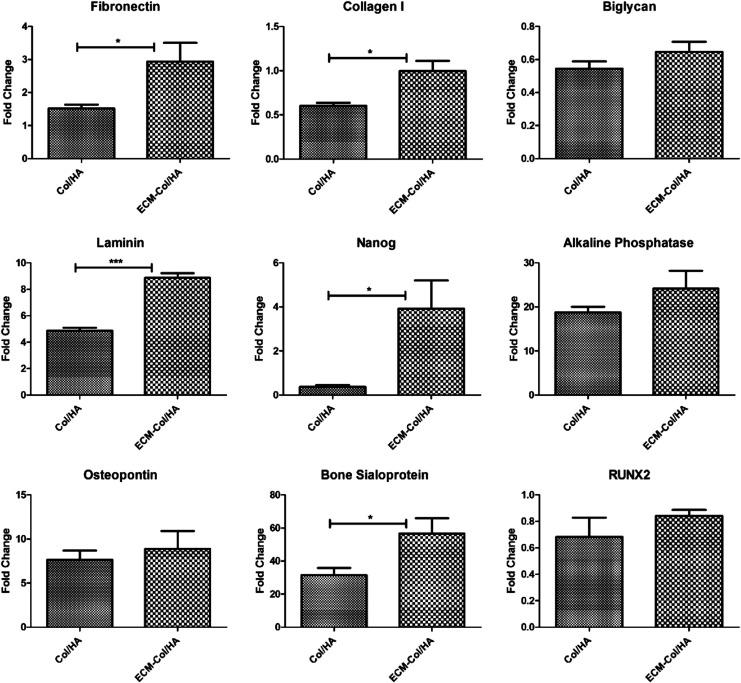
The osteogenic differentiation capacity of the MSCs cultured on the two different types of scaffolds was then evaluated (Fig. 8). The data show the fold change in mRNA expression before and after the addition of osteogenic induction media to the cells. After 21 days of cells treated with induction media, the expression of all the genes tested was higher when the cells were maintained on the ECM-Col/HA versus on the Col/HA scaffolds. Particularly, the comparisons of fibronectin, collagen type I, laminin, bone sialoprotein, and Nanog were significant. The data suggest that the stromal-cell-derived ECM on the Col/HA scaffold preserved the osteogenic differentiation capacity of MSCs.
FIG. 8.
Relative transcripts of osteoblastic cell markers and a stem cell marker expressed by MSCs cultured on Col-HA and ECM-Col/HA scaffolds for 21 days in osteogenic induction media. All tested transcripts were upregulated in cells cultured on the ECM-Col/HA scaffold, as compared with cells cultured on standard Col/HA, which suggests that the ECM-Col/HA scaffold preserved the osteogenic differentiation capacity of MSCs (n=5; *p<0.05, ***p<0.001).
In vivo bone formation
Following in vitro analysis, the two types of scaffolds (with or without carrying cells) were implanted into immunocompromised mice. Micro-CT analysis revealed that all groups significantly increased in mineralized tissue in comparison with the scaffolds before implantation (data not shown). However, the bone volume, trabecular number, and apparent density values were higher in the Col/HA groups, as compared with the ECM-Col/HA groups (Fig. 9). The data suggest that the stromal-cell-derived ECM renders the ECM-Col/HA scaffolds less osteoconductive than the Col/HA scaffolds when implanted subcutaneously, similar to bone marrow microenvironment that retains stem cell's original phenotypes rather than stimulates them for differentiation.
FIG. 9.
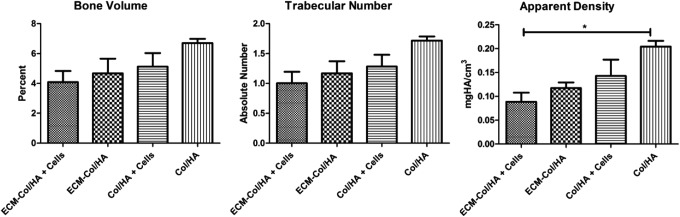
Micro-CT analyses of bone formation after 8 weeks of in vivo implantation of Col/HA and ECM-Col/HA, with or without cells, in immunocompromised mice. Scaffolds without ECM or without cells exhibited more mineral formation in comparison to the scaffolds with ECM or with cells, respectively (n=3; *p<0.05). Micro-CT, micro-computed tomography.
