Abstract
Objectives:
To identify the staining potential of acidulated phosphate fluoride (APF) foam on restorations in vitro.
Materials and Methods:
Two hundred ovine molars were used. Except 40 teeth remained unrestored as the controls, each was randomly selected to receive one of four restorative materials including preparation without restoration, glass ionomer cement (GIC), resin modified glass ionomer cement (RMGIC), or composite resin (CR). Following the procedure, topical APF was applied with a predetermined frequency. Staining formation was then evaluated.
Results:
APF-treated teeth and restorations appeared with a darker shade, an orange-colored surface and/or a brown margin. The staining rates on GIC, RMGIC, and CR were 50%, 27.5%, and 17.5%, respectively. GIC had a higher staining potential than RMGIC (χ2 = 4.266, df = 1, P = 0.039) and CR (χ2 = 9.448, df = 1, P = 0.002), whereas the difference between RMGIC and CR was indiscernible (χ2 = 1.147, df = 1, P = 0.284). Repeated applications of topical APF increased the risk of staining on RMGIC (χ2 = 8.436 df = 1, P = 0.004) and CR (χ2 = 6.873, df = 1, P = 0.009) but not on GIC (χ2 = 0, df = 1, P = 1) and the controls (χ2 = 4.051, df = 3, P = 0.256).
Conclusions:
APF-foam-related staining was confirmed in vitro. GIC was more susceptible to fluoride staining. This study suggested aesthetic implications when applying fluorides to restored teeth.
Keywords: Acidulated phosphate fluoride, composite resin, glass ionomer cement, staining
INTRODUCTION
Topical fluoride compounds containing acidulated phosphate fluoride (APF) have been the preferred vehicle in professional use in programs of caries prevention.[1,2,3,4,5] Adverse effects of professionally applied APF gels on restorative materials have been reported in literature.[6,7,8,9,10,11,12,13,14] Some previous studies revealed considerable structural alterations on resin composites, glass ionomer cement (GIC), and resin modified glass ionomer cement (RMGIC)[6,7,8,9,10,11,12,13] and this may lead to increased staining. Nevertheless, a scanning electron microscope (SEM) study suggested that surface micromorphology of restorations was not affected by APF application.[14] On the other hand, the staining potential of APF foam has not yet been widely reviewed. Such property carries significant implications in restorative dentistry, especially for the aesthetic zone. Therefore, the aim of this study was to identify the staining potential of APF foam on various restorative materials in vitro.
MATERIALS AND METHODS
In order to evaluate the staining potential of fluoride on dental restorations in biologic systems, heads of deceased sheep were used for the experiment. Sheep were chosen as the animal model because their dentition shares structural and mechanical similarities to that of humans.[15] In addition, access to these specimens was more readily available in Australia.
A fully dentate sheep has 32 teeth, including 8 lower permanent incisors, 12 mandibular permanent molars, and 12 maxillary permanent molars.[16] A sheep becomes fully dentate near 38 months. After discussion with a domestic frozen food supplier (Craig Mostyn Group), an effort was made to only select frozen heads of fully dentate sheep. Since the animals have been commercially slaughtered for their meat other than a scientific purpose, approval from the Animal Ethics Committee was waived. As it is common for farmers to grind down the lower incisors of sheep, the conditions of these teeth cannot be guaranteed by the supplier. Therefore, only molars were used in the study.
Accordingly, a random sample of 200 permanent molars from 10 deceased sheep was selected. Upon receiving the specimen, a number of preparatory steps were needed before these heads could be used in the research. Firstly, a relieving incision along the cheek was provided to allow adequate access for restorative purposes. A number 15 blade equipped with a number 3 scalpel handle was used to carry out the incision. The specimen was then rinsed with water to remove any blood attached.
Secondly, the dentition of the sheep was completely covered in a black, hard substance, most probably calculus. Therefore, such foreign material was removed before restoring the teeth. In order to reduce the damage on the tooth structure, an ultrasonic scaler was used to remove the plaque, clearing at least 4 mm × 4 mm on the mid-buccal section, to allow for restoration. Typical specimen after scale and clean is shown in Figure 1a.
Figure 1.
