Abstract
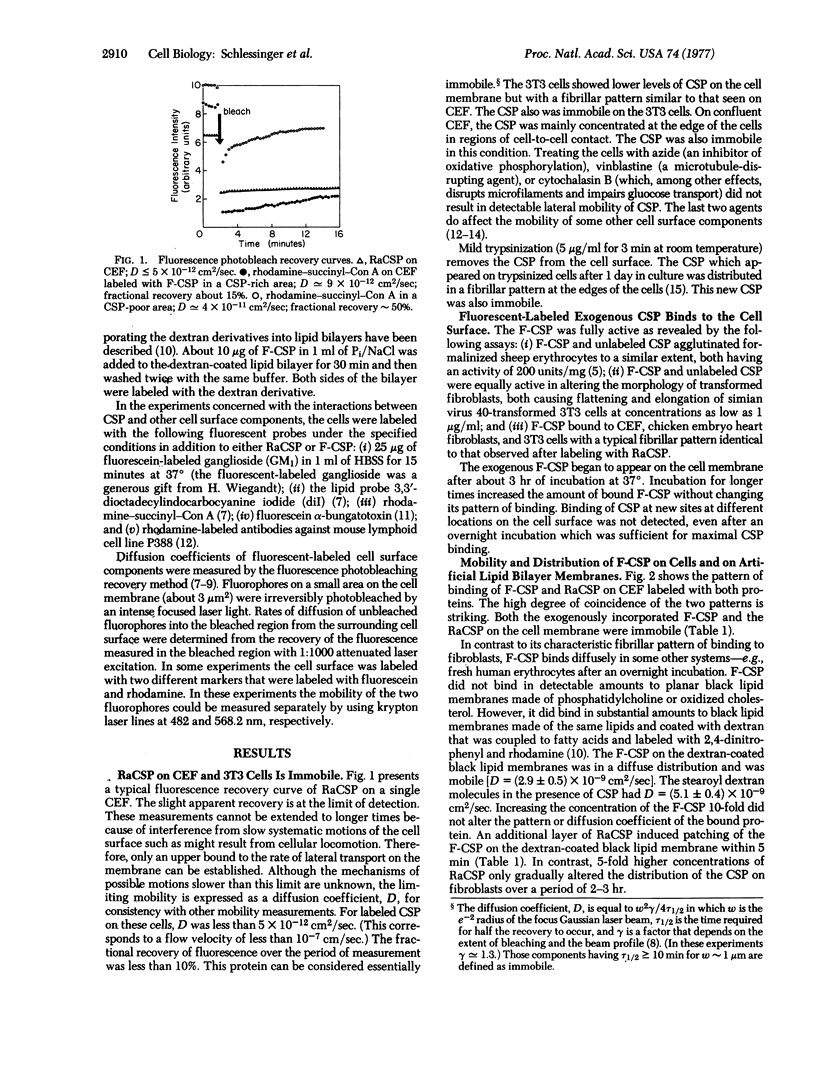
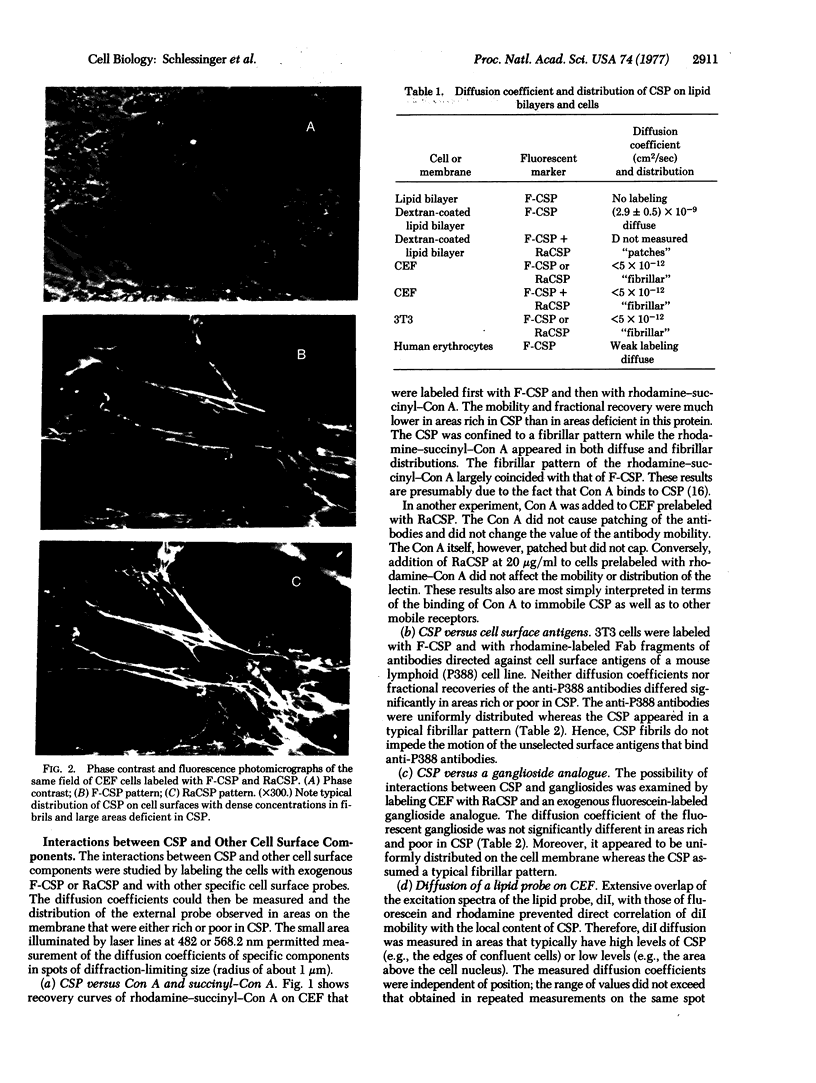
Fluorescence photobleaching recovery and immunofluorescence methods have been used to study the lateral mobility and topographical distribution of a major cell surface glycoprotein (CSP). Both endogenous CSP and fluorescent-labeled exogenous CSP bind to the cell surface in a fibrillar pattern and are immobile on the experimental time scale. Azide, vinblastine, and cytochalasin B do not alter the immobility and cell surface distribution of the CSP molecules. Therefore, oxidative phosphorylation and the cytoskeleton do not seem to be responsible for the properties of the bound glycoprotein. The presence of immobile CSP fibrils does not, however, impede the diffusion of a lipid probe, a ganglioside analogue, or various surface antigens. Therefore, the fibrils apparently do not form a “barrier” across the lipid phase of the plasma membrane. In contrast, concanavalin A binds to CSP and is largely immobile in regions rich in CSP. The presence of immobile concanavalin A receptors in areas or on cells lacking CSP indicates that other types of immobile concanavalin A receptors also exist.
CSP does not bind to lipid bilayers composed of phosphatidylcholine or oxidized cholesterol. It does bind to dextran-coated bilayers as a diffuse distribution of mobile molecules that can patch after addition of antibodies to CSP. The latter result suggests that CSP molecules do not interact strongly with other CSP molecules under these