Abstract
Background: Delayed union, nonunion, and mechanical failure is still problems encountered in limb salvage surgery (LSS) using extracorporeal irradiation (ECI). This study aimed to determine whether bone marrow mesenchymal stem cells (MSC) and recombinant human bone morphogenetic protein-2 (rhBMP-2) improve hostgraft union after osteotomy and also increase its mechanical strength.
Methods: Thirty Sprague Dawley rats were randomly divided into five groups. Group I (control) underwent LSS using ECI method with 150 Gy single doses. Similar procedures were applied to other groups. Group II received hydroxyapatite (HA) scaffold. Group III received HA scaffold and MSC. Group IV received HA scaffold and rhBMP-2. Group V received HA scaffolds, MSC, and rhBMP-2. Radiograph were taken at week-2, 4, 6, and 8; serum alkaline phosphatase and osteocalcin were measured at week-2 and 4. Histopathological evaluation and biomechanical study was done at week-8.
Results: The highest radiological score was found in group IV and V Similar result was obtained in histological score and ultimate bending force. These results were found to be statistically significant. There was no significant difference among groups in serum alkaline phosphatase and osteocalcin level.
Conclusion: Combination of MSC and rhBMP-2 was proven to accelerate union and improve mechanical strength of ECI autograft.
Keywords: Extracorporeal irradiation, Limb salvage surgery, Mesenchymal stem cell, Bone morphogenetic protein, Scaffold, Hydroxyapatite
Introduction
Limb salvage surgery (LSS) using extracorporeal irradiation (ECI) technique is an effective method to reconstruct bone defects in any cases of sarcomas in extremity and pelvis (1-7). In some Asian countries like Japan (1991) (8), Taiwan (2002) (5), Indonesia (2011) (9), even Germany (2002) (10), Australia (2005) (11), and Switzerland (2009) (12), LSS with ECI technique still remain a popular choice in surgical treatment of bone sarcoma.
ECI dosage needed to achieve free tumor cell autograft (10) varies between 30 to 300 Gy in single fraction (10,13-17).According to Davidson et al. (11) and Krieg et al. (12), the combination of chemotherapy and single fraction of ECI with dose of 50 Gy would eradicate 100% malignant bone sarcoma cells, prevent local recurrence (11,18),and yield autograft bone segment which function as a scaffold for the process of creeping substitution (11).
However, ECI with a dose of more than 50 Gy may lead to non-viable bone autograft segment (10), reduced number of mesenchymal stem cells (MSC) and osteoblasts (19), decrease in graft osteoconductive potency, delayed union or non-union, and decrease in the mechanical strength of bone (9,11,14,19).
Problem of delayed union or nonunion, decrease in the mechanical strength of bone, implant failure, and reconstruction of large bone defect are major challenges in LSS with ECI technique (20-22). Measures which have been done to improve the process of union and incorporation of the reconstructed bone defect are the application of autologous bone graft, demineralized bone matrix, bioceramics (calcium phosphate / hydroxyapatite), autologous bone marrow, and growth factor such as bone morphogenetic proteins (BMPs) (22-23).
MSC application may show promises as a therapeutic method for tissue repair and regeneration. It is able to be stimulated to differentiate into desired cell and form specific tissue for therapeutic benefit, which yield tissue with structurally and mechanically appropriate for normal tissue function and also achieve full integration with surrounding tissue (24-26).
Recent studies also show that BMPs have important roles in the process of new bone formation (21, 27) by induction of differentiation of MSC into osteoblast, promoting osteoblast maturity, and endochondral ossification (28). Sasso et al. (29) and Reddi et al. (30) stated that BMPs could heal large bone defect (29) and fracture nonunion (23). Among studied BMPs (BMP-2 to BMP-7, and BMP-9), BMP-2 has the highest osteoinductive potential (31).
Therefore, we studied the role of MSC transplantation and recombinant human BMP-2 (rhBMP-2) application in LSS with ECI technique. In this study, MSC, rhBMP-2, and their combination was expected to accelerate union of osteotomy between post ECI autograft and host bone, and also improve their mechanical strength. All of these approaches on the LSS with ECI technique have not yet been applied in humans, thus this study was conducted in Sprague Dawley (SD) rat.
Methods
Experimental Design
Thirty SD rats were randomly divided into five groups.Group I acting as the control group underwent LSS using ECI technique with a dose of 150 Gy single fractions. Similar procedures were applied to group II, with additional application of hydroxyapatite (HA) on the proximal and distal osteotomy sites. Group III received HA and bone marrow MSC transplantion of 2 x 106 cells. Group IV received HA as well as application of rhBMP-2. Group V received HA, bone marrow MSC of 2 x 106 cells, and rhBMP-2 which were transplanted at the proximal and distal osteotomy sites (Fig. 1).
Fig. 1 .

Protocol of this study. Thirty SD rats was randomly divided into 5 groups. Group I as control group underwent resection of 10 mm intercalary femoral segment, radiation with 150 Gy single fraction, and reimplantation on the next day and fixed with intramedulary 1.4 mm Kirschner Wire. Similar procedures were applied to other groups. Group II received hydroxyapatite (HA) scaffold on the proximal osteotomy (PO) and distal osteotomy (DO) sites. Group III received HA scaffold and bone marrow MSC. Group IV received HA scaffold and rhBMP-2. Group V received HA scaffolds, bone marrow MSC, and rhBMP-2. Radiological evaluation were done at week-2, 4, 6, and 8; serum alkaline phosphatase and osteocalcin were measured at week-2 and 4. All animals were sacrificed at week-8 and prepared for histopathological evaluation, immunohistochemistry, and biomechanical study to determine ultimate bending force.
All procedures undertaken in this study had been approved by Institutional Animal Care and Use Committee (IACUC) PT BimanaIndomedical Bogor number R.03-11-IR and ethical approval from University of Indonesia number 131/PT02.FK/ETIK/2011.
Evaluation of this study was performed with Lane and Sandhu radiological score (32), value of serum alkaline phosphatase (SAP) and osteocalcin (ELISA test), histological score, tissue expression of alkaline phosphatase and osteocalcin(immunohistochemistry), and ultimate force in bending test to evaluate the mechanical strength.