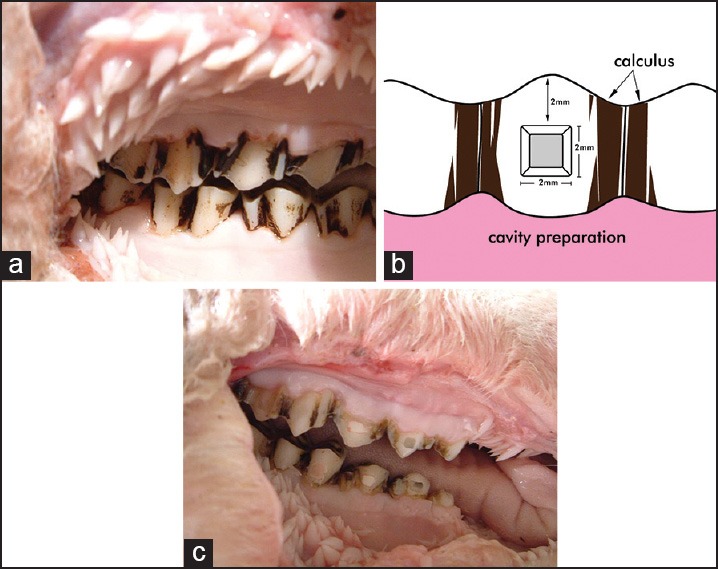
(a) A typical specimen after scale and clean. (b) Diagram of proposed cavity preparation. (c) Orange staining around marginal line angles
Cavity preparation of the following dimensions and locations was made for all specimens in order to establish consistency when inspecting the staining. The cavity preparation was located at 2mm above the gingiva on the mid-buccal surface of the teeth and designed as a square box with a depth and a width of 2mm, respectively [Figure 1b].
A 2 mm depth cut was first performed with a high-speed pear-shaped bur under water. Then a high-speed flat fissure was used to extend the box into the required cavity dimensions as outlined above. Finally, a slow speed flat fissure bur was used to smooth and refine the cavity form as necessary. The cavity was then rinsed thoroughly with water to remove any debris, and air dried, ready for restoration.
Various restorative materials were then used to restore the preparations. The experiment divided the restorative material into six groups, and the method of restoration for each group is described below:
-
Control — two groups
-
Teeth without any preparationTeeth left as original, unprepared, and unrestored.
-
Teeth with preparation but no restorative material placedTeeth prepared using the technique described above.
-
-
GIC restoration (Fuji IX GP fast capsules, GC corp, tokyo, Japan: Shade A2)
After cavity preparation is complete, Fuji IX capsule was activated and applied to cavity using a conventional applicator. The excess was wiped away using a plastic number 6 instrument. The material was allowed 3 minutes, from start of mixing for setting.
-
RMGIC Restoration (Fuji II LC Capsules, GC Corp, Tokyo, Japan: Shade A2)
After cavity preparation was complete, Fuji II capsules was activated and applied to cavity using a conventional applicator. Excess was removed using a plastic number 6 instrument before light curing for 40 seconds until set.
Composite restoration (Gradia direct anterior, 3M ESPE, saint paul, USA: Shade A2)
Cavity preparation was etched with a liquid acid etchant (Halas Acid Etch Gel: 37% phosphoric acid), applied using a microbrush for 20 seconds. This was followed by a water rinse for 20 seconds, and the area evacuated, then dried of all visible moisture. 3M Single Bond Adhesive was applied to all internal surfaces of the cavity, and then light cured for 15 seconds. The composite was then placed using a plastic number 6 instrument, the excess carved away, and the light cured for 40 seconds.
Each sheep head housed all six of these groups in an attempt to minimize individual biologic variations between specimens. The first five molars in a quadrant will be used for the experiment. The first molar contains control group 1, teeth with no preparation and no restoration. The second molar contained control group 2, teeth with preparation but no restoration. The third molar was prepared and received GIC. Fourth molar received RMGIC. The fifth molar housed the composite. None of the restorations was polished.
The restored teeth then had APF foam (Laclede Topical Fluoride Foam: Sodium fluoride and hydrofluoric acid, equivalent to 1.23% weight/weight fluoride ion) applied to them. The technique for fluoride application is described below:
Teeth were cleaned and rinsed to remove any debris that may be present on and around restoration surfaces.
