Abstract
Aims:
The aim was to compare efficacy and cost-effectiveness of bimatoprost 0.03% and brimonidine 0.2% in primary open-angle glaucoma (POAG)/ocular hypertension (OHT).
Settings and Design:
Open, randomized, cross-over, comparative study.
Materials and Methods:
Forty patients of POAG or OHT with intraocular pressure (IOP) <30 mm Hg were included in the study after a written informed consent. The patients were divided randomly into two groups of 20 patients each. Patients of group A were administered bimatoprost 0.03% eye drops once daily, and those of group B brimonidine 0.2% eye drops twice daily for a period of 4 weeks. After a washout period of 4 weeks, the patients were crossed over that is, group A was administered brimonidine 0.2% and group B bimatoprost 0.03%. Fall in IOP at 4 weeks was recorded. The daily cost of each drug was calculated by maximum retail price and the average number of drops per bottle. The cost-effectiveness was then calculated as the cost of drug/mm Hg fall in IOP.
Statistics:
Independent samples t-test was used to compare the efficacy of both drugs.
Results:
IOP lowering with bimatoprost (8.9 ± 1.598 mm Hg) was significantly (P < 0.0001) higher than brimonidine (6.55 ± 1.26 mm Hg). The number of drops/ml were 33.43 ± 0.52 and 25.49 ± 0.26, respectively, for bimatoprost and brimonidine. Treatment with bimatoprost was costlier than brimonidine with daily costs/eye Rs. 4.02 ± 0.06 and 3.14 ± 0.03, yearly costs/eye Rs. 1467.46 ± 20.74 and 1147.75 ± 11.15, respectively. Bimatoprost was more cost-effective than brimonidine with the cost-effectiveness ratio (CER) respectively Rs. 13.10 ± 2.61/mm Hg and Rs. 13.96 ± 2.86/mm Hg. Incremental CER Rs. 10.43/mm Hg implies lower costs/mm Hg extra IOP lowering by bimatoprost than Rs. 13.96 for brimonidine.
Conclusion:
In spite of being costlier, bimatoprost is more efficacious and cost-effective than brimonidine.
Keywords: Cost-effectiveness, glaucoma, pharmacoeconomics
Glaucoma is a chronic debilitating disease requiring life-long treatment. Being the largest cause of bilateral blindness, second only to cataract; glaucoma is a major public health problem.[1] Prevalence of blindness in India, according to National Survey on Blindness 2001–2002, is 1.1%.[2] Leading cause of blindness in India is cataract accounting for 62.6%, whereas glaucoma accounts for 5.8%.[3] Worldwide, it is estimated that about 66 million people have visual impairment from glaucoma, with 6.7 million suffering from blindness due to it.[4] From 2010 to 2020, the most detectable change in glaucoma worldwide would be, its increase in India. The largest absolute number of glaucoma cases have been reported in China, followed by Europe and India.[5]
Primary open-angle glaucoma (POAG) is a subset of the glaucomas defined by raised intraocular pressure (IOP) consistently above 21 mm Hg in at least one eye with typical glaucomatous visual field and/or optic nerve head damage and an open, normal appearing anterior chamber angle with no other underlying disease.[6] Ocular hypertensives are defined as a subset of patients with open angles, raised IOP but neither optic nerve head nor visual field changes.[7]
Studies provide strong evidence that high IOP plays an important role in the neuropathy of POAG. It has been demonstrated that the reduction in the level of IOP lessens the risk of visual field progression in open-angle glaucoma.[8] Treatment strategies of glaucoma aim at lowering IOP, which helps to prevent optic nerve damage and glaucoma-related blindness. An even a single unit lowering of IOP has been associated with significant clinical improvements.[9]
Pharmacotherapy is usually the first line of treatment for elevated IOP and open-angle glaucoma. Major drug classes for medical treatment of POAG include alpha-agonists (brimonidine), beta-blockers (timolol, betaxolol, levobunolol), topical carbonic anhydrase inhibitors (dorzolamide, brinzolamide), oral carbonic anhydrase inhibitors (acetazolamide), miotic agents (pilocarpine), prostaglandin (PG) analogs (travoprost, latanoprost), prostamides (bimatoprost), and sympathomimetic drugs (epinephrine, dipivefrine). PG analogs lower IOP by increasing the uveoscleral outflow of aqueous humor.[10] They are effective in reducing IOP and have the additional advantage of requiring only once a day administration. Bimatoprost, a synthetic prostamide analog, used as a 0.03% topical preparation once daily is efficacious in the treatment of open-angle glaucoma, ocular hypertension (OHT) and other forms of glaucoma.