Isolation and culture of bone marrow MSC: Five male SD rats were prepared for allogenic donor, bone marrow MSC was extracted from both tibia and femur of each rat. Bone marrow cells were taken using modified Dobson method by putting the bone in 25 mL polypropylene conical flask. The flasks were centrifuged at 750 x g for 30 minutes. After pellet was formed on the bottom of the tube, it was resuspended by adding 8 mL RPMI medium, then centrifuged at 750 x g for 10 minutes. Supernatant was removed, the pellet was added to 10 mL RPMI, and centrifuged at 750 x g for 10 minutes. Supernatant was removed again, the cell pellet was added to 3 mL of growth medium and counted by using a hemocytometer. Cells were cultured, incubated, and evaluated on 6 wells tissue culture plates with concentration of 107cells per well. Culture was incubated at 37°C with 5% CO2 concentration. Growth medium was replaced on day 7 and subsequently changed every 3 days. Observation was done by inverted microscopy (80x magnification) to evaluate the adhesion of the nucleated cells with fibroblast-like morphology to the plastic culture plate (33).
Characterization of MSC
Characterization of MSC was done using reverse transcriptase-polymerase chain reaction (RT-PCR) and immunocytochemistry assay. RT-PCR was used to detect the expression of genes which encoded some of MSC surface proteins such as CD73, CD90, CD105, CD44, and STRO-1. Beside surface protein of MSC, the marker of hematopoietic stem cells such as CD34 (a marker of primitive hematopoietic progenitor cells and endothelial cells) and CD45 (a marker of pan-leukocytes) were also checked to ensure the isolated cells were not contaminated with hematopoietic stem cells. Immunocytochemistry assays were also performed to see the expression of MSC surface protein (33).
Surgical Procedure of LSS with ECI Technique
LSS with ECI technique was performed in two stages of surgery (in two consecutive days). On day-1, resection of 10 mm diaphyseal segment of the right femur was performed using manual A letter saw (designed by author), followed by ECI exposure with dose 150 Gy single fraction. The host bone was temporarily fixed by 1.4 mm Kirschner wire (K-wire). On day-2, reimplantation of the diaphyseal segment of the femur was conducted and fixed with 1.4 mm K-wire.
After anaesthesia with ketamin(Ketamil(R), Troy laboratories PTY limited Australia) 80 mg/kg body weight and xylazine (Seton 2%(R), LaboratoriosCalier S.A. Spain) 10 mg/kg body weight intraperitoneally, disinfection was done with 10% povidone iodine and 70% alcohol from mid-body to the entire region of the right lower extremity which had been shaved previously.Incision was performed 20 mm long with anterolateral approach to the right femur.Vastuslateralis muscle was split from biceps femoris and patella was dislocated medially. Vastuslateralis and biceps femoris muscles were retracted from femoral bone meanwhile the periosteum was kept intact. Segmental osteotomy including its periosteum of 1 cm long was done at mid-diaphysis of the femur with manual a letter saw equipped with 1 mm blade (modified from Tsuchida et al.) (34), while cutting, irrigation with NaCl 0.9% was done. These femoral diaphyseal segments from group II-V were detached from surrounding soft tissue and wrapped with 3layer moist saline gauze and dry gauze at the outside and wrapped again in sterile plastic bag. Specimens were kept in dry ice and irradiated by extracorporeal irradiation at National Nuclear Centre (Badan Tenaga Nuklir Nasional).
On the second day, reimplantation of the femoral diaphyseal segments were done. After anesthesia with the same regiment as above, sutures were removed, osteotomy site were identified, and temporary K-wire was removed. The autograft segment were placed at its original position and orientation and fixed with intramedulary 1.4mm K-wire. Group II received hydroxyapatite scaffold (DrSutomo Tissue Bank, Surabaya, Indonesia) topically around both osteotomies. Group III received hydoxyapatite scaffold and 2x106 bone marrow MSC. Group IV received hydroxyapatite scaffold and 240 µg rhBMP-2 (Daewoong Pharmaceutical, Samseong-dong, Korea). Group V received hydroxyapatite scaffold, 2x106bone marrow MSC, and 240 µg rhBMP-2. Soft tissue was reapproximated, fascia and skin were sutured, and animals were kept at animal laboratory (PT BimanaIndomedical, Bogor, Indonesia).
Radiographic examination
Serial radiographs of rat femur were examined at the end of the week-2, week-4, week-6, and week-8 at Radiology Unit of Bogor Agricultural Institute Animal Hospital. Radiographic examination was done under anaesthesia using E7239X Rotanode Toshiba X-ray machine with serial number 2A009, with a maximum exposure condition of 125 kV and 500 mA. X-ray exposure used was 52 kV and 6.4 mA for 400 ms on ventrodorsal and laterolateral projection (35).Radiological evaluation was performed using radiological score developed by Lane and Sandhu (1987) (32).
Alkaline phosphatase and osteocalcin
Blood samples were collected 1 to 1.5 mL in each microtube. SAP and serum osteocalcin was examined by ELISA kit from USCN. The microtiter plate provided in that kit has been precoated with a monoclonal antibody specific to SAP and osteocalcin. Standards or samples were then added to the appropriate microtiterplate wells with a biotin-conjugated polyclonal antibody preparation specific for SAP and osteocalcin. Next, Avidin conjugated to Horseradish Peroxidase was added to each microplate well and incubated. Then a TMB substrate solution was added to each well. Only those wells that contained SAP or osteocalcin, biotin-conjugated antibody, and enzyme-conjugated Avidin had a change in color. The color change was measured spectrophotometrically at a wavelength of 450nm ± 10nm.
Histopathologic examination
After euthanasia, the right femur was resected immediately. By maintaining a K-wire inside, harvested femur was fixed in 10% neutral buffered formalin for 48 hours. K-wire was removed before decalcification. Samples were embedded in paraffin and cut longitudinally with a microtome 5 µm sections, and stained in hematoxylin eosin (HE). Stained sections were examined with light microscope Nikon Eclipse E400(R).