Teeth were air dried completely.
Fluoride was applied using a microbrush to cover all surfaces of the teeth.
Fluoride was left in place for 4 minutes.
Finger was used to wipe excess fluoride from the teeth.
All teeth within a specimen would have had received fluoride application using the above technique, with the exception of the two control groups in the second and the third quadrants. This created control groups with fluoride application, and those without. A further comparison was performed to reveal the relationship between tooth preparation and staining, using the control group with preparation but no restoration.
Fluoride was applied once a day over a 3-day period. As a previous study suggested providing fluoride therapy once per 6 months to those children who showed a higher caries risk[5], three episodes of topical fluoride therapy were applied to the ovine teeth in this study to simulate repetition of fluoride treatment that a child may receive.
The presence of staining was then evaluated. This was achieved by utilizing shade guides (VITAPAM Classifical),[6] similar to those commonly used in Prosthetics and Crown and Bridge. The evaluation was repeatedly carried out with visual inspection by three trained examiners, in a “blind” fashion evaluated shades of the restored and unrestored teeth, and noted the nature and extent of any staining. An A2 shade guide was used as the benchmark of assessment, since all restorative materials selected were of Shade A2 upon setting. Evaluation results were recorded as yes/no for staining, this being defined as a difference in shade at any portion/margin of a restoration.[6] Utilizing the control group, the original shade of the teeth was ascertained, to assist in determining the extent of staining. The examiners were not told which restorations have received fluoride application.
Storage of specimen during the experimental period was as follows. Each specimen was separately wrapped in plastic bags and stored in a large container filled with ice. The container was drained of excess water, and ice was changed on a daily basis. The average temperature inside the container, measured with a household thermometer, was 3°C.
Data entry and statistical analysis were conducted with the IBM SPSS Statistics (version 20.0, IBM Corporation,Somers, NY, USA). A chi-square method was used to examine staining potentials among restorative materials and/or frequency of APF application. The level of two-sided significance for all statistical procedures was set at 5%.
RESULTS
Fluoride staining on the teeth and/or the restorations varied, appearing with a distinguishable darker shade, an orange-colored surface or margin [Figure 1c]. The staining was most commonly observed against the marginal line angles of the restoration. This staining was subjected to continuous scratching with a sickle-scale probe, and it was evident that the staining could not be removed by scratching.
The fluoride staining rates of GIC, RMGIC, and CR were 50%, 27.5%, and 17.5%, respectively [Figure 2]. GIC had a higher staining potential than RMGIC (χ2 = 4.266, df = 1, P = 0.039) and CR (χ2 = 9.448, df = 1, P = 0.002), whereas difference of staining potential between RMGIC and CR was indiscernible (χ2 = 1.147, df = 1, P = 0.284).
Figure 2.
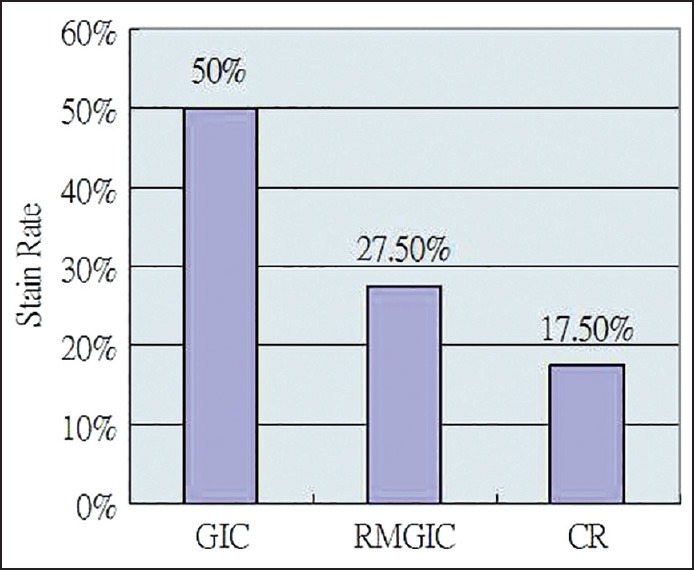
Staining rates of various restorative materials
The occurrence of staining increased with the frequency of topical APF application on RMGIC (χ2 = 8.436, df = 1, P = 0.004) and CR (χ2 = 6.873, df = 1, P = 0.009) but GIC (χ2 = 0, df = 1, P = 1). Staining was not associated with frequency of APF application on teeth without preparation and/or restoration (χ2 = 4.051, df = 3, P = 0.256).
