Abstract
Background: Timely diagnosis of liver cirrhosis is vital for preventing further liver damage and giving the patient the chance of transplantation. Although biopsy of the liver is the gold standard for cirrhosis assessment, it has some risks and limitations and this has led to the development of new noninvasive methods to determine the stage and prognosis of the patients. We aimed to design an artificial neural network (ANN) model to diagnose cirrhosis patients with non-alcoholic fatty liver disease (NAFLD) using routine laboratory data.
Methods: Data were collected from 392 patients with NAFLD by the Middle East Research Center in Tehran. Demographic variables, history of diabetes, INR, complete blood count, albumin, ALT, AST and other routine laboratory tests, examinations and medical history were gathered. Relevant variables were selected by means of feature extraction algorithm (Knime software) and were accredited by the experts. A neural network was developed using the MATLAB software.
Results: The best obtained model was developed with two layers, eight neurons and TANSIG and PURLIN functions for layer one and output layer, respectively. The sensitivity and specificity of the model were 86.6% and 92.7%, respectively.
Conclusion: The results of this study revealed that the neural network modeling may be able to provide a simple, noninvasive and accurate method for diagnosing cirrhosis only based on routine laboratory data.
Keywords: Liver cirrhosis, Non-Alcoholic Fatty Liver Disease (NAFLD), Neural Networks, Diagnosis
Introduction
Many people around the world are affected by different types of chronic liver diseases. Liver diseases occur from a variety of causes including: viral infection, hereditary disorders, drug-induced or chemical-induced conditions, autoimmune disorders, cancer, metabolic disorders and non-alcoholic fatty liver disease (NAFLD) (1). NAFLD is among the most common types of liver disease around the world. It is estimated to affect about 20–30% of adult populations in developed countries (2). Patients with NAFLD are usually asymptomatic even in cases with liver enzyme abnormalities and histologic lesions (3). The process from simple steatosis to cirrhosis is triggered by the accumulation of excessive hepatic fat (probably due to insulin resistance) and oxidative stress (4). NAFLD is becoming more common among adults between 40-60 years of age, but the disease is well described in children as well (5).
NAFLD appears most often in middle-aged and overweight people. Other associated factors are high cholesterol or triglycerides,diabetes or insulin resistance (6). NAFLD is strongly associated with metabolic syndrome and is considered as the hepatic manifestation of metabolic syndrome (7).
Cirrhosis is the advanced stage of liver fibrosis with distortion of the hepatic vasculature (8). These changes in liver tissue result in liver shrinkage and hardening and lead to the progressive loss of the biochemical function of the liver (9). It is generally accepted that early diagnosis of cirrhosis and eliminating its cause may stop further liver damage, increase the chance of successful transplantation and may also reduce mortality (8).
There are several diagnostic procedures for cirrhosis including laboratory tests, liver function tests, imaging the liver by ultrasound or CT scan and in some cases by a liver biopsy. Generally, imaging techniques are highly expensive and inaccessible in underprivileged areas (10). Liver biopsy is the gold standard for detecting cirrhosis (11). However, the risk of severe complications, patient discomfort and expensiveness, makes it inappropriate for regular monitoring of the patients (12). Therefore, a non-invasive, accurate, simple and inexpensive diagnostic method for cirrhosis may be helpful, particularly when access to an expert pathologist is limited or liver biopsy is contraindicated.
Serum markers measured in laboratory tests and liver function tests offer an attractive alternative to liver biopsy for both patients and physicians. Direct markers represent extracellular matrix components; indirect markers are based on routine laboratory data which demonstrate the consequences of the liver damage. Direct and indirect markers may be used alone or in combination to produce composite scores (13). Indirect markers have shown a high accuracy in detecting cirrhosis in comparison with intermediate stages of fibrosis (10). A major advantage of noninvasive markers is their easy reproducibility over time. Longitudinal assessment of noninvasive markers can allow the clinicians to monitor disease progression with no cost in terms of safety and patient acceptance (10).
The diagnostic value of indirect markers of liver fibrosis has been investigated in numerous studies (14). Some previous studies have attempted to diagnose cirrhosis by the artificial neural network method in different types of liver disease; however, some of them employed expensive and less accessible tests (15-17). The method has also been applied for predicting mortality and survival rates in cirrhotic patients (18,19).
