Abstract
Background: Aging can cause loss of balance, which may lead to physical and psychological problems. As the role of the otolith organs in maintaining postural stability has been emphasized in recent years, the present study investigated the effect of aging on saccular function using cervical vestibular evoked myogenic potentials (cVEMP).
Methods: The participants were assigned into two groups; group one included 31 young adults with a mean age of 22.15 (range: 19-26 yr) and group two consisted of 31 old adults with a mean age of 69.76 years (range: 61-79 yr). All participants hearing sensitivity was normal with no history of balance problems. VEMP was recorded for all subjects using tone burst 500 Hz stimuli at the threshold level and 95 dB nHL intensity level through air-conduction stimulation via an insert receiver.
Results: There was a significant difference in the cVEMP response threshold (p< 0.001), P1 wave latency (p<0.001), P1/N1 amplitude (p< 0.001), and asymmetry ratio of P1/N1 amplitude (p< 0.05) between the two groups. No significant difference was found between the left and right ears or in N1 wave latency between the two groups.
Conclusion: VEMP abnormalities observed in healthy older adults showed the sensitivity of this test in identifying early signs of vestibular dysfunction. VEMP is an easy-to-use test that requires a short time to be performed. Therefore, it can be used as a selective objective screening test to detect vestibular disorders
Keywords: Saccular function, Vestibular evoked myogenic potentials, Aging
Introduction
Age-related structural and functional deterioration of sensory and motor mechanisms, especially of the vestibular system, is a leading cause of dizziness and imbalance in older adults (1, 2). It is well established that older individuals with a history of imbalance and dizziness are at higher risk of fall related injuries, loss of independence, and even death (1), all depicting the importance of determining the effect of aging on the vestibular system (3).
The peripheral vestibular apparatus consists of the labyrinth (semicircular canals and otoliths), vestibular nerve, and a central component consisting of the vestibular nuclei (4). The otoliths (saccule and utricle) register forces related to linear acceleration (2). Vestibular evoked myogenic potential (VEMP) is a reliable clinical tool to assess the accuracy of the sacculo-collic reflex (5), first introduced by Colebatch and Halmagyi (1992) and further described by Colebatch et al. (1994) (6). VEMP is a short-latency myogenic response evoked by loud sounds and recorded using surface electrodes placed over cervical muscles (6). The VEMP response consists of a positive peak (P1) in the ipsilateral sternocleidomastoid (SCM) followed by a negative peak (N1) (6).
The effect of aging on VEMP response has been reported in previous studies (e.g. Su et al. (7), Lee et al. (5), Janky et al. (8), and Tseng et al. (9)). The evidence is accumulated that the aging influences on P1 and/or N1 latencies, although a new study by Nguyen et al. (10) did not report any significant difference between latency measurement and aging. Furthermore, the effects of aging on VEMP amplitude has also been reported in previous studies (3, 5, 9, 10). However, Janky et al. (8) did not find any effect of aging on VEMP amplitude at the threshold level, though amplitude was negatively correlated with age in suprathreshold level.
Age- dependent anatomical and physiological changes in vestibular system has also been reported in previous studies (11-19). As age-related loss of vestibular otolith-ocular function is associated with increased mediolateral measures of sway (20), characterizing the impact of aging on saccular function is important. However, little is known about the influence of aging on the function of otolith organ. In recent years, VEMP has become a standard clinical test of otolith (mainly saccular) function (6). The VEMP is an easy-to-use test that can be performed in a relatively short time, though some previous studies have contradictory recommendations (3, 5, 7-10). The aim of the present study was to evaluate the impact of aging on saccular function using cVEMP response.
Methods
This comparative survey was conducted from February to July 2013. Sixty-two subjects were including, 31 young adults (62 ears) with a mean age of 22.15 ± 1.93yr (range: 19-26yr) comprising 27 females and 4 males as control group, and 31 old adults (50 ears) with a mean age of 69.76 ± 5.14yr (over age 60) comprising 26 females and 5 males, as test group. The mean pure tone average at 500, 1000, and 2000Hz was ≤ 25dB HL for both groups. Inclusion criteria were as follows: participants with no history of middle ear pathology, and no vestibular, cervical and neurological disorders. The study protocol was approved by the Ethics Committee of ShahidBeheshti University of Medical Sciences, and informed consent was obtained from all participants.