conditions. Exogenous CSP binds to regions on the cell surface that already bear CSP. In view of the apparent weakness of CSP-CSP interactions on the lipid bilayer, it seems possible that the assembly of CSP fibrils is nucleated by cell surface components in addition to CSP.
Keywords: membrane-associated glycoprotein, cell adhesion, photobleaching recovery, protein mobility
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G., Chen L. B. Local inhibition of centripetal particle transport where LETS protein patterns appear on 3T3 cells. Nature. 1977 Mar 31;266(5601):454–456. doi: 10.1038/266454a0. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B. Alteration in cell surface LETS protein during myogenesis. Cell. 1977 Mar;10(3):393–400. doi: 10.1016/0092-8674(77)90026-5. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Bye J. M. Density and cell cycle dependence of cell surface proteins in hamster fibroblasts. Cell. 1974 Oct;3(2):113–120. doi: 10.1016/0092-8674(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Yamada K. M. Direct detection of antigens in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1977 Apr;78(2):483–490. doi: 10.1016/0003-2697(77)90108-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Wartiovaara J., Linder E., Ruoslahti E., Vaheri A. Distribution of fibroblast surface antigen: association with fibrillar structures of normal cells and loss upon viral transformation. J Exp Med. 1974 Dec 1;140(6):1522–1533. doi: 10.1084/jem.140.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]