ANN is an information processing system which acts as a combination of highly interconnected processing elements (neurons) working in parallel to solve a problem (15). Neural network can be used to extract patterns from complicated data and detect trends in huge amounts of data that could not be recognized otherwise. ANNs have the advantage of learning to predict arbitrarily complex nonlinear relationships between independent and dependent variables (20).
In this study, we developed an ANN model based on our clinical and laboratory data to predict cirrhosis in NAFLD patients.
Methods
Diagnostic variablesselection: We examined a set of data which was collected from 396 patients with NAFLD. Data had been collected by the Middle East Liver Disease Center (MELD). The liver cirrhosis condition of the patients had been diagnosed by liver biopsy. Sixteen patients were excluded because it could not be determined whether or not they had diabetes.
We extracted seven out of 27 available variables (age, sex, height, weight, BMI, Plt, AST, ALT, ALP, Albumin, Bil T, Bil D, PT, INR, FBS, diabetes, Chol, TG, HDL, serum iron, TIBC, ferritin, TSH, T4, T3, uric acid, Total NAS) in our database by means of a specific feature extraction software (KNIME2.6), and we also asked two experts to evaluate these variables in order to select those which were significantly different between cirrhotic andnon-cirrhotic patients with NAFLD. We handled about 30% of the missing data by the average method. Data for diabetes were discrete as 0 for non-diabetic patients and 1 for the diabetic patients. The BMI variable (weight divided by the square of height) was obtained, and AST/ALT ratio was also calculated in this stage.
Finally, the following variables plus age and BMI were used as the inputs of the models. These inputs were age (years), platelet count (1/μl), albumin (g/dl), AST/ALT ratio, AST (IU/l), diabetes (yes= 1, no= 0) and BMI (kg /m2) (Table1). The outputs of the model were supposed to be the absence (y= 1) or presence (y= 2) of the cirrhosis and (y= 3) means indeterminate.
Table1 . Characteristics of Markers Used for DiagnosingCirrhosis with the ANN Model .
| Input | Variable | Train Group (n = 300) | Test Group (n = 80) | ||||||
| Average | SD | Max | Min | Average | SD | Max | Min | ||
| X 1 | Age (years) | 39.9 | 7.75 | 60 | 15 | 41.5 | 8.7 | 58 | 24 |
| X 2 | BMI (kg /m2) | 29.4 | 4.56 | 78.32 | 21.45 | 29.5 | 3.99 | 50.7 | 21.8 |
| X 3 | Platelet count (1/μl) | 231186 | 52130 | 449000 | 110000 | 236206 | 64011 | 431000 | 113000 |
| X 4 | AST(IU/l) | 56.1 | 39.40 | 350 | 12 | 54.2 | 28.19 | 197 | 19 |
| X 5 | Albumin (g/dl) | 4.3 | 0.42 | 5.9 | 2.7 | 4.4 | 0.43 | 5.4 | 3.4 |
| X 6 | AST/ALT ratio | 0.8 | 0.47 | 4.17 | 0.38 | 0.8 | 0.28 | 1.76 | 0.328 |
| X 7 |
Diabetes (yes=1, no=0) |
83 patients have diabetes | 36 patients have diabetes | ||||||
The patients were divided into two groups: the first group (about 80% of the total records) included the data of 52 cirrhotic patients and 192 non-cirrhotic and 56 indeterminate cases. This group was used to train the models (ANN). The second group (about 20% of the total records) included the remainder of 15 cirrhotic persons, 43 non-cirrhotic and 22 indeterminate cases which were used to test the models. The characteristics of the markers used for diagnosing cirrhosis ANN train and test models are demonstrated in Table 1.
Model Development: In order to design the optimal ANN, we examined the toolbox of the MATLABsoftwareversion 7.10.0.499 (R2010a) in combination with the MATLAB codes, and we then compared the performance of several two or three layers of back-propagation and feed-forward networks with different number of neurons and permutations of TANSIG (Tan-Sigmoid Transfer Function) and LOGSIG (Log-Sigmoid Transfer Function) for the hidden layer to obtain optimum network performance. Finally, the input, hidden and output layers of the desired network contained eight neurons in total. The default transfer function for the hidden layers and the output layer was TANSIG and PURELIN (Linear Transfer Function), respectively. The default back-propagation training algorithm was TRAINLM (Leven berg-Marquardt back propagation). It is a network training function that updates weight and bias values of the network. TANSIG, LOGSIG and PURELIN functions calculate the layers’ output from their net input.