Procedures
Adherence to inclusion criteria was confirmed by having all volunteers complete a case history, Berg balance test (to evaluate total balance status), otoscopy and acoustic immittance evaluation (to test middle ear health using an impedance-interacoustic AT235 audiometer, Denmark), and pure tone and speech audiometry (to evaluate the integrity of auditory pathway-interacoustic AC40 audiometer, Denmark). Participants also underwent a cervical VEMP (cVEMP) test performed using electrophysiologic equipment (ICS Charter EP, USA, software version 6.20). To activate the SCM muscle, participants were asked to sit in a chair and turn their head toward the contralateral side shoulder.
For cVEMP recording, 150 responses to air-conducted 500 Hz tone bursts with two-cycle rise/fall and no plateau (Blackman gated) were presented monaurally with rarefaction polarity via an insert receiver. These were averaged with a stimulation rate of 5.1/s. The responses were amplified (5000×) and band-pass filtered (10 Hz-2000Hz). The skin was prepared and the active electrode was placed at the midpoint of the SCM muscle belly; the reference electrode was set on the sternoclavicular junction, and the ground electrode was placed on the forehead. The cVEMP responses were obtained at threshold level (VEMP threshold was defined as the lowest level at which VEMP parameters were repeatable and distinctive) and at 95dB nHL intensity level. VEMP response thresholds were obtained using a down 10, up 5 dB step manner. Each recording was repeated twice to assure reproducibility. To avoid distortion of the results by neck muscle fatigue, participants were trained to rest between trials. The VEMP threshold, P1 and N1 latency, P1/N1 amplitude, and asymmetry ratio were determined for all participants after the VEMP responses were recorded. The asymmetry ratio (AR) between the right and left ears was computed according to the following formula (21).
Data were summarized as means (± SD). All statistical analysis was performed using SPSS software (IBM SPSS Statistics 21). Kolmogorov-Smirnov was used to test normal distribution of the data. The data was analyzed using a paired-samples t-test and independent-samples t-test. Differences were considered significant at p< 0.05
Results
To examine the effect of aging on saccular function, cVEMP results for the older group were compared with the controls. There was no significant difference between VEMP parameters for the right and left ears, therefore, the results for both ears were combined. The VEMP responses revealed that 23 participants had bilateral VEMP response and 4 participants had unilateral VEMP response (in 112 of 124 ears in the 62 participants). Four participants had no VEMP responses in both ears. The VEMP was also recorded for all controls).
Fig. 1 shows the cVEMP waveforms obtained at 95 dB nHL from the right ear for a young and an old adult. The independent-samples t-test demonstrated a significant difference in VEMP response threshold between the two groups (p<0.001). There was also a significant difference in P1 wave latency between the two groups (p<0.001). No significant difference existed for N1 wave latency between the two groups (p=0.06). The independent t-test showed a significant difference in P1/N1 amplitude (p<0.001), and asymmetry ratio of P1/N1 amplitude showed a significant difference between the two groups (p=0.01). The standard deviation, mean threshold level, latency, amplitude, and asymmetry ratio of P1/N1 amplitude of VEMP for the two groups are shown in Table 1.
Fig. 1 .
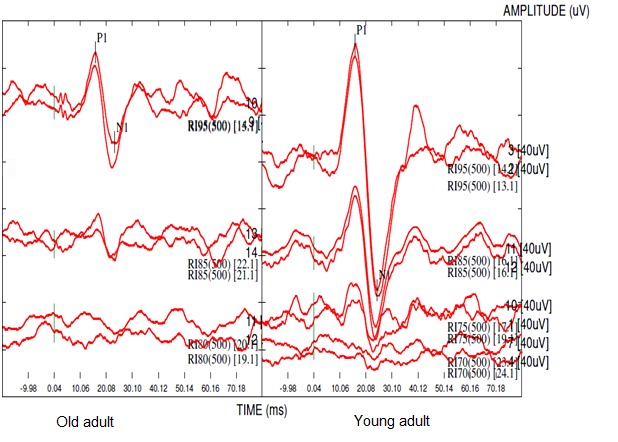
The cervical vestibular evoked myogenic potentials recorded in the right ear of a young and an old adult at 95 dB nHL and threshold intensity level.