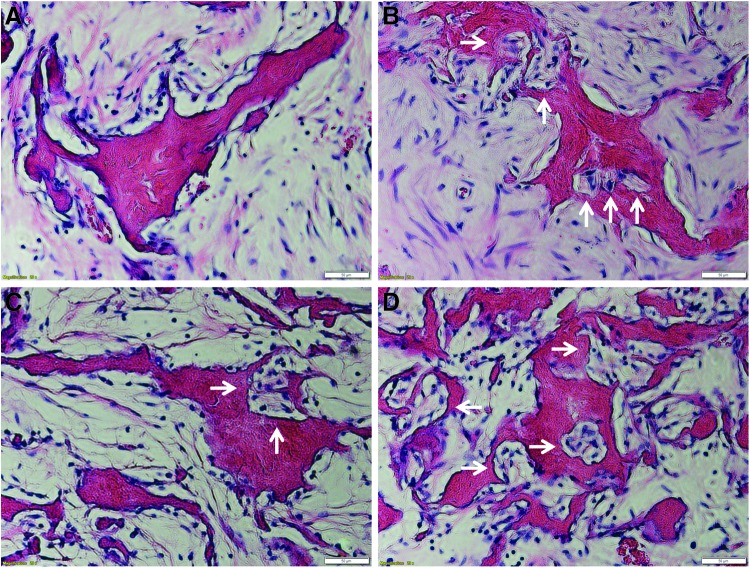
Histological analysis also revealed that the remodeling activities were higher in scaffolds without ECM or cells (Fig. 10), indicated by massive host cell infiltration, dense ECM synthesis, and formation of basic multicellular unit, suggesting that the cells dissolved an area of the scaffold and began to fill it with new bone. The observation is consistent with the results of quantification of mineralization shown in Figure 9.
FIG. 10.
Hematoxylin and eosin staining showing massive host cell infiltration and early bone formation within the implants of (A) ECM-Col/HA+MSCs, (B) ECM-Col/HA only, (C) Col/HA+MSCs, and (D) Col/HA only. White arrows indicate basic multicellular unit (BMU), suggesting that the cells dissolved an area of the scaffolds and began to fill it with new bone. The remodeling activities were increased by A<B<C<D, which is consistent with the observation of skeletal quantification shown in Figure 9. Scale bars are 50 μm. Color images available online at www.liebertpub.com/tec
Discussion and Conclusion
We have reported that a stromal-cell-derived ECM system on 2D substrates can preserve the stemness of MSCs.23–25,28,37 To further mimic an in vivo situation, we took this technology to the next level by developing a 3D tissue-specific scaffold and investigated its effect on the proliferation and differentiation capacity of MSCs. Such 3D scaffolds coated with a native ECM synthesized by bone marrow stromal cells exhibited a biomimetic bone-marrow-like microenvironment (or “niche”) in vitro. Due to the large differences in materials, geometry, patterns of architectures,38,39 stiffness,40,41 and even cell seeding42–44 between 2D tissue culture plastic versus 3D Col/HA scaffolds, it is unreasonable to directly compare the properties of a native ECM coated on 2D tissue culture plastic versus on 3D Col/HA scaffold in controlling the fate of MSCs. Therefore, in the present study, we only focused on comparing the behavior of MSCs maintained on a 3D scaffold made by the same materials with or without coating a native bone-marrow-derived ECM.
An extensive deposition of the ECM network was shown by both histology and ESEM images. Cells began synthesizing ECM on the Col/HA scaffold as early as 3 days after culture, well before cell confluence was reached. It is well known that the cells maintained on 2D tissue culture plastic do not secrete much ECM until they reach confluence. This indicates that the 3D scaffold strongly promotes marrow stromal cells to make their own ECM. The fact that the scaffold was primarily made of bovine collagen type I, and thus reduced the requirements of de novo synthesis, may account for the relative decreased expression of human collagen type I, as shown by IHC analysis.
MSCs cultured on the ECM-Col/HA and Col/HA scaffolds behave quite differently. Already at day 1 postseeding, the cells were highly aligned along the direction of the ECM proteins on the ECM-Col/HA scaffolds in comparison to the random distribution of the cells on the Col/HA scaffolds. Within a few days, the cells were fully embedded within the ECM, a phenomenon similar to that shown in our previous 2D ECM studies.24,25 Our unpublished data indicate that the ECM components provided MSCs with topographical and biological cues for directing cell migration and facilitating the proliferation of MSCs by preventing their contact inhibition.
MSCs proliferated significantly faster on the ECM-Col/HA scaffolds than on the Col/HA scaffolds. The doubling time (DT) of cells grown on the ECM-Col/HA was 65.8 h compared with a DT of 70.1 h for MSCs cultured on the Col/HA scaffolds. The proliferation data were consistent with the results of the CFU-F assay. MSCs cultured on the ECM-Col/HA scaffolds formed a greater number of colonies when compared with MSCs cultured on the Col/HA scaffolds. Primitive MSCs are known to possess shorter DT,14 which results in faster proliferation and a higher overall cell number.