A major problem of neural networks is their tendency to over-fit the data. Over-fitting occurs when a model is trained by maximizing its performance on some set of training data, but its ability is determined by its good performance on unseen data .To deal with over-fitting, we divided the data into a training set and a testing set by stratified random selection according to the target variable (presence/absence of cirrhosis). Our network performed a proper response to the testing set (second group of data). In addition, both the train and test error rates decreased after 38 training times (epoch 38) in the network performance plot, so it can be claimed that over-fitting had not occurred in the training phase. We normalized data to gain better performance before training the network.
Model Evaluation: Considering the experts’ opinion, we merged the indeterminate and non-cirrhotic cases to calculate accuracy (the number of correct predictions divided by total predictions), sensitivity (the proportion of actual positives which are correctly identified), specificity (the proportion of negatives which are correctly identified), positive predictive value (PPV), negative predictive value (NPV), likelihood ratio positive (LR+) and likelihood ratio negative (LR−) of the obtained model. The discriminatory power was measured by area under curve (AUC) of ROC plot to evaluate the model. An ideal model would have an AUC of 1.0 and thus having the highest sensitivity and specificity (100%).
Several models with different and higher accuracy than our optimum network were developed, and the ones with more number of neurons or higher test error (false positive rate) were ignored preferentially. To facilitate the usage of the final model, we designed a simple GUI by MATLAB at the last step.
Results
In this study, several different ANNs were developed based on the extracted features and their performances were compared to achieve the best possible model. The best obtained model was developed with two layers, eight neurons, TANSIG and PURLIN functions for the layer1; and the output layer presented the train error of (MSE_train) 0.0526 and the test error of (MSE_test) 0.0732 in 10 validation checks, respectively. As demonstrated in Table 2, our ANN model was able to classify 86.6% of the cases correctly. Among the cases in which cirrhosis was ruled out by liver biopsy, 97% were correctly classified; and among the cases with biopsy-proven cirrhosis, the ANN model correctly detected 66% of the cases. Also, the model classified 74% of the cases with indeterminate liver biopsy results in the same group.
Table 2 . The Prediction Results of the Model in Each Category of Liver Biopsy Reports .
| Result of Liver Biopsy | |||||
| Not Cirrhosis | Cirrhosis | Indeterminate | Total | ||
| Result of the ANN model | Not Cirrhosis | 227 | 7 | 17 | 251 |
| Cirrhosis | 1 | 44 | 3 | 48 | |
| Indeterminate | 7 | 16 | 58 | 81 | |
| Total | 235 | 67 | 78 | 380 | |
We assumed the cases with indefinite diagnosis (the indeterminate group) as non-cirrhotic based on the experts’ opinion and merged the two groups. The model had 66% sensitivity and 34% false negative rate, 99% specificity with 1% false positive rate. PPV and NPV were 92% and 93%, respectively. Other measures of the model performance are demonstrated in Table 4.
Table 4 . The Predictive Performance of ANN in Diagnosing Cirrhosis .
| Measure | Estimate | 95% Confidence Interval |
| Sensitivity | 66% | 54% -76% |
| Specificity | 99% | 97% - 100% |
| PPV | 92% | 80% - 97% |
| NPV | 93% | 90% - 95% |
| LR+ | 51.5 | 19.1 - 138.2 |
| LR- | 0.35 | 0.25 – 0.48 |
Table 3 . The Prediction Results of the Model after Merging the Indeterminate and Non-Cirrhotic Groups.
| Biopsy | |||
| Cirrhotic(N = 67) | Non-Cirrhotic(N = 313) | ||
| ANN | Cirrhotic | 44 | 4 |
| Non-Cirrhotic | 23 | 309 | |
The AUC is a measure of a model’s discriminatory power, and in our study, it was 0.89 and 0.73 for validation and test data, respectively (Fig. 1).
Fig. 1 .