Table 1 . Comparison of the means (SDs) of the VEMP parameters in the two study groups .
| VEMP parameters | Age Group | Mean ± SD | Range | p | |
| Threshold (dBnHL) | Older adults (n=49 ears) | 87.65 | ± 5.69 | 75 – 95 | <0.001 |
| Young adults (n=49 ears) | 78.88 | ±5.23 | 65–85 | ||
| p1 latency (ms) | Older adults (n=50 ears) | 16.46 | ± 1.73 | 14.40 – 23.25 | <0.001 |
| Young adults (n=62 ears) | 15.43 | ± 1.23 | 13.50 – 18.40 | ||
| n1 latency (ms) | Older adults (n=50 ears) | 24.09 | ± 2.00 | 20.92 – 31.27 | 0.06 |
| Young adults (n=62 ears) | 24.77 | ± 1.88 | 19.50 – 27.93 | ||
| p1-n1 amplitude(μV) | Older adults (n=50 ears) | 63.79 | ± 41.79 | 22.70 – 199.95 | <0.001 |
| Young adults ( n=62 ears) | 144.14 | ± 55.31 | 34.42 –258.06 | ||
| Asymmetry Ratio (AR%) | Older adults (n=23 persons) | 15.90 | ± 11.83 | 0.00 – 38.33 | 0.01 |
| Young adults (n=31 persons) | 8.38 | ± 8.47 | 0.05 – 34.53 |
SD: standard deviation, ms: millisecond, μV: microvolt, and dB nHL: decibel normalized hearing level.
Discussion
Dizziness and imbalance are common complaints by older adults. Although, in recent years, research has demonstrated the role of otolith function in postural stability (20), few studies have investigated age-related loss of otolith organ function. The purpose of this study was to examine the effect of aging on saccular function in the elderly. The cVEMP measurements were recorded using tone burst 500 Hz stimuli delivered through air conduction and compared between the two study groups.
Unilateral or bilateral omission of cVEMP response in some of the elderly has also been reported in the previous studies (7, 8, 22), which might be due to age- dependent changes in the course of producing the required response.
Effects of age on cVEMP threshold:The results showed that cVEMP thresholdsincreases with an increase in the age, which is in association with previous studies (8, 22, 23). Ochi and Ohashi (23) reported the effects of age on VEMP response in 60 healthy adults. Their study showed a significant correlation between age and VEMP threshold. Welgampola and Colebatch (22) investigated the influence of aging on the vestibule-collic reflex in response to click stimuli and demonstrated that VEMP thresholds increased as age increased. Janky and Shepard studied 46 normal adults ranging in age from 20 to 76 yr. cVEMP responses were recorded at threshold with a click for 250, 500, 750, and 1000 Hz tone burst stimuli and at a suprathreshold level for a 500 Hz tone burst stimuli. They documented a significant difference between age groups for threshold. The increase in cVEMP threshold may be caused by age-related functional changes in the sensory and neural components involved in the projection of cVEMP responses. In fact, aging may affect the cVEMP response pathway from the saccule, scarpa ganglion, inferior vestibular nerve, lateral vestibular nucleus and lateral vestibule-spinal tract to the SCM muscle.
