Abstract
A 27-year-old woman developed bilateral acute angle closure glaucoma (AACG) and transient myopia after taking oseltamivir for four days. On the fourth day, she received systemic and topical intraocular pressure (IOP)-lowering agents, and IOP decreased in both eyes. However, her visual acuity was unchanged. A myopic shift of -5.25 D OD and -5.0 D OS was estimated to have occurred in the acute phase. A-scan ultrasonography and Pentacam showed markedly shallow anterior chambers and increased lens thickness. Ultrasound biomicroscopy revealed an annular ciliochoroidal effusion with forward displacement of the lens-iris diaphragm. Ciliochoroidal effusion and transient myopia were resolved after discontinuation of oseltamivir.
Keywords: Acute angle closure glaucoma, ciliochoroidal effusion, oseltamivir, transient myopia
Attention has focused on oseltamivir (Tamiflu; Roche, Nutley, NJ, USA) due to the era of the influenza A (H1N1) pandemic. Oseltamivir is an ester prodrug of oseltamivir carboxylate, a selective inhibitor of influenza viruses A and B neuraminidase.[1] The most frequent adverse events are nausea and vomiting, but neuropsychiatric events and skin and hypersensitivity reactions are often also observed.[1] However, acute angle-closure glaucoma (AACG) and transient myopia attributed to oseltamivir have not been reported previously. Here, we report on a patient with oseltamivir-induced AACG and transient myopia with an ultrasound biomicroscopic description of the pathogenic mechanism.
Case Report
A 27 year old woman experienced acute-onset blurry vision and ocular pain in both eyes four days after beginning oseltamivir therapy (75 mg twice daily) for suspected influenza. Her medical and ocular history was unremarkable and she had never worn glasses. Before arriving at our hospital, her intraocular pressure (IOP) measured 60 mmHg OU. A diagnosis of bilateral AACG was made by her local ophthalmologist because of increased IOP and severely shallow anterior chambers with cornea edema. She was treated with oral acetazolamide, topical timolol/dorzolamide combination, and brimonidine and was transferred to our hospital the next day. On examination at our hospital, her uncorrected visual acuity (UCVA) was 20/400 OU. The manifest refraction was −5.0D-1.0D × 170° OD and −4.5D-1.0D × 20° OS, yielding a best-corrected visual acuity (BCVA) of 20/32 OD and 20/40 OS. IOPs were 9 mm Hg OD and 11 mm Hg in OS. Slit-lamp examination revealed mild cornea edema and markedly shallow anterior chambers without inflammation in both eyes. Gonioscopy revealed grade 0 angles on Shaffer classification OU. Undilated fundus examination revealed that maculas and optic discs were unremarkable. Fluorescein angiography and indocyanine green angiography were normal. Sclera and choroidal thickening and edema in Tenon's space were not detected on B-scan ultrasonography. However, ultrasound biomicroscopy (UBM) confirmed a 360° ciliochoroidal effusion and anterior rotation of the ciliary processes with forward displacement of the lens-iris diaphragm in both eyes [Figs. 1a and b]. A-scan ultrasonography and Pentacam were used to measure the axial length, anterior chamber depth (ACD), lens thickness (LT), chamber volume (CV), iridocorneal angle, and central corneal thickness (CCT). A-scan ultrasonography and Pentacam revealed markedly shallow anterior chambers and lens thickening in both eyes [Table 1] [Figs. 2a and b].
Figure 1.
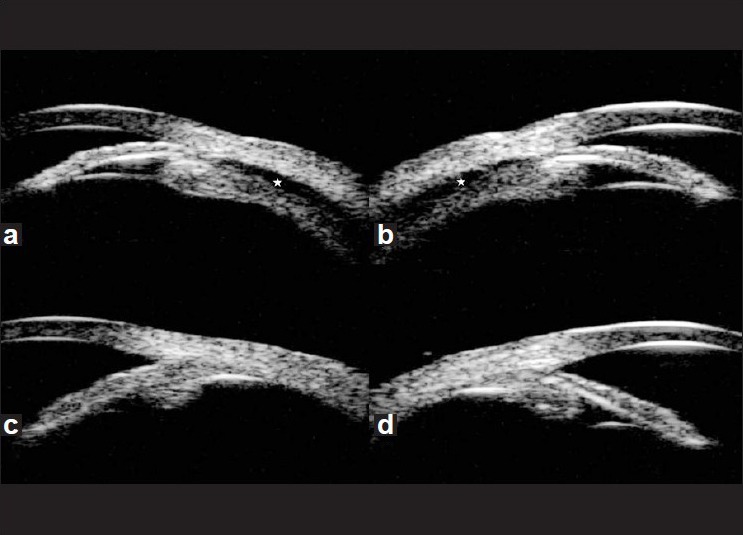
Ultrasound biomicroscopy on day 1 showed ciliochoroidal effusion (star) from the scleral spur, detachment of the ciliary body and an anterior rotation of the ciliary processes in the right (a) and left eyes (b), Ultrasound biomicroscopy on day 15 showed resolution of ciliochoroidal effusion and deepening of the anterior chamber in the right (c) and left eyes (d)
Table 1.