The evaluation of the osteogenic differentiation capacity of MSCs cultured on the two types of scaffolds showed higher expression of bone markers by MSCs cultured on the ECM-Col/HA under the induction of osteogenic differentiation medium, indicating that the stromal-cell-derived ECM facilitated the retention of MSC function and osteogenic differentiation capacity. In general, differentiation of MSCs into osteoblasts can be divided into three main stages: cell proliferation, matrix maturation, and matrix mineralization. During proliferation, MSCs mainly secrete collagen type I, fibronectin, and laminin. During matrix maturation, high levels of alkaline phosphatase are secreted, whereas in matrix mineralization, genes for bone sialoprotein and osteopontin proteins are highly expressed.45 Thus, the increased expression of all these markers shown in our study suggests that MSCs cultured on the ECM-Col/HA progressed through the path of osteogenesis but have not yet fully committed to the osteogenic lineage. Interestingly, this is also supported by the expression of Nanog, which is a transcription factor that inhibits differentiation and maintains the pluripotent capacity of embryonic and adult stem cells.46 Therefore, a significantly higher Nanog expression implies that at least some of the MSC population still retained their primitive properties when they were maintained on the ECM-Col/HA scaffold. Taken together, these in vitro data suggest that the ECM-Col/HA scaffold promoted the proliferation, while retaining primitive MSCs, similar to the in vivo situation.
Micro-CT analysis of the scaffolds after 8-week subcutaneous implantation in immunocompromised mice revealed a large amount of mineralized tissue formation in both types of scaffolds when compared with controls (i.e., before implantation). It is important to note that the Col/HA groups exhibited significantly more mineral formation than the ECM-Col/HA groups. It is possible that the Col/HA coated by the stromal ECM formed a unique microenvironment (similar to that of bone marrow) and therefore diminished the inductivity of the Col/HA for bone formation. The reason why the scaffold loaded with cells formed less skeletal tissue is not very clear. The lack of blood supply to the scaffold may cause a significant cell death after implantation. These dead cells could release harmful substances influencing bone remodeling.
To conclude, a marrow stromal-cell-derived ECM was deposited on a Col/HA scaffold to create a unique biomimetic 3D microenvironment in vitro. In this microenvironment, the proliferation of MSCs was promoted while their differentiation capacity was better preserved. In addition to MSCs, this microenvironment may potentially support the growth of a variety of bone-marrow-resident cells, such as the hematopoietic stem cells. Hematopoiesis requires the stroma, which provides both the structural and functional framework for the production of blood cells, as well as for maintaining the appropriate stem cell pool.15 Thus, the stromal-cell-derived ECM scaffolds can potentially be utilized for supporting hematopoiesis as well as for enriching the pool of other bone-marrow-derived stem cells. The established ECM-Col/HA scaffold could serve as a powerful in vitro model to better mimic the in vivo niche for elucidation of cell behavior, such as migration, proliferation, differentiation, and apoptosis of MSCs. Further, a biomimetic scaffold that preserves MSCs in an undifferentiated state could provide a relatively more homogeneous population of multipotent MSCs for basic stem cell research as well as clinical applications.
Acknowledgment
This work was supported by the National Heart, Lung, and Blood Institute: R21 HL102775.
Disclosure Statement
No competing financial interests exist.