Effects of age on cVEMP latency: The present research demonstrated a difference for P1 wave latency between the two study groups where P1 wave latency increased as age increased. Analysis of the data also indicated that there was no N1 wave latency difference between the two study groups. This may be because the spread of central transmission and the time required to activate the vestibulo-collic reflex that causes the VEMP response changed as age increased. Previous studies have reported inconsistent results on age-related changes in the latency of cVEMP responses. The findings of the present study are in agreement with those of Janky and Shepard (8) who reported no difference between groups for N23 latency in response to click and tone burst stimuli. Su et al (7) reported that the P13 latency increased as age increased, although this difference was not significant. These results, however, are in disagreement with those of Nguyen et al. (10), who found no correlation between latency of VEMP response and age. Possible explanations for this discrepancy are differences in recording techniques, such as variations in stimuli rise/fall time, and filter setting, for instance, in Nguyen et al., tone bursts stimuli with a linear envelope and 1ms rise/fall time and 2 ms plateau time were used. Age - dependent variations observed in latency of cVEMP response might probably be due to the created changes in processing messages from otolith organs by central nervous system but might not be related to reduced function in peripheral vestibular system.
Effects of age on cVEMP P1/N1amplitude: The results of the present study showed a difference in P1/N1 amplitude between the two groups. In other words, the P1/N1 amplitude decreased as age increased. These findings confirm the results of other studies (3, 5, 7, 22-25). This decrease may be caused by several factors, such as age-dependent anatomical and physiological changes in the vestibular end organ and central pathways or age-related changes in the tonic SCM muscle electromyogram level.
Effects of age on cVEMPasymmetry ratio: The results of the present study also showed no difference between left-right cVEMP parameters, which again confirms the results of the previous research (5, 8, 22, 23). Although the mean asymmetry ratio for P1/N1 amplitude in each group was within normal range, the mean asymmetry ratio showed a significant difference between the two groups.
The cVEMP response is an easy-to-use test that can be performed in a short time. Thus, cVEMP response can be used as an objective screening tool to identity the first symptoms of vestibular disorders. Numerous pathological and non-pathological factors can affect VEMP parameters. Pathologies affecting VEMP parameters include: acute vestibular neuritis (26), benign positioning vertigo (27), Meniere’s disease (28), vestibular schwannoma (29), otosclerosis (30), middle ear disease and hearing loss (31, 32), superior canal dehiscence syndrome (33), gentamicin toxicity (34), central vestibular disorders (35-37). Non-pathological factors include aging and recording techniques. It is proposed that audiology clinics establish normative data for different age groups using standard protocols for recording VEMP responses similar to auditory evoked potential and vestibular-ocular reflex function tests.
A limitation of the present study was inability of the device to record the EMG level, which required maintaining muscle tone using the feedback method (38).
Conclusion
The results of the present study showed that aging can influence the parameters of the VEMP response. The cVEMP response threshold, latency (especially P1), and asymmetry ratio of P1/N1amplitude increased, while the P1/N1amplitude decreased. VEMP abnormalities observed in healthy older adults indicated the sensitivity of this test in identifying the first signs of vestibular dysfunction. The VEMP response is easy to use, can be performed in a short time and only requires equipment for evaluating auditory evoked brainstem responses. Therefore, the cVEMP response can be used as a selective objective screening test to detect vestibular disorders. The results of the present study can provide normative data for correct interpretation of VEMP response results in the identification of saccular disorders, which are representative of vestibular disease.
Conflict of interest
The authors declared no competing interests.
Acknowledgments
The authors are grateful to the Audiology Department of ShahidBesheshti University for their assistance in the implementation of this study. The authors warmly appreciate the assistance of Ms. ElhamManbaJoud and cooperation of all participants in this study.
Cite this article as: Maleki M, Jafari Z, Zarrinkoob H, Akbarzadeh Baghban A. Effect of aging on Saccular function. Med J Islam RepubIran 2014 (22 October). Vol. 28:117.
References
- 1.Barin K, Dodson E. Dizziness in the elderly. Otolaryngol Clin North Am. 2011;44(2) doi: 10.1016/j.otc.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 2. Herdman S. Vestibular rehabilitation. Third ed: Philadelphia, PA: F; 2007.