Brimonidine, a selective alpha-2-agonist, available as 0.2% ophthalmic solution to be administered twice daily, causes a suppressed aqueous humor production. With IOP lowering efficacy comparable to β-blockers, brimonidine confers neuroprotection in glaucoma by stimulating an ongoing neuronal survival pathway.[11] It is used as one of the first-line therapies in patients who have contraindications to β-blockers.
The ophthalmologists have a wide range of choices for management of glaucoma, in terms of cost, efficacy, and adverse effects. There is an increased demand from society and health care payers that clinical medicine in particular when aimed at treatment of chronic life-long disease should justify its cost. Taking into consideration, the broadening gap between therapeutic possibilities and resources available, the choices have to be made by prioritizing (rationing) all treatment strategies.[12] Economic evaluation of glaucoma therapy needs to be targeted at assessment of efficiency, that is, health effects weighed against the sacrifices or costs incurred for attaining them.[13]
Therapeutic decisions for glaucoma therapy should be taken with due consideration of cost of drug along with efficacy and safety. It is no longer enough that an intervention is clinically effective; it needs to be cost-effective as well if we are to make the most of our finite resources. Contemplating the importance of cost-effectiveness, the idea of our study is to update the ophthalmologists’ knowledge regarding the cost-effectiveness along with daily and yearly cost of treating glaucoma with bimatoprost, an effective prostamide and brimonidine, an alpha-2-agonist.
Materials and Methods
In this open, randomized, cross-over, comparative study, 40 subjects of POAG or OHT attending the outpatient Department of Ophthalmology, were included after obtaining written informed consent. The inclusion criteria were a minimum age of 18 years and unilateral or bilateral POAG or OHT with IOP < 30 mm Hg. Patients with bilateral POAG were treated for both eyes, but only left eye of every patient was considered as the study eye.
Patients with closed anterior chamber angle or acute angle closure glaucoma, any history of intraocular surgery within 6 months of study, ocular infection or inflammation, history of allergy to the drugs or other side effects with the drugs or with established diagnosis of secondary glaucoma were excluded from the study. Apart from this, the female patients who were pregnant, lactating or not employing adequate measures to prevent conception or patients with a history of chronic obstructive pulmonary disease/asthma were also excluded.
Intraocular pressure lowering (effectiveness) determination
In this study conducted over 12 weeks, 40 eyes (left side) of these 40 patients were included with the main treatment outcome as the number of mm Hg fall in IOP. The patients were randomly divided into two groups of 20 each. Baseline IOP was recorded on 1st visit, day 0 at 9 am. Patients in group A were instructed to instill one drop of bimatoprost 0.03% (Lumigan) once a day at 9 pm daily for 4 weeks. Patients in group B were instructed to instill one drop of brimonidine 0.2% (Alphagan) twice a day at 9 am and 9 pm for 4 weeks. Measurement of IOP was done subsequently at 2 weeks and 4 weeks. The patients were counseled for regular instillation of eye drops at first visit and compliance to treatment ensured at subsequent visits at 2 weeks and 4 weeks by questioning the patient.
The 4 weeks of therapy were followed by a 4 week washout period when no drug was administered to the patients in either of the groups. The 4-week washout period was decided after literature search. Most of the studies with bimatoprost and brimonidine were carried out with a washout period of 4–6 weeks.[14,15,16]
The drugs were then crossed over, that is, group A was instilled brimonidine 0.2% and group B was instilled bimatoprost 0.03% for a period of another 4 weeks after a baseline IOP measurement and subsequent measurements on 2nd week and 4th week. Effectiveness data used for this economic analysis were the number of mm Hg of IOP reduction at 4 weeks compared with the baseline.
Statistical analysis
The efficacy in terms of IOP lowering was subjected to statistical analysis using independent samples t-test.