Changes in refraction, biometric measurements, and intraocular pressure after discontinuation of oseltamivir in the patient in the study
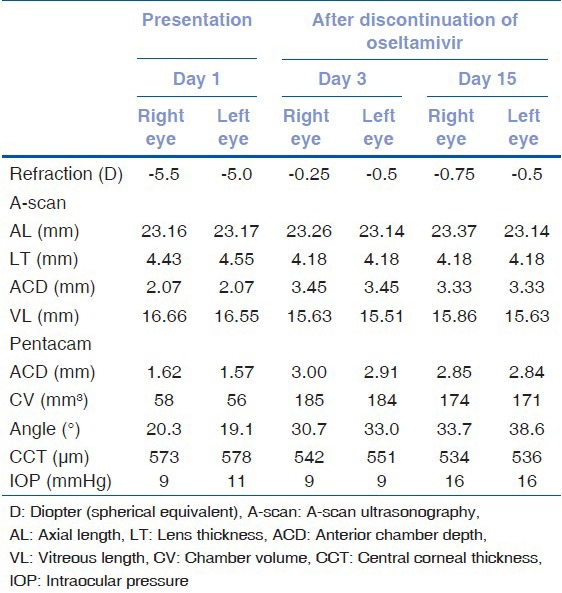
Figure 2.
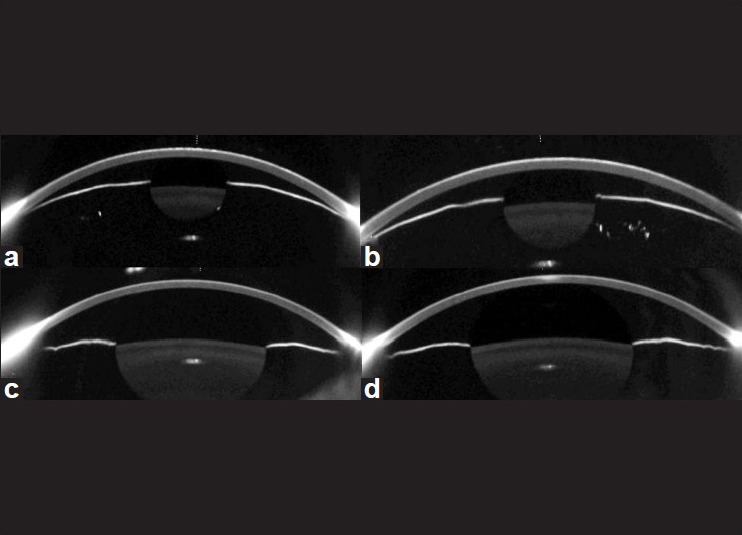
Pentacam images of the right (a) and the left eyes (b) on day 1 showed markedly shallow anterior chambers. Compared with day 1, Pentacam images of the right eye (c) and the left eye (d) on day 15 revealed that chamber volume, anterior chamber depth, and angle had increased
At that time, real-time reverse transcription polymerase chain reaction (RT-PCR) using an AB H1N1 PCR Kit (Applied Biosystems, CA, USA) for the specimen obtained from the nasopharyngeal swab was negative for influenza A virus antigen. The swine origin influenza A/H1N1 was not detected in influenza A virus PCR. The diagnosis of probable oseltamivir-induced AACG was suspected because there was no other detectable origin of the AACG, and the clinical features of our patient appeared similar to those of other drug-induced cases of AACG. Oseltamivir was the only medication being used by the patient when she developed acute-onset blurry vision. Oseltamivir was discontinued, and she was treated with topical prednisolone acetate and cycloplegic agents. Three days later, the UCVA was 20/20 OU with deep anterior chambers and IOP was measured to be 9 mm Hg OU. The spherical equivalent improved from −5.5 to −0.25D OD and from −5.0 to −0.5D OS. A-scan ultrasonography revealed that the ACD increased from 2.07 to 3.45 mm OU and that the LT decreased from 4.43 to 4.18 mm OD and from 4.55 to 4.18 mm OS. Pentacam showed that CV increased from 58 to 185 mm3 OD and from 56 to 184 mm3 OS, angle increased from 20.3 to 30.7OD and from 19.1 to 33.0°OS, and CCT decreased from 573 to 542 μm OD and from 578 to 551 m OS [Table 1]. Gonioscopy revealed grade 4 angles on Shaffer classification OU. UBM revealed that the annular ciliochoroidal effusion had resolved in both eyes. Anti-glaucoma medications, a corticosteroid, and cycloplegic eye drops were subsequently discontinued.