- 3.Akin FW, Murnane OD, Tampas JW, Clinard CG. The effect of age on the vestibular evoked myogenic potential and sternocleidomastoid muscle tonic electromyogram level. Ear hear. 2011 Sep-Oct;32(5):617–22. doi: 10.1097/AUD.0b013e318213488e. [DOI] [PubMed] [Google Scholar]
- 4.Katz J. Handbook of clinical audiology. Recherche. 2009;67:2. [Google Scholar]
- 5.Lee SK, Cha CI, Jung TS, Park DC, Yeo SG. Age-related differences in parameters of vestibular evoked myogenic potentials. Acta Otolaryngol. 2008 Jan;128(1):66–72. doi: 10.1080/00016480701387108. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: Past, present and future. Clinical Neurophysiology. 2010;121(5):636–51. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Su HC, Huang TW, Young YH, Cheng PW. Aging effect on vestibular evoked myogenic potential. Otology & Neurotology. 2004;25(6):977–80. doi: 10.1097/00129492-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Janky KL, Shepard N. Vestibular evoked myogenic potential (VEMP) testing: normative threshold response curves and effects of age. J Am Acad Audiol. 2009;20(8):514. doi: 10.3766/jaaa.20.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng CL, Chou CH, Young YH. Aging effect on the ocular vestibular-evoked myogenic potentials. Otol Neurotol. 2010 Aug;31(6):959–63. doi: 10.1097/MAO.0b013e3181e8fb1a. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010 Jul;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baloh RW, Enrietto J, Jacobson KM, Lin A. Age‐Related Changes in Vestibular Function. Ann N Y Acad Sci. 2001;942(1):210–9. doi: 10.1111/j.1749-6632.2001.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 12.Hajioff D, Barr‐Hamilton R, Colledge N, Lewis S, Wilson J. Re‐evaluation of normative electronystagmography data in healthy ageing. Clin Otolaryngol. 2000;25(4):249–52. doi: 10.1046/j.1365-2273.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi M, Saito R, Mizukoshi K, Alford BR. Otoconia in young and elderly persons: a temporal bone study. Acta Otolaryngol. 1993;113(S504):26–9. doi: 10.3109/00016489309128117. [DOI] [PubMed] [Google Scholar]
- 14.Lopez I, Honrubia V, Baloh RW. Aging and the human vestibular nucleus. J Ves Res. 1997;7(1):77. [PubMed] [Google Scholar]
- 15.Park JJ, Tang Y, Lopez I, Ishiyama A. Age‐related change in the number of neurons in the human vestibular ganglion. J Comp Neurol. 2001;431(4):437–43. doi: 10.1002/1096-9861(20010319)431:4<437::aid-cne1081>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Peterka RJ, Black F, Schoenhoff M. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J Ves Res. 1990 [PubMed] [Google Scholar]
- 17.Rauch SD, Velazquez‐Villaseñor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Ann N Y AcadSci. 2001;942(1):220–7. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- 18.Richter E. Quantitative study of human Scarpa's ganglion and vestibular sensory epithelia. Acta Otolaryngol. 1980;90(1-6):199–208. doi: 10.3109/00016488009131716. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Lopez I, Baloh RW. Age-related change of the neuronal number in the human medial vestibular nucleus: a stereological investigation. J Ves Res. 2001;11(6):357–64. [PubMed] [Google Scholar]
- 20.Serrador JM, Lipsitz LA, Gopalakrishnan GS, Black FO, Wood SJ. Loss of otolith function with age is associated with increased postural sway measures. Neurosci lett 2009 Nov. 6;465(1):10–5. doi: 10.1016/j.neulet.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall Jw. New handbook of auditory evoked responses: pearson education,Inc; 2007.