Cost analysis
To determinate the cost, five bottles each of bimatoprost (Lumigan) and brimonidine (Alphagan) were taken and number of drops per bottle calculated with bottles held at 135° (the angle at which the drops are instilled in the eyes) and drops collected in graduated measuring cylinder. The actual, not labeled volume was determined for each bottle. Thus, the number of drops/ml were determined by counting the number of drops in one bottle and dividing by the total actual volume measured.
Daily cost of particular anti-glaucoma medication was calculated by dividing the cost of one bottle by total number of drops in a bottle and multiplying by number of drops required daily. Thereafter, 4 weekly and yearly costs of both drugs were calculated.

Cost-effectiveness analysis
Cost-effectiveness i.e. cost/mm Hg of IOP reduction was calculated as:
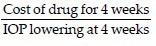
Where one treatment strategy is both more expensive and more effective than its comparator, an incremental cost-effectiveness ratio (ICER) can be calculated that depicts the extra cost per unit of outcome obtained in comparing one treatment option to another.[17]

Ethics
The study was approved by the institutional ethical committee.
Observations
The age- and gender-related characteristics of study groups were as shown by Table 1. The baseline IOP of group A and group B before recruitment was 26.45 ± 1.98 and 26 ± 1.89 mm Hg, respectively; and after washout was 26 ± 1.65 and 25.75 ± 1.74 mm Hg.
Table 1.
Age and gender distribution in both groups
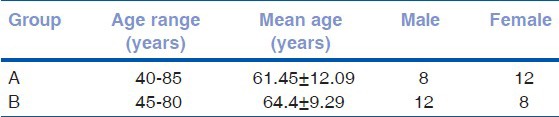
Efficacy of both drugs was compared by measuring IOP lowering effect of bimatoprost and brimonidine. Statistical analysis was done using independent samples t-test. As shown in Table 2, absolute fall in IOP with bimatoprost was by 8.9 ± 1.598 mm Hg by the final visit. This was significantly (P < 0.0001) higher than the reduction of 6.55 ± 1.26 mm Hg seen in brimonidine-treated patients. Among the patients on bimatoprost, 85% (34/40) showed IOPs ≤ 18 mm Hg at 4 weeks as against 25% (10/40) with brimonidine.
Table 2.
Statistical analysis of IOP reduction with bimatoprost and brimonidine using independent samples t-test

Fig. 1 summarizes the mean IOP at visit 0, 1, 2 and Fig. 2 summarizes the IOP fall with a standard deviation. The number of drops per bottle, the number of drops/ml and overfilling/underfilling of bottles were as shown by Table 3. Thus, bimatoprost had more drops/ml than brimonidine.
Figure 1.
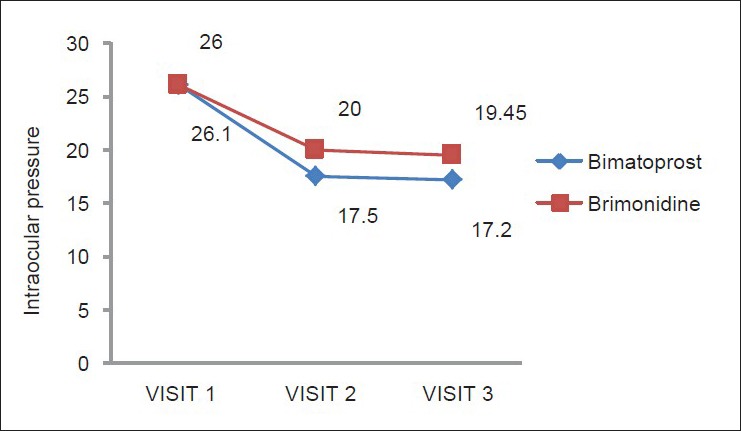
IOP changes in patients on Bimatoprost and Brimonidine on subsequent visits
Figure 2.

4-weekly cost s of both drugs
Table 3.
Volumetric analysis

In our study, we found treatment with bimatoprost to be costlier than brimonidine with daily costs for each eye, Rs. 4.02 ± 0.06 and 3.14 ± 0.03, respectively. The yearly costs for the drugs per eye were Rs. 1467.46 ± 20.74 and 1147.75 ± 11.15 for bimatoprost and brimonidine, respectively. The 4 weekly costs for both drugs used for calculation of CER were as shown by [Fig. 2].