Immunohistochemistry Staining of Alkaline Phosphatase and Osteocalcin
Immunohistochemical examination of alkaline phosphatase and osteocalcin was performed by modification of Yoshiki procedure (36). The slide was deparaffinized in xylol I-III respectively for 5 minutes and dehydrated in xylene and graded alcohol for each 4 minutes. After that blocking was done with 0.5% H2O2 in methanol for 30 minutes and then washed in water for 5 minutes. Pretreatment of the slide was performed with citrate buffer in microwave Cook I and Cook II for each 5 minutes, followed by blocking background target to block non-specific antigens and then incubated for 15 minutes. Then it was given primary antibody to alkaline phosphatase / osteocalcin and incubated for 1 hour. The slide was given a universal ink secondary antibody to bind to the primary antibody for 15 minutes. After that, counterstaining was performed with haematoxylin for 1-2 minutes. Positive alkaline phosphatase and osteocalcin expression referred to the positive control of alkaline phosphatase and osteocalcin from osteosarcoma tissues.
Bone Mechanical Strength Test
The mechanical strength of bone was examined by bending test. The test was carried out using universal testing machine Schenck-Trebe type RME100 (Germany) with Yokogawa XY recorder type 3023 through KWS amplifier type 3073 (Japan), and a supporting bone holder.
Measurements taken were callus diameter, callus cross-sectional area, and the ultimate force in the bending test until the specimen was broken. Protocol of this study was resumed in Fig. 1.
Statistical analysis
Statistical analysis was performed with repeated One Way Analysis of Variance (ANOVA) test and ANOVA test using SPSS software. Differences between the means were considered statistically significant when p< 0.05.
Results
Radiological Score
Repeated ANOVA test with sphericity assumed showed significant difference between the radiological mean score of proximal osteotomy (PO) at week-2 to week-8 (F(3,8) = 62.593, p<0.0001). The same test also showed significant difference in radiological mean scores of the distal osteotomy (DO) from week-2 to week-8 (F(3,87)= 74.604, p< 0.0001). In Dunnett’s test, we found an increase in the radiological mean score of PO and DO from week-2 to week-8 (3.12 ± 0.15; 3.39 ± 0.15) and (2.93 ± 0.13; 3.0 ± 0.15); however it did not show any significant difference (Figs. 2 and 3). Fig. 4(a, b, and c) shows the pictures of the radiographic examination in latero-lateral projection of samples in group I, III, and V respectively.
Fig. 2 .
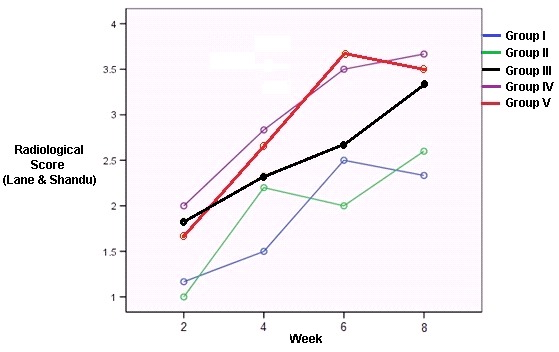
Graphic of proximal osteotomy radiological mean score in each group from week-2 to 8.
Fig. 3 .

Graphic of distal osteotomy radiological mean score in each group from week 2 to 8.
Fig. 4.
Fig. 4a .
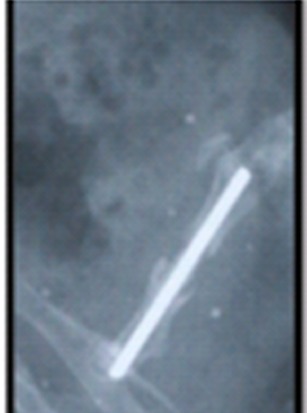
Lateral projection radiograph of a sample in group I at 8th week. There was nonunion.
Fig. 4b .

Lateral projection radiograph of a sample in group III at 8th week. There was union with moderate amount of callus.
Fig. 4c .

Lateral projection radiograph of a sample in group V at 8th week. There was union with enormous callus formation.
Serum Alkaline Phosphatase and Osteocalcin
SAP level in group I-IV at week-4 was lower than its level at week-2; while, SAP level in group V was higher(Table 1). Mean SAP level was 10.529,4 ± 3.792,70pg/µ and 8.732,9 ± 2.189,76pg/µl at week-2 and week-4 respectively. ANOVA test showed no significant difference at week-2 (p=0.059), however, it was statisticallydifferent at week-4 (p=0.027). Dunnett’s test revealed significant difference at week-4, it was between group V and II (p=0.023). hatase or osteocalcin, (+): positive expression of alkaline phosphatase or osteocalcin, F: failure
Table 1 . Mean histological score, serum alkaline phosphatase and osteocalcin level, and biomechanical study.R .
| Group | Mean Histological Score# | Mean Serum Alkaline Phosphatase level (ng/μL) | Mean Serum Osteocalcine Level (pg/μL) | Biomechanical Study at 8th Week | |||||
| PO | DO | 2nd week | 4th week | 2nd week | 4th week | Mean Callus Diameter (mm) | Mean Callus Crossectional Area (mm2) | Mean Ultimate Strength (Newton) | |
| I (control) | 4 | 3.8 | 13.18 | 9.09 | 189.1 | 270.7 | 2.98 | 5.01 | 111 |
| II (HA) | 1.4 | 4 | 12.93 | 6.86 | 227.8 | 279.3 | 4.94 | 9.31 | 120 |
| III (HA+MSC) | 9.5 | 5.75 | 8.85 | 8.29 | 272.9 | 358.8 | 3.24 | 5.57 | 111 |
| IV (HA+rhBMP-2) | 12 | 12.7 | 10.89 | 8.04 | 283.6 | 322.3 | 8.37 | 16.85 | 232 |
| V (HA+MSC +rhBMP-2) | 12 | 13.25 | 6.8 | 11.38 | 270.8 | 270.7 | 8.86 | 17.94 | 161 |
| Contralateral leg | 3.04 | 5.13 | 159.3 | ||||||
#: Histological score of union according to Salkeld and Marino at 8th week, PO: Proximal osteotomy, DO: Distal osteotomy, HA: Hydroxyapatite scaffold, MSC: Bone marrow mesenchymal stem cells, rhBMP-2: Recombinant human bone morphogenetic protein-2
Mean serum osteocalcin level was 248.8 ± 57.09 pg/µl and 300.4 ± 117.69pg/µl at week-2 and week-4 respectively (Table 1). ANOVA test found no significant difference among the groups at week-2 (p = 0.082) and week-4 (p= 0.818). In contrast to the result of SAP, serum osteocalcinlevel of all groups were higher at week-4 than its level at week-2.