- 22.Welgampola M, Colebatch J. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001;112(11):1971–9. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- 23.Ochi K, Ohashi T. Age-related changes in the vestibular-evoked myogenic potentials. Otolaryngol Head Neck Surg. 2003;129(6):655–9. doi: 10.1016/s0194-5998(03)01578-x. [DOI] [PubMed] [Google Scholar]
- 24.Basta D, Todt I, Ernst A. Normative data for P1/N1-latencies of vestibular evoked myogenic potentials induced by air- or bone-conducted tone bursts. Clin Neurophysiol. 2005;116(9):2216–9. doi: 10.1016/j.clinph.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Basta D, Todt I, Ernst A. Characterization of age-related changes in vestibular evoked myogenic potentials. J Ves Res. 2007;17(2-3):93–8. [PubMed] [Google Scholar]
- 26.Kim H-A, Hong J-H, Lee H, Yi H-A, Lee S-R, Lee S-Y. et al. Otolith dysfunction in vestibular neuritis Recovery pattern and a predictor of symptom recovery. Neurology. 2008;70(6):449–53. doi: 10.1212/01.wnl.0000297554.21221.a0. [DOI] [PubMed] [Google Scholar]
- 27.Hong SM, Park DC, Yeo SG, Cha CI. Vestibular evoked myogenic potentials in patients with benign paroxysmal positional vertigo involving each semicircular canal. Am J Otolaryngol. 2008;29(3):184–7. doi: 10.1016/j.amjoto.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Kim-Lee Y, Ahn JH, Kim YK, Yoon TH. Tone burst vestibular evoked myogenic potentials: diagnostic criteria in patients with Meniere's disease. Acta Otolaryngol. 2009;129(9):924–8. doi: 10.1080/00016480802495412. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y.F, Cheng P.W, Young Y.H. Comparison of vestibular function between large cerebellopontine angle meningioma and schwannoma. Acta Otolaryngol. 2009;129(2):161–5. doi: 10.1080/00016480802126553. [DOI] [PubMed] [Google Scholar]
- 30.Halmagyi GM, Aw ST, McGarvie LA, Todd MJ, Bradshaw A, Yavor RA. et al. Clinical Records Superior semicircular canal dehiscence simulating otosclerosis. J Laryngol Otol. 2003;117:553–7. doi: 10.1258/002221503322113003. [DOI] [PubMed] [Google Scholar]
- 31.Licameli G, Zhou G, Kenna MA. Disturbance of vestibular function attributable to cochlear implantation in children. Laryngoscope. 2009;119(4):740–5. doi: 10.1002/lary.20121. [DOI] [PubMed] [Google Scholar]
- 32.Seo T, Miyamoto A, Saka N, Shimano K, Nishida T, Hashimoto M. et al. Vestibular evoked myogenic potential induced by bone-conducted stimuli in patients with conductive hearing loss. Acta Otolaryngol. 2008;128(6):639–43. doi: 10.1080/00016480701635183. [DOI] [PubMed] [Google Scholar]
- 33.Brantberg K, Verrecchia L. Testing vestibular-evoked myogenic potentials with 90-dB clicks is effective in the diagnosis of superior canal dehiscence syndrome. Audiol Neurotol. 2008;14(1):54–8. doi: 10.1159/000153435. [DOI] [PubMed] [Google Scholar]
- 34.Ozluoglu LN, Akkuzu G, Ozgirgin N, Tarhan E. Reliability of the vestibular evoked myogenic potential test in assessing intratympanic gentamicin therapy in Meniere's disease. Acta Otolaryngol. 2008;128(4):422–6. doi: 10.1080/00016480701808988. [DOI] [PubMed] [Google Scholar]
- 35.Baier B, Stieber N, Dieterich M. Vestibular-evoked myogenic potentials in vestibular migraine. J Neurol. 2009;256(9):1447–54. doi: 10.1007/s00415-009-5132-4. [DOI] [PubMed] [Google Scholar]
- 36.Deftereos S, Panagopoulos G, Eleftheriadou A, Korres S, Georgonikou D, Kandiloros D. et al. Using vestibular evoked myogenic potentials to localise brainstem lesions A preliminary report. B-ENT. 2008;4(4):215. [PubMed] [Google Scholar]
- 37.Patkó T, Simó M, Arányi Z. Vestibular click-evoked myogenic potentials: sensitivity and factors determining abnormality in patients with multiple sclerosis. Multiple Sclerosis. 2007;13(2):193–8. doi: 10.1177/1352458506070940. [DOI] [PubMed] [Google Scholar]
- 38.Vanspauwen R, Wuyts FL, Van de Heyning PH. Improving vestibular evoked myogenic potential reliability by using a blood pressure manometer. Laryngoscope. 2006;116(1):131–5. doi: 10.1097/01.mlg.0000187405.57567.ae. [DOI] [PubMed] [Google Scholar]


