Abstract
Bovine vascular endothelial cells do not grow when cultured at low density unless fibroblast growth factor is included in the culture medium. When endothelial cells obtained from the intimal surface of fetal and adult aortas were seeded at low density (8 cells per cm2), they formed small colonies of large, irregular, vacuolated cells. At very low density (0.3 cells per cm2) they did not survive. The addition of fibroblast growth factor to endothelial cells maintained at such low densities resulted in the formation of vigorously growing colonies of small, uniform cells. Electron microscopy showed that the cultured endothelial cells had the fine structure characteristics of endothelial cells. Immunofluorescence microscopy revealed antihemophilic factor (Factor VIII) antigen in the cells. Our results demonstrated that fibroblast growth factor permits the survival of endothelial cells plated at extremely low cell density. With the use of fibroblast growth factor, endothelial cell clones are easily produced.
Full text
PDF
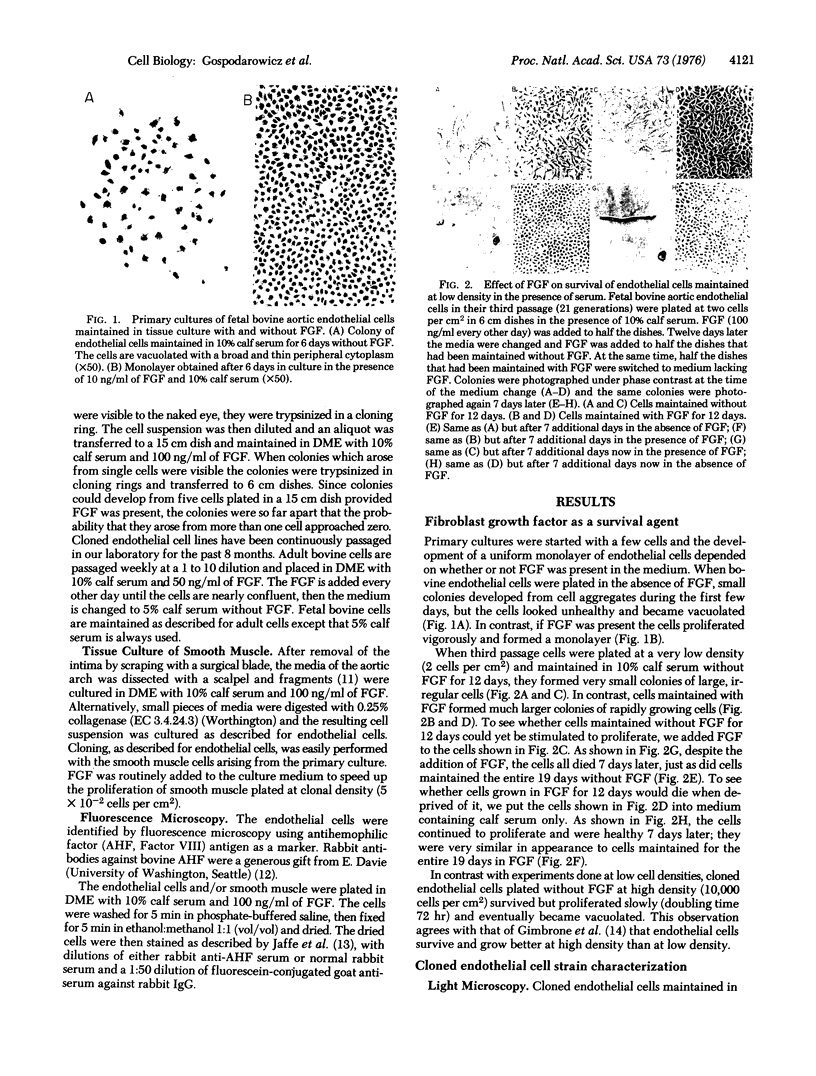


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aursnes I. Increased permeability of capillaries to protein during thrombocytopenia. An experimental study in the rabbit. Microvasc Res. 1974 May;7(3):283–295. doi: 10.1016/0026-2862(74)90016-8. [DOI] [PubMed] [Google Scholar]
- Boone C. W., Takeichi N., Paranjpe M., Gilden R. Vasoformative sarcomas arising from BALB/3T3 cells attached to solid substrates. Cancer Res. 1976 May;36(5):1626–1633. [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Buonassisi V. Sulfated mucopolysaccharide synthesis and secretion in endothelial cell cultures. Exp Cell Res. 1973 Feb;76(2):363–368. doi: 10.1016/0014-4827(73)90388-1. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer D. G., Birnbaum G., Luttrell C. N. Human endothelium in cell culture. J Atheroscler Res. 1966 Mar-Apr;6(2):151–163. doi: 10.1016/s0368-1319(66)80019-4. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Aster R. H., Cotran R. S., Corkery J., Jandl J. H., Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969 Apr 5;222(5188):33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
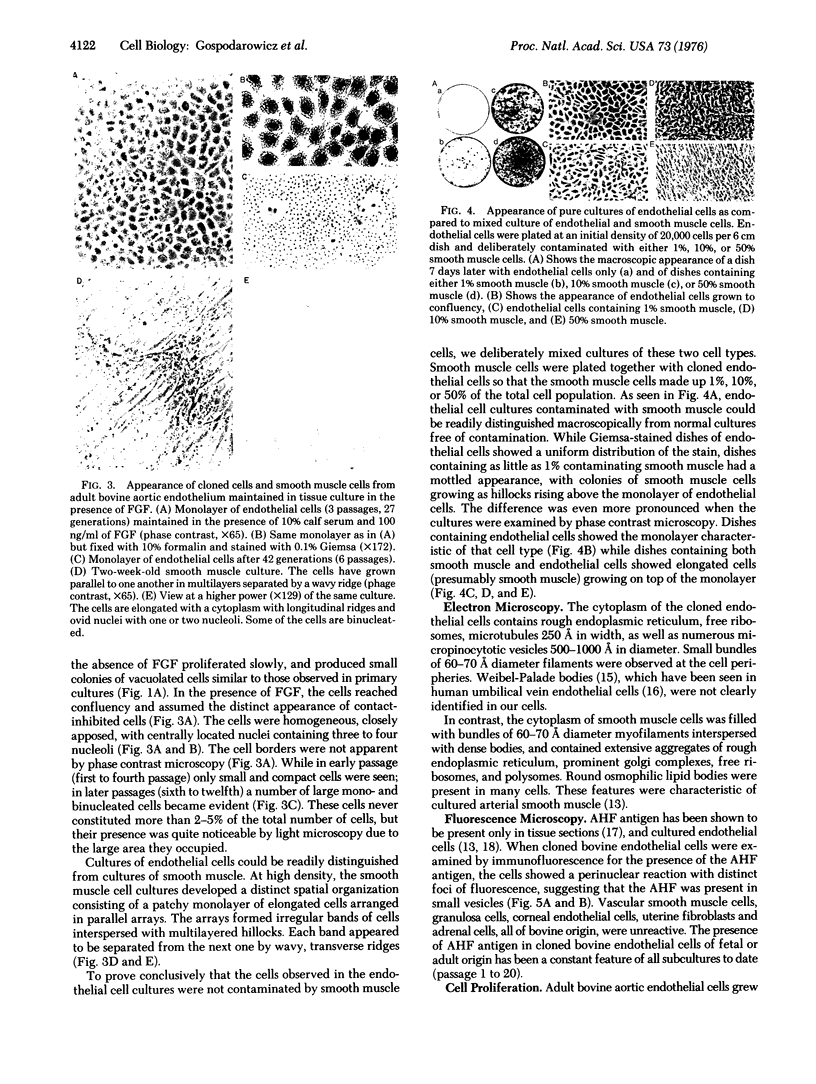
- Gore I., Takada M., Austin J. Ultrastructural basis of experimental thrombocytopenic purpura. Arch Pathol. 1970 Sep;90(3):197–205. [PubMed] [Google Scholar]