Histopathologic Examination
The mean histological scores of PO and DO were 7.4 ± 5.2 and 7.7 ± 5.2 respectively; mean histological score is shown in table 1.ANOVA test showed significant differences among groups in the histological scores of PO and DO with respective p values of <0.001 and <0.001. Dunnett’s test showed significant difference in the histological mean score of PO in group IV and V compared to group I (control) (p = 0.001 and 0.003) as well as DO in group IV and V to the group I (control) (p = 0.007 and p = 0.005). The mean histological score of PO and DO of the group III was higher than control (9.5 ± 2.6 compared to 4.0 ± 3.8 for the PO histological score; 5.8 ± 4.2 compared to 3.8 ± 3.8 for DO histological score), although they did not show a statistically significant difference (p= 0.064 and p= 1).
Histological images in each treatment group were shown in Fig. 5(a, b, and c). Union and healing by extensive connective tissue (score 0) appeared in some control groups (Fig. 5a). Most other control groups experienced union with fibrocartilage tissue. The majority of group III has reached union with mature bone or partial union with mineralized cartilage (figure 5b). Unlike the other groups, in general, group IV and V were generally healed with lamellar bone. In group IV and V lamellar bone aggressively grew surrounding cortical wall (Fig. 5c).
Fig. 5.
Fig. 5a .

Histopathological imaging with HE staining at the osteotomy area in control group. Union does not occur, 50x magnification.
Fig. 5b .

Histopathologic imaging with HE staining in group III with total histological score 7. Group III (bone marrow MSC + HA scaffold) gives better histological scores compared with the control group and group II (HA scaffold only). 200x magnification.
Fig. 5c .

Histopathologic imaging with HE staining in Group V with histological score 14. In group IV and V, there were an aggressive growth of cortical lamellar bone in response to rhBMP-2 and HA scaffold, forming a lamellar bone surrounding cortical wall, 50x magnification.
Immunohistochemistry of alkaline phosphatase and osteocalcin
Alkaline phosphatase and osteocalcin expression of tissue showed positive results in group I-III and negative in group IV-V. Alkaline phosphatase expression was most commonly found in group III (80%), followed by group II (67%), and group I (33%). Osteocalcin expression were found the most in group I (67%), followed by group II (50%) and III (40%). The expression of alkaline phosphatase and the tissue osteocalcin are summarized in Table 2.
Table 2 . Expression of tissue alkaline phosphatase and osteocalcin.
| Group | Σn | Alkaline Phosphatase | Osteocalcin | ||||
| - | + | F | - | + | F | ||
| I | 6 | 3 | 2 | 1 | 2 | 4 | 0 |
| II | 6 | 2 | 4 | 0 | 3 | 3 | 0 |
| III | 5 | 1 | 4 | 0 | 3 | 2 | 0 |
| IV | 5 | 5 | 0 | 0 | 5 | 0 | 0 |
| V | 4 | 4 | 0 | 0 | 4 | 0 | 0 |
Description: n: number of samples; (-): negative expression of alkaline phosphatase or osteocalcin, (+): positive expression of alkaline phosphatase or osteocalcin, F: failure
Tissue expression of alkaline phosphatase and osteocalcin was positive in group I, II, and III. Group IV and V had perfect healing and remodelling, so that they no longer express alkaline phosphatase and osteocalcin (Fig. 6a, b, and c).
Fig. 6.
Fig. 6a .

Immunohistochemistry analysis of alkaline phosphatase of a sample on group I (control group). There was positive tissue expression of alkaline phosphatase on cells and extracellular matrix (white arrow), 200x magnification.
Fig. 6b .

Immunohistochemistry analysis of alkaline phosphatase of a sample on group III (bone marrow MSC group). There was positive tissue expression of alkaline phosphatase on cells and extracellular matrix (green arrow), 200x magnification.
Fig. 6c .
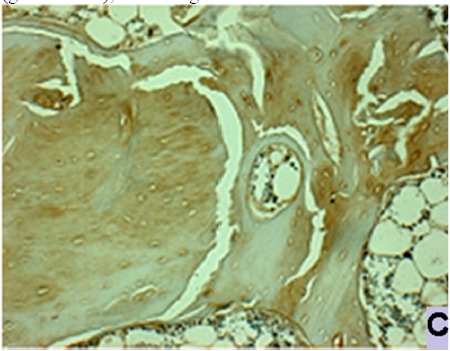
Immunohistochemistry analysis of alkaline phosphatase of a sample on group V (bone marrow MSC + rhBMP-2 group). There was negative tissue expression of alkaline phosphatase on cells and extracellular matrix, 200x magnification.
Mechanical Strength of Bone
Bone mechanical strength test was used for different samples. The mean diameter of the bone (callus) was 5.8 ± 2.57 mm, the cross-sectional area of bone (callus) was 11.3 ± 5.66 mm2. The mean of ultimate force on bending test was 151.8 ± 52.66 N. The highest ability to withstand the bending force was found in group IV. The average diameter of the callus, callus cross-sectional area, and ultimate force of each treatment group are shown in Table 4. Diameter and cross-sectional area of callus in group IV and V are greater than the other groups.
Discussions
In primary malignant bone tumor with indication of LSS with ECI technique, fracture could occur due to bone destruction by osteoclasts, the tumor itself (bone destruction before surgery), ordue to complication of ECI exposure. In this model the various factors mentioned above was eliminated, so we evaluated the effect of MSC, rhBMP-2, and their combination to the union of osteotomy and its mechanical properties.The use of autogenous bone as a graft after devitalized with ECI exposure as a preferred method of LSS continued to increase, because this method was simple and inexpensive (3,6,11,13)and also the size and shape of the graft suits individual patient (matching) (3,37-38).
The defining characteristics of MSC are inconsistent among investigators. Many have their own methods to isolate and expand MSC, which show quite significant differences (39-41). In 2005, The International Society of Cellular Therapy (ISCT) issued a consensus that MSC should have the following three criteria (42). First, MSC must be plastic-adherent when maintained in standard culture conditions. Second, MSC must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface molecules. Third, MSC could differentiate to osteoblasts, adipocytes, and chondroblasts in vitro. In order to prove that, we performed characterization with RT-PCR and immunocytochemistry assays. RT-PCR showed that cultured cells had positive gene for surface markers CD73, CD90, CD105, and STRO-1, and negative for CD34 and CD45. Those results were further supported by immunocytochemistry assay that showed positive expression of CD73 and CD105. It was the limitation of this study that we could only confirm two of three ISCT criteria. Considering their fibroblast-like morphology, plastic adherent properties, ability of cells to grow and proliferate on appropriate medium for mesenchymal stem cells, and the result of RT-PCR and immunocytochemistry, we concluded that those mononuclear cells with fibroblast like morphology are very likely MSC (33).