DISCUSSION
Fluoride-releasing dental materials are effective in preventing secondary caries or caries on adjacent teeth.[17] Some fluoride releasing materials can reduce enamel solubility and dissolution and plaque formation by bacteria that initiate caries, thereby preventing enamel demineralization. The etching of GIC with phosphoric acid increases the surface roughness.[18] This may account for the increased stain rate of GIC materials in the present study.
This staining of the restorative materials by APF gels reported in scientific literature was confirmed by this study.[14] Repeated application of APF foam can lead to stain formation, particularly along the marginal line angles.
Other studies have also demonstrated the change in surface roughness of various restorative materials after application of APF gel.[18] This may help explain the stain rates obtained in this study. In previous studies, Fuji IX GP was reported as demonstrating a very high surface roughness after APF application. This correlates with the high stain rates obtained by GIC in the present study.
On the other hand, Fuji II LC has been reported as displaying no significant morphological changes before and after APF treatment.[18] This may be due to the light-cured resin matrix, which makes RMGIC more resistant to the effects of the APF action. This correlates in the present with RMGIC displaying a lower stain rate when compared to GIC.
With regards to composites, evidence exists as to the method by which surface attracted stain. Sposetti et al.[19] suggested that silicon dioxide, which is a component of glass, is susceptible to hydrofluoric acid. Furthermore, Bowen and Cleed[20] reported that fluoride may cause depolymerization of the matrix-particle interface. Hydrofluoric acid, a well-known glass etchant, dissolves the composite filler particles resulting in a pitted surface. This roughened surface may contribute to plaque accumulation and may produce surface staining of the materials.[21]
The effect of APF agents on composite surface may depend on the type of filler. Macro-inorganic filled composites have been reported to be more affected than other composites.[7,22] Microfilled or hybrid resin-based composites may be a better choice, when APF treatment is to be used routinely. However, the present study shows that microfilled composites, such as one used in the experiment, can still produce staining after application of APF foam treatment.
CONCLUSIONS
The staining of APF foam reported in the literature was confirmed in vitro. GIC was more susceptible to fluoride staining than RMGIC and CR. This study suggested aesthetic implications when topically applying fluorides to restored teeth. Further investigation on human teeth is indicated.
ACKNOWLEDGEMENT
The publication was supported with an Australian Dental Research Foundation (ADRF) Undergraduate Research Grant. The authors would like to show appreciation to those staff and students who helped in this project. In addition, this paper is indebted to Craig Mostyn Group for their kind sponsorship of frozen sheep heads. Special thanks to Dr. Mei-lan Chen for her assistance in preparation of electronic artwork.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bryan ET, Williams JE. The cariostatic effectiveness of a phosphatefluoride gel administered annually to school children. I. The results of the first year. J Public Health Dent. 1968;28:182–5. doi: 10.1111/j.1752-7325.1968.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz HS. Effect on dental caries of topically applied acidulated phosphate-fluoride: Results after two years. J Am Dent Assoc. 1969;78:568–72. doi: 10.14219/jada.archive.1969.0116. [DOI] [PubMed] [Google Scholar]