References
- 1.Banfi A., Muraglia A., Dozin B., Mastrogiacomo M., Cancedda R., and Quarto R.Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 28,707, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Kim J., Kang J.W., Park J.H., Choi Y., Choi K.S., Park K.D., et al. . Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res 32,117, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Rosland G.V., Svendsen A., Torsvik A., Sobala E., McCormack E., Immervoll H., et al. . Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69,5331, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Giordano A., Galderisi U., and Marino I.R.From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol 211,27, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Baksh D., Davies J.E., and Zandstra P.W.Soluble factor cross-talk between human bone marrow-derived hematopoietic and mesenchymal cells enhances in vitro CFU-F and CFU-O growth and reveals heterogeneity in the mesenchymal progenitor cell compartment. Blood 106,3012, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Li Z., and Cui Z.Three-dimensional perfused cell culture. Biotechnol Adv 32,243, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Meng X., Leslie P., Zhang Y., and Dong J.Stem cells in a three-dimensional scaffold environment. Springerplus 3,80, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M., Kawashima N., Takashino N., Koizumi Y., Takimoto K., Suzuki N., et al. . Three-dimensional spheroid culture promotes odonto/osteoblastic differentiation of dental pulp cells. Arch Oral Biol 59,310, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Carletti E., Motta A., and Migliaresi C.Scaffolds for tissue engineering and 3D cell culture. Methods Mol Biol 695,17, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Chan B.P., and Leong K.W.Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17Suppl 4,467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikada Y.Challenges in tissue engineering. J R Soc Interface 3,589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvir T., Timko B.P., Kohane D.S., and Langer R.Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6,13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daley W.P., Peters S.B., and Larsen M.Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121,255, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kolf C.M., Cho E., and Tuan R.S.Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9,204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scadden D.T.Rethinking stroma: lessons from the blood. Cell Stem Cell 10,648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoggatt J., and Scadden D.T.The stem cell niche: tissue physiology at a single cell level. J Clin Invest 122,3029, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause D.S., and Scadden D.T.Deconstructing the complexity of a microenvironmental niche. Cell 149,16, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng C.P., Sharif A.R., Heath D.E., Chow J.W., Zhang C.B., Chan-Park M.B., et al. . Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials 35,4046, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Song J.J., and Ott H.C.Organ engineering based on decellularized matrix scaffolds. Trends Mol Med 17,424, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Sellaro T.L., Ravindra A.K., Stolz D.B., and Badylak S.F.Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng 13,2301, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Vorotnikova E., McIntosh D., Dewilde A., Zhang J., Reing J.E., Zhang L., et al. . Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol 29,690, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Gattazzo F., Urciuolo A., and Bonaldo P.Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 1840,2506, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X.D., Shi S., Xu T., Robey P.G., and Young M.F.Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res 17,331, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Chen X.D., Dusevich V., Feng J.Q., Manolagas S.C., and Jilka R.L.Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res 22,1943, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lai Y., Sun Y., Skinner C.M., Son E.L., Lu Z., Tuan R.S., et al. . Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev 19,1095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund A.W., Yener B., Stegemann J.P., and Plopper G.E.The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev 15,371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin H., Yang G., Tan J., and Tuan R.S.Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials 33,4480, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Sun Y., Li W., Lu Z., Chen R., Ling J., Ran Q., et al. . Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J 25,1474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haycock J.W.3D cell culture: a review of current approaches and techniques. Methods Mol Biol 695,1, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Braccini A., Wendt D., Jaquiery C., Jakob M., Heberer M., Kenins L., et al. . Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells 23,1066, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Sikavitsas V.I., Bancroft G.N., Lemoine J.J., Liebschner M.A.K., Dauner M., and Mikos A.G.Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng 33,63, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Zhao F., Chella R., and Ma T.Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: experiments and hydrodynamic modeling. Biotechnol Bioeng 96,584, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zhao F., and Ma T.Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: dynamic cell seeding and construct development. Biotechnol Bioeng 91,482, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Sadr N., Pippenger B.E., Scherberich A., Wendt D., Mantero S., Martin I., et al. . Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix. Biomaterials 33,5085, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Antebi B., Cheng X., Harris J.N., Gower L.B., Chen X.D., and Ling J.Biomimetic collagen-hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng Part C Methods 19,487, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianco P., Riminucci M., Gronthos S., and Robey P.G.Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19,180, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Chen X.D.Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells. Birth Defects Res Part C Embryo Today 90,45, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Holthaus M.G., Stolle J., Treccani L., and Rezwan K.Orientation of human osteoblasts on hydroxyapatite-based microchannels. Acta Biomater 8,394, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Pilia M., Guda T., Shiels S.M., and Appleford M.R.Influence of substrate curvature on osteoblast orientation and extracellular matrix deposition. J Biol Eng 7,23 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert P.M., Havenstrite K.L., Magnusson K.E., Sacco A., Leonardi N.A., Kraft P., et al. . Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329,1078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cukierman E., Pankov R., Stevens D.R., and Yamada K.M.Taking cell-matrix adhesions to the third dimension. Science 294,1708, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Lode A., Bernhardt A., and Gelinsky M.Cultivation of human bone marrow stromal cells on three-dimensional scaffolds of mineralized collagen: influence of seeding density on colonization, proliferation and osteogenic differentiation. J Tissue Eng Regen Med 2,400, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Erickson I.E., Kestle S.R., Zellars K.H., Farrell M.J., Kim M., Burdick J.A., et al. . High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater 8,3027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talukdar S., Nguyen Q.T., Chen A.C., Sah R.L., and Kundu S.C.Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials 32,8927, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lian J.B., and Stein G.S.Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med 3,269, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Han J., Mistriotis P., Lei P., Wang D., Liu S., and Andreadis S.T.Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential. Stem Cells 30,2746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]