In this study, we used allogenic bone marrow MSC from the previous culture results (our preliminary study) due to several considerations: (i) autogenic MSC donor is not possible, because it is obtained from the femur and tibia of rats via surgical hip joint disarticulation, (ii) surgery on the rest of the contralateral femur would cause injury and pain that violate the animal welfare, (iii) the use of allogenic MSC relatively did not cause rejection due to lack of expression of HLA class II antigens on the bone marrow MSC (25). The low rate of immunorejection of the allogenic MSC was reported by Koc et al (43). The study showed no T cell reaction or rejection on the use of allogenic MSC in patients with Hurler's syndrome. Horwitz et al. (44) reported allogenic MSC transplantation in three children with osteogenesisimperfecta did not result in immunorejection. That result was also supported by Bartholomew et al. (45) and Ryan et al. (26) which showed allogenic MSC administration on wound healing gave no rejection reaction.
Our study showed that on the second week of observation, the radiological result of proximal and distal osteotomy in group I and II only showed signs of new callus formation (score 1), whereas group III, IV and V more than 60% of rats reached the beginning of healing by bone tissue (score 2). The osteotomy line was almost disappeared (score 3) in more than 60% of samples in group III, IV and V (score 3) on the fourth week, however only 30% of samples on group I and II had reached score 3. It means that transplantation of bone marrow MSC, application of rhBMP-2, and its combination improved radiological score and accelerated union. It was supported then, on the sixth week, more than 50% rats on group IV and V had achieved complete healing (score 4).
Radiological result was supported by histopathological examinations. Healing in group I-III varied from fibrous to mature bone tissue. In group I and II, there was fibrous tissue between graft and host bone expanding into medullary canal of femur. A few osteotomy in group I and II healed with cartilage or osteoid. In general, it could be concluded that group III had better histological score compared to control or group II. It was due to the effect of MSC transplantation on osteogenesis and endochondral ossification at the osteotomy site. This result was supported by research done by Cuomo et al. (46) which showed that tissue healing on control group and MSC transplantation group varied from fibrous tissue to mature bone.
In group IV and V (rhBMP-2 and bone marrow MSC + rhBMP-2), there were extensive bridging callus, contained many trabecular bone, which bridge the host bone from proximal to distal covering the femoral autograft. Asymmetric callus on one side of the femur was mainly the influence of the rhBMP-2 implantation area. Both bony osteotomizedarea underwent union with mature bone, by forming lamellar bone that surround the cortical wall. The combination of bone marrow MSC and rhBMP-2 resulted in better histological score compared to the groups which received bone marrow MSC or rhBMP-2 alone.
Hydroxyapatite administration alone which functioned as an osteoconductive scaffold did not improve radiological score or accelerate the healing process. In fact, radiological mean score of group II at the sixth week was lower compared with control group. The results of post hoc statistical analysis also showed no significant difference between groups that received hydroxyapatite and the control group. These results are similar to studies conducted by Ozturk et al. (47) which showed that the addition of hydroxyapatite does not improve fracture healing both radiologically and histopatologically. According to Finkemeier (21), hydroxyapatite administration would give good results when administered on a gap or bone defects in areas that have good blood supply such as in metaphysis.
Theoretically, hydroxyapatite has the same chemical composition with bone mineral fraction. It may fill gap between graft and host bone and also stimulate bone growth. Hydroxyapatite is a nonresorbablescaffold, it is a good substrate for adhesion, proliferation, and differentiation of MSC and osteoblasts. Furthermore, differentiated cells would produce extracellular matrix and integrated with host tissue (24,48,49). However, according to some researcher, hydroxyapatite may cause osteolysis if it was exposed to bone marrow and soft tissue. Hydroxyapatite debris was allegedly caused implant failure by stimulating phagocytosis (by macrophage) and release of various cytokines (TNF-α, IL-ß, IL-6, dan prostaglandin E2 (PGE2)). After that, there would be inflammatory process, triggering differentiation of osteoclast precursor into mature osteoclast, and it might cause impairment in bone remodeling and result in osteolysis (50). The healing of osteotomy (union between graft and host bone) was influenced by; biologic activity (MSC, viable osteoblast and its product), osteoinductive potential (growth factors such as BMP-2) (31,51), and osteoconductive potential (graft and scaffold) (51-52), and also local host condition (53).
Bone marrow MSC transplantation alone (group III) had increased radiological scores, with radiologic mean score higher than group I and II. At week-8, it gave significant difference in mean radiological scores of distal osteotomy compared to group I. Theoretically bone marrow MSC might provide response to biological and mechanical environment and could differentiate into cells which provide component required for fracture healing (54). But bone marrow MSC cannot work alone, and very dependent on the extracellular matrix, intercellular signal (54), and also neovascularization to sustain its viability (55).
The limitation of bone marrow MSC in this study is thought to be caused by in vivo environment which does not support their proliferation and differentiation. Bone marrow MSC which had been previously growth in artificial medium must adapt to the new environment after surgical resection and re-implantation procedure, which was poor in oxygen and growth factors. LSS may cause extensive tissue damage (after fracture, oxygen level at fracture site would decrease into 0-2%) (56). Transplanted stem cell was avascular on the state of extensive tissue damage and inadequate nutrition, until there was vascular invasion from the surrounding tissue (57).Decrease of O2 concentration at the osteotomy site, extensive tissue damage, and loss of hematome (54) were not an ideal environment for the proliferation and differentiation of MSC. It was also explained by Cancedda et al. (57)which stated the results of stem cell transplantation cannot be predicted and would fail when transplanted into unsuitable microenvironment.
