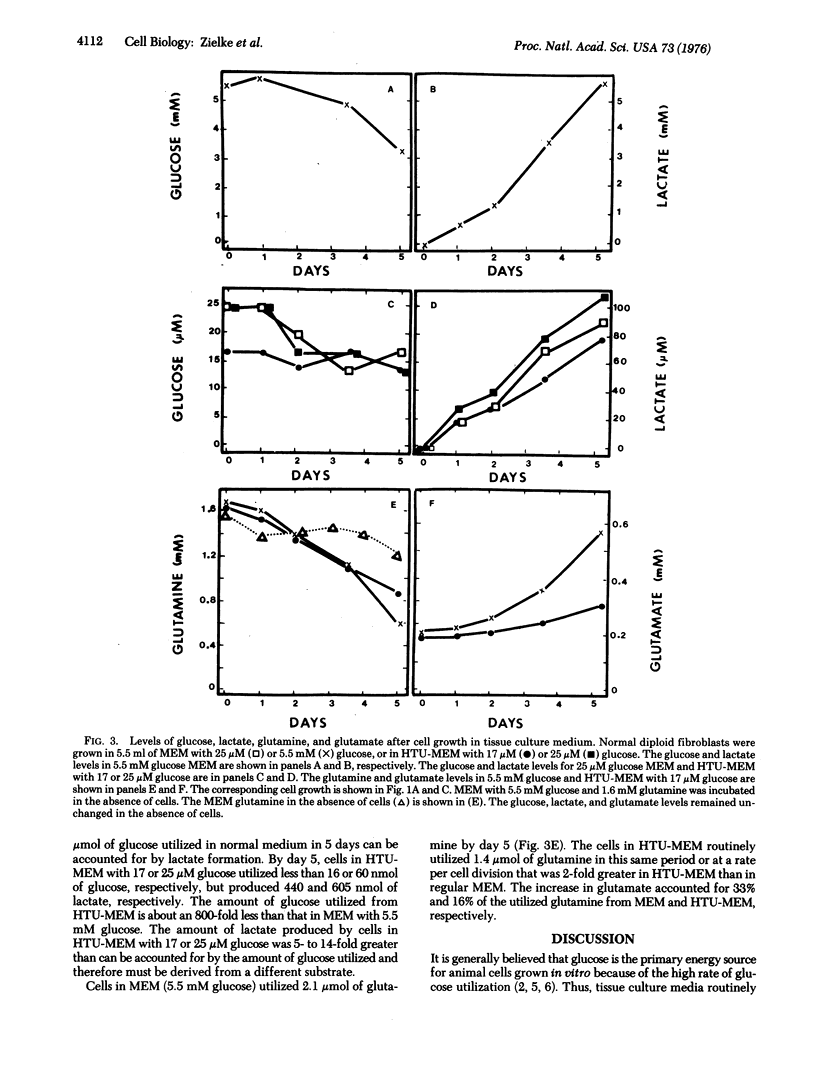
Abstract
Normal human diploid fibroblasts were able to undergo one to two cell divisions without glucose utilization in Eagle's minimum essential medium plus 10% dialyzed fetal calf serum if the medium was supplemented with hypoxanthine, thymidine, and uridine (supplemented medium termed HTU-MEM). Under these conditions, the added purine and pyrimidines were required for nucleic acid synthesis, as shown by the inability of Lesch-Nyhan fibroblasts to grow in HTU-MEM. Normal human diploid fibroblasts continued to produce lactate in HTU-MEM, but at a greatly reduced rate. Since cells grew in HTU-MEM without glucose utilization, the probable energy and carbon source was glutamine, which is present in relatively high concentration. Furthermore, the rate of glutamine utilization per cell division was 2-fold greater in HTU-MEM than in medium with 5.5 mM glucose. These results suggest that glutamine can be a major energy source for cells grown in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT J. C., SCHILLING E. L., EARLE W. R. Massive fluid-suspension cultures of certain mammalian tissue cells. II. Glucose utilization and cell proliferation. J Natl Cancer Inst. 1958 Aug;21(2):349–364. [PubMed] [Google Scholar]
- CRISTOFALO V. J., KRITCHEVSKY D. GROWTH AND GLYCOLYSIS IN THE HUMAN DIPLOID CELL STRAIN WI-38. Proc Soc Exp Biol Med. 1965 Apr;118:1109–1113. doi: 10.3181/00379727-118-30057. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Kritchevsky D. Respiration and glycolysis in the human diploid cell strain WI-38. J Cell Physiol. 1966 Feb;67(1):125–132. doi: 10.1002/jcp.1040670114. [DOI] [PubMed] [Google Scholar]
- EAGLE H., BARBAN S., LEVY M., SCHULZE H. O. The utilization of carbohydrates by human cell cultures. J Biol Chem. 1958 Sep;233(3):551–558. [PubMed] [Google Scholar]
- EAGLE H. The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J Exp Med. 1955 Jul 1;102(1):37–48. doi: 10.1084/jem.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Marliss E., Pozefsky T., Cahill G. F., Jr Amino acid metabolism in the regulation of gluconeogenesis in man. Am J Clin Nutr. 1970 Jul;23(7):986–992. doi: 10.1093/ajcn/23.7.986. [DOI] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Glucose utilization, pH reduction and density dependent inhibition in cultures of chick embryo fibroblasts. J Cell Physiol. 1975 Jun;85(3):635–642. doi: 10.1002/jcp.1040850316. [DOI] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Stimulation of lactic acid production in chick embryo fibroblasts by serum and high pH in the absence of external glucose. J Cell Physiol. 1975 Dec;86(3 Pt 1):453–457. doi: 10.1002/jcp.1040860303. [DOI] [PubMed] [Google Scholar]