Cost-effectiveness that is, cost/mm reduction of IOP was calculated. The costs and effectiveness included in the calculation were the 4 weekly costs (28 days) and IOP lowering at V3 that is, at 4 weeks. CER analysis as shown by Fig. 3 shows a lower cost incurred by bimatoprost than brimonidine per millimeter lowering of IOP. Thus, bimatoprost is superior to brimonidine in cost-effectiveness analysis.
Figure 3.

Comparision of cost-effectiveness of bimatoprost and brimonidine
Incremental cost-effectiveness ratio is the ratio of the difference in costs and the difference in IOP lowering by both drugs. It represents an additional cost for each unit of additional fall in IOP. The ICER was calculated as 10.43 which means extra Rs. 10.43 were required for each additional mm Hg IOP reduction given by bimatoprost for 4 weeks as compared with brimonidine. Selecting brimonidine, the less costly alternative, implies a willingness to pay Rs. 13.96/mm Hg (CER of brimonidine). An additional IOP reduction obtained with bimatoprost costs Rs. 10.43, which is below willingness to pay the amount for brimonidine. Therefore, on the basis of its incremental CER, bimatoprost could be considered a cost-effective strategy as compared with brimonidine.
The adverse drug reactions reported by the patients were as shown in Table 4. No patient in either group had any serious adverse drug reactions warranting discontinuation of therapy or requirement of additional medication for the treatment of adverse effects. Adverse events were mostly mild in both groups, and the drugs were well-tolerated.
Table 4.
Adverse effects of drugs

Discussion
An ophthalmologist has a wide range of choices for the medical management of glaucoma. Hypotensive lipids (PG analogues/prostamides) have high efficacy, a favorable safety profile, ease of once daily regimen and are often reasonable on a cost per day basis. They have thus fast become a favorite among both physicians and patients despite their higher costs. Bimatoprost has been shown to be most cost-effective among PG analogues.[18] Alpha-agonists like brimonidine are available at lower maximum retail price (MRP's) than PG analogs and are being studied for additional advantages of neuroprotection.[19] Bimatoprost and brimonidine, both are commonly prescribed drugs in glaucoma, each with its own advantages. As the treatment is life-long, studies have been done to compare the efficacy and cost of various anti-glaucoma drugs previously. In our study, the efficacy of drugs as shown by IOP lowering of 8.9 ± 1.6 (34.10%) and 6.55 ± 1.26 (25.19%), respectively, for bimatoprost and brimonidine was comparable to studies by Thomas et al., 2003 and Whitcup et al., 2003 which showed IOP lowering 8 (32.4%) and 6 ± 3.3 (21%) for bimatoprost and brimonidine, respectively.[20,21]
Several ways have been to devised by the drug manufacturers to minimize the wastage of eye drops, like overfilling of the bottles, bottle design, medication dispensing mechanisms and administration techniques. Hence, a lot of preparations may be cheaper, but due to large drop size, they may end up being less cost-effective. The dispensing angles, and viscosity of the medication are the other factors that may influence drop size.[22] The MRP of an anti-glaucoma agent is just one of the multitudes of factors to consider when choosing a medication for a patient. The products with higher actual volume, smaller drop size; hence, the larger number of drops/ml may in reality cost less. In the present study, the average number of drops/ml was 33.43 ± 0.52 for bimatoprost (Lumigan) and 25.49 ± 0.26 for brimonidine (Alphagan) whereas, in study by Rylander an Vold, 2008 these were, respectively, 32.27 ± 0.45 and 24.83 ± 1.57.[23]
The daily costs in the present study, Rs. 4.02 ± 0.06 and Rs. 3.14 ± 0.03, respectively, for bimatoprost and brimonidine were comparable with the cost analysis studies by Fiscella et al., 1999 and Fiscella et al., 2003; which showed daily costs to be $0.95 and $0.90 for bimatoprost and brimonidine, respectively.[22,24] Thus, bimatoprost showed higher daily costs in all these studies. But, cost analysis alone is not a complete economic analysis as the treatment with higher costs may be more efficacious too. On the other hand, a comparatively cheaper therapy may have the disadvantage of lower efficacy and higher adverse effects.