Withoutexogenousapplication of rhBMP-2, healing of osteotomy occurred slower(delayedunion) or might develop non-union. When thenumber ofosteogeniccellswas sufficient, healing of osteotomy/fracturewould stilltook placeeven without theadministration ofBMP-2 (58). This canbe seen insome of theratsin group I-III which had completehealingwith score 4. From several BMPs (BMP-2 to BMP-7, and BMP-9) which was known for their osteoinductive properties, BMP-2 showed the strongest osteoinductive potency. BMP-2 had important roles in the process of differentiation, from the differentiation of bone marrow MSC into osteoprogenitor cells, and then became pre-osteoblast, and eventually the differentiation of osteoblast into osteocyte (23). So far in developed country, only rhBMP-2 and rhBMP-7 were available for clinical application and also had been approved by FDA (59). Based on its potential and availability, the researcher select the application of rhBMP-2 for the intervention in group IV and V.
Application of rhBMP-2 alone and their combination with bone marrow MSC had accelerated healing, increased bridging callus, and also prevented implant failure. This was proven by thickness of callus formation and higher radiological score on the group IV and V compared to the other three groups. These results are consistent with studies of Cuomo et al. (46)that showed addition of rhBMP-2 as an osteoinductive component caused MSC differentiated more effectively by giving signal to the cells and produce 100% healing rates.
Radiological assessment at week-2 and week-4 did not show any association with serum alkaline phosphatase level. Group III-V showed better radiological score than group I and II, but the serum alkaline phosphatase level of group III-V were lower than serum alkaline phosphatase level in group I and II at week-2 and week-4. It was assumed due to different individual response (inter-individual variations were very large) and higher inflammatory reaction in group I and II. The large inter-individual variation can also be seen from the results of serum osteocalcin level (60-61).
Variations in the level of serum alkaline phosphatase and osteocalcin were influenced by various factors, such as; different research protocol (clinical and experimental studies), fixation method, treatment method, type of animal, and also diurnal variation. Diurnal variation in bone turnover marker had been documented in several species including rats, mice, rabbits, and horses (62).
This study found positive alkaline phosphatase expression on osteoblast in group I-III (80% in group III, 67% in group II, and 33% in group I), but negative in group IV and V. These results can be explained because the healing of osteotomy (enchondral ossification) in group I-III were still ongoing; so there were still many active osteoblasts producing alkaline phosphatase. Immunohistochemistry also showed positive tissue osteocalcin expression in group I-III and negative in group IV-V. Osteocalcin expression were found the most in group I (67%), followed by group II (50%), and group III (40%).
Implantation of rhBMP-2 and its combination with bone marrow MSC had increased the cross-sectional area of callus and ultimate bending force (25,30-31). Bone marrow MSC transplantation alone did not lead to significant increase in ultimate bending force compared to control group, although the cross-sectional area of callus in group III was 11.2% greater than control group. Compared to normal bones, ultimate force in group III is lower than the normal bones. Specimens in group IV and V have cross-sectional area which were 228.5% and 249.7% larger than the specimens in control group and healthy bone. Their ultimate bending force were 45.5% and 1.1% greater than healthy bones. In order to achieve the same ultimate bending force as healthy bone, it required addition of rhBMP-2. Nevertheless, in order to achieve the same ultimate bending force as healthy bone, it required 228% greater cross-sectional area.
Conclusion
The conclusions of this study are: (1) transplantation of bone marrow MSC alone was proven to accelerate union of osteotomy but inadequate to improve the mechanical strength of ECI autograft, (2) combination of bone marrow MSC and rhBMP-2 was proven to accelerate union of osteotomy and improve the mechanical strength of ECI autograft.
Acknowledgments
We would like to express our deep gratitude to Professor SarwonoWaspadji for advice and suggestion in methodological aspect of this research, Ms. SilmyMariya for assistance in laboratory works, Kurniadi for recording and typing research data, and Daewoong Pharmaceutical for the donation of rhBMP-2. I hereby affirm that there is no conflict of interest in this research.
Cite this article as: Fauzi Kamal A, Hadisoebroto Dilogo I, Untung Hutagalung E, Iskandriati D, Susworo R, Chaerani Siregar N, Aulia Yusuf A, Bachtiar A. Transplantation of mesenchymal stem cells, recombinant human BMP-2, and their combination in accelerating the union after osteotomy and in-creasing, the mechanical strength of extracorporeally irradiated femoral autograft in rat models. Med J Islam Repub Iran 2014 (15 November). Vol. 28:129.
References
- 1.Futani H, Minamizaki T, Nishimoto Y, Abe S, Yabe H, Ueda T. Long-term follow-up after limb salvage in skeletally immature children with a primary malignant tumor of the distal end of the femur. J Bone Joint Surg (Am) 2007;88-A:596–603. doi: 10.2106/JBJS.C.01686. [DOI] [PubMed] [Google Scholar]
- 2.Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a scandinavian sarcoma group study of 118 patients. J Bone Joint Surg (Br) 2008;90-B:786–94. doi: 10.1302/0301-620X.90B6.19805. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs B, Ossendorf C, Leerapun T, Sim FH. Intercalary segmental reconstruction after bone tumor resection. Eur J SurgOncol. 2008;34:1271–6. doi: 10.1016/j.ejso.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Bielack S, Carle D, Jost L. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19Suppl 2:94–6. doi: 10.1093/annonc/mdn102. [DOI] [PubMed] [Google Scholar]