- GORHAM L. W., WAYMOUTH C. DIFFERENTIATION IN VITRO OF EMBRYONIC CARTILAGE AND BONE IN A CHEMICALLY-DEFINED MEDIUM. Proc Soc Exp Biol Med. 1965 May;119:287–290. doi: 10.3181/00379727-119-30160. [DOI] [PubMed] [Google Scholar]
- GRAFF S., MOSER H., KASTNER O., GRAFF A. M., TANNENBAUM M. THE SIGNIFICANCE OF GLYCOLYSIS. J Natl Cancer Inst. 1965 Apr;34:511–519. [PubMed] [Google Scholar]
- Griffiths J. B. The quantitative utilization of amino acids and glucose and contact inhibition of growth in cultures of the human diploid cell, WI-38. J Cell Sci. 1970 May;6(3):739–749. doi: 10.1242/jcs.6.3.739. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., TAYLOR E. The ability of purine and thymine derivatives and of glycine to support the growth of mammalian cells in culture. J Biol Chem. 1959 Jan;234(1):126–128. [PubMed] [Google Scholar]
- Lavietes B. B., Regan D. H., Demopoulos H. B. Glutamate oxidation of 6C3HED lymphoma: effects of L-asparaginase on sensitive and resistant lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3993–3997. doi: 10.1073/pnas.71.10.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell B., Froesch E. R. Fibroblasts as an experimental tool in metabolic and hormone studies. I. Growth and glucose metabolism of fibroblasts in culture. Eur J Clin Invest. 1973 Mar;3(2):112–118. doi: 10.1111/j.1365-2362.1973.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Ozand P. T., Hawkins R. L., Collins R. M., Jr, Tildon J. T., Cornblath M. A micro-autoanalytic procedure developed for the determination of ketone bodies, gluconeogenic amino acids, pyruvate, lactate, and glucose in metabolic studies. Biochem Med. 1975 Oct;14(2):170–183. doi: 10.1016/0006-2944(75)90034-4. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Growth of cultured mammalian cells on secondary glucose sources. Cell. 1974 Aug;2(4):287–293. doi: 10.1016/0092-8674(74)90023-3. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SEBRING E. D. Utilization of precurosors for nucleic acid synthesis by human cell cultures. Arch Biochem Biophys. 1959 Sep;84:143–150. doi: 10.1016/0003-9861(59)90563-6. [DOI] [PubMed] [Google Scholar]
- Stoner G. D., Merchant D. J. Amino acid utilization by L-M strain mouse cells in a chemically defined medium. In Vitro. 1972 Mar-Apr;7(5):330–343. doi: 10.1007/BF02661723. [DOI] [PubMed] [Google Scholar]
- Wein J., Goetz I. E. Asparaginase and glutaminase activities in culture media containing dialyzed fetal calf serum. In Vitro. 1973 Nov-Dec;9(3):186–193. doi: 10.1007/BF02618436. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]