The CER for both drugs in the present study was calculated for the study period of 4 weeks and cost/mm Hg lowering found to be Rs. 13.10 ± 2.61/mm Hg and Rs. 13.96 ± 2.86/mm Hg for bimatoprost and brimonidine, respectively. Thus in the present study, bimatoprost, in spite of being costlier had a more favorable CER. Another study showing comparison of cost-effectiveness of bimatoprost and brimonidine by Galindo-Ferreiro et al. too shows similar results placing bimatoprost at a superior position to brimonidine with respect to CER, with CER being 9.1 for bimatoprost and 9.6 for brimonidine.[25] The gross difference in numerical values of CER in both of above studies is due to variability in monetary units, MRP's and cost of patient visits in different countries.
In the present study, ICER of bimatoprost was Rs. 10.43/mm Hg which is lower than cost/mm Hg reduction incurred by using brimonidine Rs. 13.96 ± 2.86/mm Hg). Selecting brimonidine, the less costly alternative, implies “willingness to pay” Rs. 13.96 ± 2.86/mm Hg lowering of IOP. An additional reduction in IOP obtained with bimatoprost costs Rs. 10.43/mm Hg which is less than the “willingness to pay” amount for brimonidine. Therefore, on the basis of ICER, using bimatoprast over the brimonidine would be considered a cost-effective strategy.
We have compared cost-effectiveness of bimatoprost 0.03% (Lumigan, Allergan) and brimonidine 0.2% (Alphagan, Allergan). The findings of the study thus may not be generalized to other brands and concentrations of these drugs. Similar studies could be carried out to prioritize the anti-glaucoma therapy based on cost-effectiveness analysis.
Conclusion
Bimatoprost and brimonidine are efficacious drugs in the treatment of POAG/OHT, but in spite of daily costs being higher, bimatoprost is a better choice than brimonidine because of:
Greater cost-effectiveness
Greater reduction in IOP
Greater ocular hypotensive efficacy
Better compliance due to once daily dosing.
Physicians consider many factors when prescribing patients with glaucoma. Ultimately, the goal of eye care providers is to give the best, most cost-effective care to their patients taking into consideration efficacy, tolerability, medication response, compliance, and dosing regimens along with the cost of medication.
Economic evaluation of glaucoma therapy needs to be targeted at assessment of efficiency, that is, health effects weighed against the sacrifices or costs incurred for attaining them. The deciding criterion should be cost-effectiveness of treatment strategy rather than efficacy or cost alone. As glaucoma management requires life-long therapy and the options available are many, future studies would be needed to update the rapidly changing economic information pertaining to the medical management of glaucoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Palimkar A, Khandekar R, Venkataraman V. Prevalence and distribution of glaucoma in central India (Glaucoma Survey 2001) Indian J Ophthalmol. 2008;56:57–62. doi: 10.4103/0301-4738.37597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy GV, Gupta S, Tewari HK, Jose R, Bachani D. Report. New Delhi: National Programme for Control of Blindness, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2002. National Survey on Blindness and Visual Outcomes after Cataract Surgery; 2001-2002. [Google Scholar]
- 3.Park K. Park's Textbook of Preventive and Social Medicine. 19th ed. Jabalpur: Banarsidas Bhanot Publishers; 2007. Noncommunicable diseases: Blindness; pp. 336–62. [Google Scholar]
- 4.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Shields MB. Textbook of Glaucoma. 5th ed. Baltimore: Lippincott Williams and Wilkins; 2005. Classification of the glaucomas; pp. 155–62. [Google Scholar]
- 7.Das J, Bhomaj S, Chaudhuri Z, Sharma P, Negi A, Dasgupta A. Profile of glaucoma in a major eye hospital in north India. Indian J Ophthalmol. 2001;49:25–30. [PubMed] [Google Scholar]