- 5.Chen WM, Chen TW, Huang CK, Chiang CC, Lo WH. Treatment of malignant bone tumours by extracorporeally irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg (Br) 2002;84-B:1156–61. doi: 10.1302/0301-620x.84b8.13508. [DOI] [PubMed] [Google Scholar]
- 6.Sewell MD, Spilberg BGI, Hanna SA, Meswania JM, Blunn GW, Henry C. Non-invasive extendible endoprostheses for limb reconstruction in skeletally-mature patients. J Bone Joint Surg (Br) 2009;91-B:1360–5. doi: 10.1302/0301-620X.91B10.22144. [DOI] [PubMed] [Google Scholar]
- 7.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J Bone Joint Surg (Am) 2005;87:2449–55. doi: 10.2106/JBJS.D.02170. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi S, Kotoura Y, Yamamuro T, Oka M, Shibamoto Y, Takahashi M. Incorporation of cortical bone autografts following intraoperative extracorporeal irradiation in rabbits. Int J Radiat Oncol Biol Phys. 1991;21:1221–30. doi: 10.1016/0360-3016(91)90279-d. [DOI] [PubMed] [Google Scholar]
- 9.Kamal AF, Ismail Ismail, Mi’raj F, Hutagalung EU. Outcome of stage IIB osteosarcoma treated by limb salvage surgery using extracorporeally irradiated (ECI) autograft. Med J Indones. 2011;20(2):131–7. [Google Scholar]
- 10.Bohm P, Fritz J, Thiede S, Budach W. Reimplantation of extracorporeal irradiated bone segments in musculoskeletal tumor surgery: clinical experience in eight patients and review of the literature. Langenbecks Arch Surg. 2003;387:355–65. doi: 10.1007/s00423-002-0332-8. [DOI] [PubMed] [Google Scholar]
- 11.Davidson AW, Hong A, McCarthy SW, Stalley PD. En-bloc resection, extracorporeal irradiation, and reimplantation in limb salvage for bony malignancies. J Bone Joint Surg (Br) 2005;87-B:851–6. doi: 10.1302/0301-620X.87B6.15950. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AH, Mani M, Speth BM, Stalley PD. Extracorporeal irradiation for pelvic reconstruction in ewing's sarcoma. J Bone Joint Surg (Br) 2009;91-B:395–9. doi: 10.1302/0301-620X.91B3.21164. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Okudaira S, Sasai K, Kotoura Y. En bloc resection, extracorporeal irradiation, and reimplantation of an entire tibia. J Orthop Sci. 2006;11:298–302. doi: 10.1007/s00776-006-1011-3. [DOI] [PubMed] [Google Scholar]
- 14.Boston SE, Duerr F, Bacon N, Larue S, Ehrhart E, Withrow S. Intraoperative radiation for limb sparing of the distal aspect of the radius without transcarpal plating in five dogs. Vet Surg. 2007;36:314–23. doi: 10.1111/j.1532-950X.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen TH, Chen WM, Huang CK. Reconstruction after intercalary resection of malignant bone tumours: comparison between segmental allograft and extracorporeally irradiated autograft. J Bone Joint Surg (Br) 2005;87-B:704–9. doi: 10.1302/0301-620X.87B5.15491. [DOI] [PubMed] [Google Scholar]
- 16.Liptak JM, Dernell WS, Duncan B, Lascelles X, Larue SM, Jameson VJ. Intraoperative extracorporeal irradiation for limb sparing in 13 dogs. Vet Surg. 2004;33:446–56. doi: 10.1111/j.1532-950x.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto T, Akisue T, Marui T, Nagira K, Kurosaka M. Osteosarcoma of the distal radius treated by intraoperative extracorporeal irradiation. J Hand Surg. 2002;27-A:160–4. doi: 10.1053/jhsu.2002.29482. [DOI] [PubMed] [Google Scholar]
- 18.Singh VA, Nagalingam J, Saad M, Pailoor J. Which is the best method of sterilization of tumour bone for reimplantation? a biomechanical and histopathological study. Biomed Eng. 2010;9:48–50. doi: 10.1186/1475-925X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoni BG, Turner AS, Wheeler DL, Nicholas RW, Anchordoquy TJ, Ehrhart N. Gene therapy to enhance allograft incorporation after host tissue irradiation. Clin Orthop Relat Res. 2008;466:921–9. doi: 10.1007/s11999-008-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong J, Li LX, Mu WD, Wang YH, Zhou DS, Hao W. et al. Bone regeneration with BMP-2 gene-modified mesenchymal stem cells seeded on nano-hydroxyapatite/collagen/poly(l-lactic Acid) scaffolds. J Bioactive Compatible Polymers. 2010;25:547–65. [Google Scholar]
- 21.Finkemeier CG. Current concepts review: bone grafting and bone graft substitutes. J Bone Joint Surg (Am) 2002;84-A:454–63. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Salkeld SL, Patron LP, Barrack RL, Cook SD. The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg (Am) 2001;83:803–16. doi: 10.2106/00004623-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 23.James D, Heckman WE, Brooks BP, Aufdemorte TB, Lohmann CH, Morgan T. et al. Bone morphogenetic protein but not transforming growth factor-β enhances bone formation in canine diaphysealnonunions implanted with a biodegradable composite polymer. J Bone Joint Surg (Am) 1999;81:1717–29. doi: 10.2106/00004623-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Vats A, Tolley NS, Buttery LDK, Polak JM. The stem cell in ortopaedic surgery. J Bone Joint Surg (Br) 2004;86-B:159–64. doi: 10.1302/0301-620x.86b2.14756. [DOI] [PubMed] [Google Scholar]
- 25.Hee HT, Dilogo IH, Lim CT, Goh JC, Wong HK. Effects of implantation of bone marrow mesenchymal stem cells, disc distraction, and combined therapy on reversing degeneration of the intervertebral disc. J Bone Joint Surg (Br) 2010;92:726–36. doi: 10.1302/0301-620X.92B5.23015. [DOI] [PubMed] [Google Scholar]
- 26.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflam. 2005;2:1–11. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldberg VM, Stevenson S. Biology of bone and cartilage allografts. In: Czitrom AA, Gross AE, editors. Allografts in orthopaedicpratice. Baltimore: Williams & Wilkins; 1992. pp. 1-14.