- Gospodarowicz D., Greene G., Moran J. Fibroblast growth factor can substitute for platelet factor to sustain the growth of Balb/3T3 cells in the presence of plasma. Biochem Biophys Res Commun. 1975 Jul 22;65(2):779–787. doi: 10.1016/s0006-291x(75)80213-0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Stimulation of division of sparse and confluent 3T3 cell populations by a fibroblast growth factor, dexamethasone, and insulin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4584–4588. doi: 10.1073/pnas.71.11.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Gospodarowicz D., Rudland P., Lindstrom J., Benirschke K. Fibroblast growth factor: its localization, purification, mode of action, and physiological significance. Adv Metab Disord. 1975;8:301–335. doi: 10.1016/b978-0-12-027308-9.50026-3. [DOI] [PubMed] [Google Scholar]
- Haudenschild C. C., Cotran R. S., Gimbrone M. A., Jr, Folkman J. Fine structure of vascular endothelium in culture. J Ultrastruct Res. 1975 Jan;50(1):22–32. doi: 10.1016/s0022-5320(75)90004-0. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens C. S., Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood. 1975 Oct;46(4):567–578. [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- MARUYAMA Y. The human endothelial cell in tissue culture. Z Zellforsch Mikrosk Anat. 1963;60:69–79. doi: 10.1007/BF00329383. [DOI] [PubMed] [Google Scholar]
- POMERAT C. M., SLICK W. C. Isolation and growth of endothelial cells in tissue culture. Nature. 1963 Jun 1;198:859–861. doi: 10.1038/198859a0. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmer G., Kirby E. P., Teller D. C., Davie E. W. The isolation nd characterization of bovine factor VIII (antihemophilic factor). J Biol Chem. 1972 Apr 25;247(8):2512–2521. [PubMed] [Google Scholar]
- Smith A. R., Wolpert L. Nerves and angiogenesis in amphibian limb regeneration. Nature. 1975 Sep 18;257(5523):224–225. doi: 10.1038/257224a0. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Spaet T. H. The subendothelium and thrombogenesis. Bull N Y Acad Med. 1972 Feb;48(2):289–301. [PMC free article] [PubMed] [Google Scholar]
- Suddith R. L., Kelly P. J., Hutchison H. T., Murray E. A., Haber B. In vitro demonstration of an endothelial proliferative factor produced by neural cell lines. Science. 1975 Nov 14;190(4215):682–684. doi: 10.1126/science.171768. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark B., Wasteson A. A platelet factor stimulating human normal glial cells. Exp Cell Res. 1976 Mar 1;98(1):170–174. doi: 10.1016/0014-4827(76)90476-6. [DOI] [PubMed] [Google Scholar]
- Westermark B., Wasteson A. The response of cultured human normal glial cells to growth factors. Adv Metab Disord. 1975;8:85–100. doi: 10.1016/b978-0-12-027308-9.50012-3. [DOI] [PubMed] [Google Scholar]