- 8.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, et al. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Lachaine J, Hodge WG, Steffensen I, Murray C, Barnes D, Foerster V, et al. Prostaglandin analogues for ophthalmic use: A cost-effectiveness analysis. Can J Ophthalmol. 2008;43:33–41. doi: 10.3129/i07-182. [DOI] [PubMed] [Google Scholar]
- 10.Hodge WG, Lachaine J, Steffensen I, Murray C, Barnes D, Foerster V, et al. The efficacy and harm of prostaglandin analogues for IOP reduction in glaucoma patients compared to dorzolamide and brimonidine: A systematic review. Br J Ophthalmol. 2008;92:7–12. doi: 10.1136/bjo.2007.123737. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JC, Chang HW. Comparison of the effects of brimonidine 0.2% and timolol 0.5% on retinal nerve fiber layer thickness in ocular hypertensive patients: A prospective, unmasked study. J Ocul Pharmacol Ther. 2005;21:475–82. doi: 10.1089/jop.2005.21.475. [DOI] [PubMed] [Google Scholar]
- 12.Gray JA. 2nd ed. Edinburgh: Churchill Livingstone; 2001. Evidence-based healthcare. In: How to Make Health Policy and Management Decisions? [Google Scholar]
- 13.Drummond MF, Richardson WS, O’Brien BJ, Levine M, Heyland D. Users’ guides to the medical literature. XIII. How to use an article on economic analysis of clinical practice. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1997;277:1552–7. doi: 10.1001/jama.277.19.1552. [DOI] [PubMed] [Google Scholar]
- 14.Lim KS, Nau CB, O’Byrne MM, Hodge DO, Toris CB, McLaren JW, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008;115:790–5.e4. doi: 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstas AG, Holló G, Irkec M, Tsironi S, Durukan I, Goldenfeld M, et al. Diurnal IOP control with bimatoprost versus latanoprost in exfoliative glaucoma: A crossover, observer-masked, three-centre study. Br J Ophthalmol. 2007;91:757–60. doi: 10.1136/bjo.2006.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day DG, Sharpe ED, Beischel CJ, Jenkins JN, Stewart JA, Stewart WC. Safety and efficacy of bimatoprost 0.03% versus timolol maleate 0.5%/dorzolamide 2% fixed combination. Eur J Ophthalmol. 2005;15:336–42. doi: 10.1177/112067210501500304. [DOI] [PubMed] [Google Scholar]
- 17.Shiell A, Donaldson C, Mitton C, Currie G. Health economic evaluation. J Epidemiol Community Health. 2002;56:85–8. doi: 10.1136/jech.56.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenkel RE, Frenkel M, Toler A. Pharmacoeconomic analysis of prostaglandin and prostamide therapy for patients with glaucoma or ocular hypertension. BMC Ophthalmol. 2007;7:16. doi: 10.1186/1471-2415-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galanopoulos A, Goldberg I. Clinical efficacy and neuroprotective effects of brimonidine in the management of glaucoma and ocular hypertension. Clin Ophthalmol. 2009;3:117–22. [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas R, Parikh R, Muliyil J, George R, Paul P, Abraham LM. Comparison between latanoprost and brimonidine efficacy and safety in Indian eyes. Indian J Ophthalmol. 2003;51:123–8. [PubMed] [Google Scholar]
- 21.Whitcup SM, Cantor LB, VanDenburgh AM, Chen K. A randomised, double masked, multicentre clinical trial comparing bimatoprost and timolol for the treatment of glaucoma and ocular hypertension. Br J Ophthalmol. 2003;87:57–62. doi: 10.1136/bjo.87.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiscella RG, Geller JL, Gryz LL, Wilensky J, Viana M. Cost considerations of medical therapy for glaucoma. Am J Ophthalmol. 1999;128:426–33. doi: 10.1016/s0002-9394(99)00235-4. [DOI] [PubMed] [Google Scholar]
- 23.Rylander NR, Vold SD. Cost analysis of glaucoma medications. Am J Ophthalmol. 2008;145:106–13. doi: 10.1016/j.ajo.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Fiscella RG, Green A, Patuszynski DH, Wilensky J. Medical therapy cost considerations for glaucoma. Am J Ophthalmol. 2003;136:18–25. doi: 10.1016/s0002-9394(03)00102-8. [DOI] [PubMed] [Google Scholar]
- 25.Galindo-Ferreiro A, Sánchez-Tocino H, Fernández-Muñoz M, Iglesias Cortiñas D. Cost-effectiveness of antihypertensive drugs most commonly used in glaucoma. Arch Ophthalmol Soc Esp. 2004;79:379–84. doi: 10.4321/s0365-66912004000800005. [DOI] [PubMed] [Google Scholar]