- 28.Yamaguchi A, Katagiri T, Ikeda T. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991;133:681–7. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasso RC, Williams JI, Dimasi N. Postoperative drains at the donor sites of iliac-crest bone grafts: a prospective, randomized study of morbidity at donor site in patients who had a traumatic injury of the spine. J Bone Joint Surg (Am) 1998;80:631–5. doi: 10.2106/00004623-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Reddi A. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg (Am) 2001;83-A:S1–6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- 31.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L. et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg (Am) 2003;85:1544–52. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Lane JM, Sandhu HS. Current approaches to experimental bone grafting. OrthopClin North Am. 1987;18(2):213–25. [PubMed] [Google Scholar]
- 33.Kamal AF, Iskandriati D, Dilogo IH, Siregar NC, Hutagalung EU, Yusuf AA, Mariya S, Husodo K. Comparison of cultured mesenchymal stem cells derived from bone marrow or peripheral blood of rats. J ExpIntegr Med. 2014;4(1):17–22. [Google Scholar]
- 34.Tsuchida H, Hashimoto J, Crawford E, Manske P, Low J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J Orthop Res. 2002;21:44–53. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 35.Yue B, Lu B, Dai KR, Zhang XL, Yu CF, Lou JR. et al. BMP-2 gene therapy on the repair of bone defects of aged rats. Calcif Tissue Int. 2005;77:395–403. doi: 10.1007/s00223-005-0180-y. [DOI] [PubMed] [Google Scholar]
- 36.Yoshiki S, Umeda T, Kurahashi Y. An effective reactivation of alkaline phosphatase in hard tissue completely decalcified for light and electron microscopy. Histochemie. 1972;29(4):296–304. doi: 10.1007/BF00279812. [DOI] [PubMed] [Google Scholar]
- 37.Abed YY, Beltrami G, Campanacci DA, Innocenti M, Capanna R. Biological reconstruction after resection of bone tumours around the kneeLong-term follow-up. J Bone Joint Surg (Br) 2009;91-B:1366–72. doi: 10.1302/0301-620X.91B10.22212. [DOI] [PubMed] [Google Scholar]
- 38.Ahlmann ER, Menenzes IR. Intercalary endoprosthetic reconstruction for diaphysealtumours. J Bone Joint Surg (Br) 2006;88-B:1487–91. doi: 10.1302/0301-620X.88B11.18038. [DOI] [PubMed] [Google Scholar]
- 39.Cheng MT, Yang HW, Chne TH, Lee OKS. Isolation and characterization of multipotent stem cells from human cruciate ligaments. Cell Prolif. 2009;42:448–60. doi: 10.1111/j.1365-2184.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villaron EM, Almeida J, Holgado NL, Alcoceba M, Sanchez-Abarca LI, Sanchez-Guijo FM. et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogenic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421–7. [PubMed] [Google Scholar]
- 41. Bernacki SH, Wall ME, Loboa EG. Isolation of human mesenchymal stem cells from bone and adipose tissue. In: Mather JP (ed) Methods in Cell Biology, Amsterdam: Elsevier; 2008. pp.257-277. [DOI] [PubMed]
- 42.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS. et al. Minimal criteria for defining multipotentmesenchymal stromal cellsThe International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 43.Koc O, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrphy (MLD) and Hurler's syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–22. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 44.Horwitz EM, Prockop DJ, Gordon PL. et al. Clinical responses to bone marrow transplantation in children with severe osteogenesisimperfecta. Blood. 2001;97:12227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 45.Bartholomew A, Stuegeon C, Siatskas M, Ferrer K, McIntosh K, Patil S. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vitro. ExpHematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 46.Cuomo AV, Virk M, Petrigliano F, Morgan EF, Lieberman JR. Mesenchymal stem cell concentration and bone repair: potential pitfalls from bench to bedside. J Bone Joint Surg (Am) 2009;91:1073–83. doi: 10.2106/JBJS.H.00303. [DOI] [PubMed] [Google Scholar]
- 47.Ozturk A, Yetkin H, Memis L, Cila E, Bolukbasi S, Gemalmaz C. Demineralized bone matrix and hydroxyapatite/tri-calcium phosphate mixture for bone healing in rat. Int Orthop. 2006;30:147–52. doi: 10.1007/s00264-006-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg (Am) 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 49. Kakar S, Einhorn T. Tissue engineering of bone. In: Hollinger JO, Einhorn TA, Doll BA, Sfeir C, editors. Bone tissue engineering. 1st edition. Washington (DC): CRC Press; 2005. pp.277-302.
- 50.Nuss KRM, Rechenberg V. Biocompatibility issues with modern Implants in bone - a review for clinical orthopedics. Open Orthop J. 2008;2:66–78. doi: 10.2174/1874325000802010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nather A, Han YY. Biology of healing of bone allograft. In: Nather A, Yusof N, Hilmy N, editors. Allograft procurement, processing, and transplantation acomprehensive guide for tissue banks. Singapore: World Scientific; 2010. pp.175-93.
- 52. Lieberman JR, Stevenson S. Bone grafts. In: Pellici PM, Tria AJ, Garvin KL, editors. Orthopaedic knowledge update hip and knee reconstruction 2. Illinois: AAOS; 2000. pp.35-42.
- 53.Beaman FD, Peterson JJ, Kransdorf J. Bone graft materials and synthetic substitutes. RadiolClin N Am. 2006;44:451–61. doi: 10.1016/j.rcl.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Kraus KH, Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006;35:232–42. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 55. Tomford WW, Bloem RM. The biology of autograft and allograft. In: Conrad EU, Eckardt JJ, Finn HA, Genhardt MC, Gitelis S, Malawer MM, et al, editors. Surgery for bone and soft tissue tumors. Philadelphia: Lippincott-Raven; 1998. pp.481-6.
- 56. Lepperdinger G, Singh S, Kloss F. Reponses of MSC to varying oxygen availability in vitro and in vivo. In: Artmann GM, Hescheler J, Minger S, editors. Stem cell engineering, principles & applications. Berlin: Springer; 2010. pp.199-211.
- 57.Cancedda R, Bianchi G, Derubeis A, Quarto R. Cell therapy for bone disease: a review of current status. Stem Cells. 2003;21:610–19. doi: 10.1634/stemcells.21-5-610. [DOI] [PubMed] [Google Scholar]
- 58.Seeherman H, Wozney J. Delivery of bone morphogenetic proteins for orthopaedic tissue regeneration. Cytokine Growth Factor Rev. 2005;16:329–45. doi: 10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Jansen TB, Overgaard S, Lind M, Rahbek O, Bunger C, Soballe K. Osteogenic protein-1 increases the fixation of implants grafted with morcellised bone allograft and ProOsteon bone substitute: an experimental study in dogs. J Bone Joint Surg (Br) 2007;89(1):121–6. doi: 10.1302/0301-620X.89B1.17077. [DOI] [PubMed] [Google Scholar]
- 60.Allen MJ. Biochemical markers of bone metabolism in animals: uses and limitations. Vet ClinPathol. 2003;32:101–13. doi: 10.1111/j.1939-165x.2003.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 61.Stoffel K, Engler H, Kuster M, Riesen W. Changes in biochemical markers after lower limb fractures. ClinChem. 2007;53(1):131–4. doi: 10.1373/clinchem.2006.076976. [DOI] [PubMed] [Google Scholar]
- 62.Paskalev M, Krastev S, Sotirov L. Variation of serum bone alkaline phosphatase activities and osteocalcin concentrations in dogs with experimental osteotomy fixed by two different osteosynthesis techniques. Revue Med Vet. 2008;159:444–9. [Google Scholar]