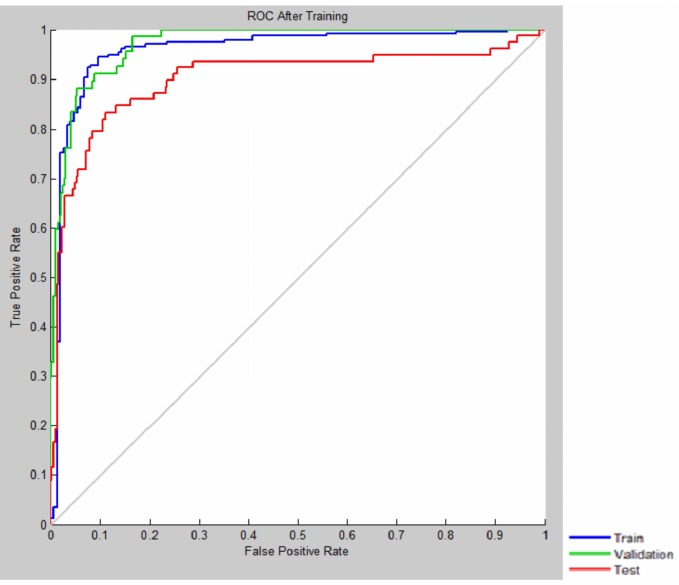
ROC Curve Shows the Model’s Discriminatory Power
Discussion
Although liver biopsy is the gold standard for cirrhosis assessment (21), it has several limitations and risks (22). Successful individualized management and follow-up of liver cirrhosis and determination of response to treatment depends on performing non-invasive, reproducible and affordable evaluations. A number of non-invasive techniques ranging from serum biomarker to advanced imaging techniques are proposed for situations in which liver biopsy could not or should not be performed. However, many of such techniques are not accessible for underprivileged areas. Neural network method which is based on some available and accessible laboratory serum markers can be applied in such circumstances.
ANN has been applied for designing diagnostic and prognostic models in different health conditions. Raoufy et al. have designed a model to detect cirrhosis in hepatitis B patients (15). They achieved sensitivity of 87.5% and specificity of 92%. In another study, an ANN based model was constructed to successfully predict the mortality risk of patients with cirrhosis (19).
In this study, we developed a predictive ANN model to detect cirrhosis in NAFLD patients. The model had a low mean standard error both in train and test groups. Also, the AUC which reflects the discriminatory power of the model had acceptable diagnostic values in both groups. The specificity of the model was high which means that our model was significantly efficient to detect the non- cirrhotic cases. With the prevalence of 17.6%, NPV and PPV of the model were both high. This utility reduces the number of patients who need definitive evaluation of liver cirrhosis, and it can also help the clinicians to focus on the resources and conduct their evaluations with a more cost effective method.
Conclusion
The results of this study revealed that the neural network modeling is a simple, noninvasive and accurate method of diagnosing cirrhosis only based on routine laboratory data.
Acknowledgments
Special thanks to Mr. Rowhanimanesh (Department of Electrical Engineering, Ferdowsi University of Mashhad) who provided us with his invaluable advices.
Cite this article as: Pournik O, Dorri S, Zabolinezhad H, Alavian S.M, Eslami S. A diagnostic model for cirrhosis in patients with nonalcoholic fatty liver disease: an artificial neural network approach. Med J Islam Repub Iran 2014 (21 October). Vol. 28:116.