- 3.DePaola PF, Aasenden R, Brudevold F. The use of topically applied acidulated phosphate-fluoride preceded by mild etching of the enamel: A one-year clinical trial. Arch Oral Biol. 1971;16:1155–63. doi: 10.1016/0003-9969(71)90044-6. [DOI] [PubMed] [Google Scholar]
- 4.Ripa LW. Professionally (operator) applied topical fluoride therapy: A critique. Int Dent J. 1981;31:105–20. [PubMed] [Google Scholar]
- 5.Jiang H, Bian Z, Tai BJ, Du MQ, Peng B. The effect of a bi-annual professional application of APF foam on dental caries increment in primary teeth: 24-month clinical trial. J Dent Res. 2005;84:265–8. doi: 10.1177/154405910508400311. [DOI] [PubMed] [Google Scholar]
- 6.Wang E, Huang B. Discolouration of glass-ionomer cement at different fluoride concentration levels. Oral Health Dent Manag. 2014;13:1–4. [PubMed] [Google Scholar]
- 7.Kula K, Nelson S, Kula T, Thompson V. In vitro effect of acidulated phosphate fluoride gel on the surface of composites with different filler particles. J Prosthet Dent. 1986;56:161–9. doi: 10.1016/0022-3913(86)90465-8. [DOI] [PubMed] [Google Scholar]
- 8.Papagiannoulis L, Tzoutas J, Eliades G. Effect of topical fluoride agents on the morphologic characteristics and composition of resin composite restorative materials. J Prosthet Dent. 1997;77:405–13. doi: 10.1016/s0022-3913(97)70166-5. [DOI] [PubMed] [Google Scholar]
- 9.Neuman E, Garcia-Godoy F. Effect of APF gel on a glass ionomer cement: An SEM study. ASDC J Dent Child. 1992;59:289–95. [PubMed] [Google Scholar]
- 10.Yap AU, Mok BY. Effects of professionally applied topical fluorides on surface hardness of composite-based restoratives. Oper Dent. 2002;27:576–81. [PubMed] [Google Scholar]
- 11.Abate PF, Bertacchini SM, Garcia-Godoy F, Macchi RL. Barcoll hardness of dental materials treated with an APF foam. J Clin Pediatr Dent. 2001;25:143–6. doi: 10.17796/jcpd.25.2.rw03351p32336r25. [DOI] [PubMed] [Google Scholar]
- 12.Kula K, Webb EL, Kula TJ. Effect of 1- and 4-minute treatments of topical fluorides on a composite resin. Pediatr Dent. 1996;18:24–8. [PubMed] [Google Scholar]
- 13.Kula K, Kula TJ. The effect of topical APF foam and other fluorides on veneer porcelain surfaces. Pediatr Dent. 1995;17:356–61. [PubMed] [Google Scholar]
- 14.García-Godoy F, García-Godoy A, García-Godoy F. Effect of APF Minute-Foam on the surface roughness, hardness, and micromorphology of high-viscosity glass ionomers. J Dent Child (Chic) 2003;70:19–23. [PubMed] [Google Scholar]
- 15.O’Brien S, Keown AJ, Constantino P, Xie Z, Bush MB. Revealing the structural and mechanical characteristics of ovine teeth. J Mech Behav Biomed Mater. 2014;30:176–85. doi: 10.1016/j.jmbbm.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Frandson RD, Wilke WL, Fails AD. 7th ed. Hoboken: Wiley; 2009. Anatomy and physiology of farm animals. [Google Scholar]
- 17.Garcia-Godoy F, Jensen ME. Artificial recurrent caries in glass ionomer-lined amalgam restorations. Am J Dent. 1990;3:89–93. [PubMed] [Google Scholar]
- 18.Cehreli ZC, Yazici R, Garcia-Godoy F. Effect of 1.23 percent APF gel on fluoride-releasing restorative materials. ASDC J Dent Child. 2000;67:330–7. 302. [PubMed] [Google Scholar]
- 19.Sposetti VJ, Shen C, Levin AC. The effect of topical fluoride application on porcelain restorations. J Prosthet Dent. 1986;55:677–82. doi: 10.1016/0022-3913(86)90441-5. [DOI] [PubMed] [Google Scholar]
- 20.Bowen RL, Cleek GW. A new series of x-ray-opaque reinforcing fillers for composite materials. J Dent Res. 1972;51:177–82. doi: 10.1177/00220345720510011301. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe A, Zalkind M. The effect of topical application of fluoride on composite resin restorations. J Prosthet Dent. 1981;45:59–62. doi: 10.1016/0022-3913(81)90013-5. [DOI] [PubMed] [Google Scholar]
- 22.Kula K, Nelson S, Thompson V. In vitro effect of APF gel on three composite resins. J Dent Res. 1983;62:846–9. doi: 10.1177/00220345830620071901. [DOI] [PubMed] [Google Scholar]