References
- 1.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World journal of gastroenterology WJG. 2011;17(29):3377. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clinical Science. 2008;115:141–50. doi: 10.1042/CS20070402. [DOI] [PubMed] [Google Scholar]
- 3.Patt CH, Yoo HY, Dibadj K, Flynn J, Thuluvath PJ. Prevalence of transaminase abnormalities in asymptomatic, healthy subjects participating in an executive health-screening program. Dig Dis Sci. 2003;48(4):797–801. doi: 10.1023/a:1022809430756. [DOI] [PubMed] [Google Scholar]
- 4.Mehta K, Van Thiel DH, Shah N, Mobarhan S. Nonalcoholic Fatty Liver Disease: Pathogenesis and the Role of Antioxidants. Nutrition Reviews. 2002;60(9):289–93. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]
- 5.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: An overview of the epidemiological evidence. World Journal of Gastroenterology. 2011 Aug;17(29):3377–89. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehreh MEG, Vahedi M, Pourhoseingholi MA, Ashtari S, Khedmat H, Amin M. et al. Estimation of diagnosis and treatment costs of non-alcoholic fatty liver disease: a two-year observation. Hepatitis Monthly. 2013;13(5) doi: 10.5812/hepatmon.7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Current opinion in lipidology. 2005 Aug;16(4):421–7. doi: 10.1097/01.mol.0000174153.53683.f2. [DOI] [PubMed] [Google Scholar]
- 8.Schuppan D, Afdhal NH. Liver cirrhosis. The Lancet. 2008;371(9615):838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I Diagnosis and evaluation. Am Fam Physician. 2006 Sep 1;74(5):756–62. [PubMed] [Google Scholar]
- 10.Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011 Jan;53(1):325–35. doi: 10.1002/hep.24013. [DOI] [PubMed] [Google Scholar]
- 11.Lefkowitch JH. Liver Biopsy Assessment in Chronic Hepatitis. Archives of Medical Research. 2007;38(6):634–43. doi: 10.1016/j.arcmed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Raoufy M, Vahdani P, Alavian S, Fekri S, Eftekhari P, Gharibzadeh S. A Novel Method for Diagnosing Cirrhosis in Patients with Chronic Hepatitis B: Artificial Neural Network Approach. Journal of Medical Systems. 2011 2011/02/01;35(1):121–6. doi: 10.1007/s10916-009-9348-8. [DOI] [PubMed] [Google Scholar]
- 13.Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: Clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clinica Chimica Acta. 2007;381(2):107–13. doi: 10.1016/j.cca.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Baranova A, Lal p, Birerdinc A, Younossi z. Non-Invasive markers for hepatic fibrosis. BMC Gastroenterology. 2011;11(91) doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raoufy MR, Vahdani P, Alavian SM, Fekri S, Eftekhari P, Gharibzadeh S. A novel method for diagnosing cirrhosis in patients with chronic hepatitis B: artificial neural network approach. Journal of Medical Systems. 2011;35(1):121–6. doi: 10.1007/s10916-009-9348-8. [DOI] [PubMed] [Google Scholar]
- 16.Haydon GH, Jalan R, Ala-Korpela M, Hiltunen Y, Hanley J, Jarvis LM. et al. Prediction of cirrhosis in patients with chronic hepatitis C infection by artificial neural network analysis of virus and clinical factors. J Viral Hepat. 1998 Jul;5(4):255–64. doi: 10.1046/j.1365-2893.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 17.Poon TC, Hui AY, Chan HL, Ang IL, Chow SM, Wong N. et al. Prediction of liver fibrosis and cirrhosis in chronic hepatitis B infection by serum proteomic fingerprinting: a pilot study. Clin Chem. 2005 Feb;51(2):328–35. doi: 10.1373/clinchem.2004.041764. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee R, Das A, Ghoshal UC, Sinha M. Predicting mortality in patients with cirrhosis of liver with application of neural network technology. Journal of Gastroenterology and Hepatology. 2003;18(9):1054–60. doi: 10.1046/j.1440-1746.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 19.Cucchetti A, Vivarelli M, Heaton ND, Phillips S, Piscaglia F, Bolondi L. et al. Artificial neural network is superior to MELD in predicting mortality of patients with end-stage liver disease. Gut. 2007 February 1 2007;56(2):253–8. doi: 10.1136/gut.2005.084434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chong C-F, Li Y-C, Wang T-L, Chang H, editors. Stratification of adverse outcomes by preoperative risk factors in coronary artery bypass graft patients: an artificial neural network prediction model. AMIA Annual Symposium Proceedings; 2003: American Medical Informatics Association. [PMC free article] [PubMed]
- 21.Tarantino G. Is Assessing the Presence of NASH by Liver Histology or Surrogate Markers Always Advisable? Hepat Mon. 2012;13(2):e7560. doi: 10.5812/hepatmon.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pournik O, Alavian SM, Ghalichi L, Seifizarei B, Mehrnoush L, Aslani A. et al. Inter-observer and intra-observer agreement in pathological evaluation of Non Alcoholic Fatty Liver Disease suspected liver biopsies. Hepatitis Monthly. 2013;13(12):e15167. doi: 10.5812/hepatmon.15167. [DOI] [PMC free article] [PubMed] [Google Scholar]