Two weeks after discontinuation of oseltamivir, UCVA was 20/20 OU and IOP was 16 mm Hg OU. Pentacam revealed that ACD increased by 1.23 mm OD and 1.27 mm OS, CV increased by 116 mm3 OD and 115 mm3 OS, and angle increased by 13.4° OD and 19.5° OS compared with the measures at the initial visit [Table 1] [Figs. 2c and d]. UBM showed no annular ciliochoroidal effusion in either eye [Figs. 1c and d].
Discussion
Bilateral AACG and transient myopia may be induced by drugs, such as topiramate,[2] 3,4-methylenedioxymethamphetamine,[3] and cabergoline.[4] On the basis of the previous reports, different possible mechanisms of drug-induced AACG have been proposed, including ciliary body swelling[2,3] and ciliochoroidal effusion.[2,3,4] In the presented patient, UBM findings of ciliochoroidal effusion during the acute stage with resolution of these findings during the convalescent stage indicated that the mechanism of bilateral AACG and transient myopia was related to the ciliochoroidal effusion. Ciliochoroidal effusion causes an anterior rotation of the ciliary body, which results in the anterior displacement of the lens-iris diaphragm. The annular ciliochoroidal effusion reduces the distance between the opposing ciliary processes and slackens the lens zonules, which results in an increase in the lens thickness. These changes may lead to AACG associated with shallow anterior chambers and myopia, as seen in our patient.
Ciliochoroidal effusion has been reported in association with dopaminergic drugs.[3,4] The systemic administration of oseltamivir increased dopamine release in the rat medial prefrontal cortex in the previous study.[5] The authors postulated that the increase in the dopamine during oseltamivir treatment may have caused an abnormal behavior in young patients.[5] Dopaminergic neuron in the eye and the presence of aqueous humor dopamine and adenylate cyclase linked dopamine receptors in the ciliary body have been reported in the experimental study.[6,7,8] Therefore, fluid movement in ciliochoroidal effusion in the presented patient may be related to the membrane potential alteration induced by the effects of dopamine on adenylate cyclase.[3,9]
Conclusion
To our knowledge, this is the first reported case of ciliochoroidal effusion associated with oseltamivir. The results obtained from this patient suggest that a history of oseltamivir must be determined in a patient with bilateral AACG and transient myopia due to ciliochoroidal effusion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Khazeni N, Bravata DM, Holty JE, Uyeki TM, Stave CD, Gould MK. Systematic review: Safety and efficacy of extended-duration antiviral chemoprophylaxis against pandemic and seasonal influenza. Ann Intern Med. 2009;151:464–73. doi: 10.7326/0003-4819-151-7-200910060-00143. [DOI] [PubMed] [Google Scholar]
- 2.Banta JT, Hoffman K, Budenz DL, Ceballos E, Greenfield DS. Presumed topiramate-induced bilateral acute angle-closure glaucoma. Am J Ophthalmol. 2001;132:112–4. doi: 10.1016/s0002-9394(01)01013-3. [DOI] [PubMed] [Google Scholar]
- 3.Kumar RS, Grigg J, Farinelli AC. Ecstasy induced acute bilateral angle closure and transient myopia. Br J Ophthalmol. 2007;91:693–5. doi: 10.1136/bjo.2006.099986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razmjoo H, Rezaei L, Dehghani A, Peyman A, Akhlaghi M. Bilateral angle-closure glaucoma in a young female receiving cabergoline: A case report. Case Report Ophthalmol. 2011;2:30–3. doi: 10.1159/000324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshino T, Nisijima K, Shioda K, Yui K, Kato S. Oseltamivir (Tamiflu) increases dopamine levels in the rat medial prefrontal cortex. Neurosci Lett. 2008;438:67–9. doi: 10.1016/j.neulet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 7.Cooper RL, Constable IJ, Davidson L. Aqueous humor catecholamines. Curr Eye Res. 1984;3:809–13. doi: 10.3109/02713688409000792. [DOI] [PubMed] [Google Scholar]
- 8.De Vries GW, Mobasser A, Wheeler LA. Stimulation of endogenous cyclic AMP levels in ciliary body by SK&F 82526, a novel dopamine receptor agonist. Curr Eye Res. 1986;5:449–55. doi: 10.3109/02713688609015114. [DOI] [PubMed] [Google Scholar]
- 9.Grega DS, Macdonald RL. Activators of adenylate cyclase and cyclic AMP prolong calcium-dependent action potentials of mouse sensory neurons in culture by reducing a voltage-dependent potassium conductance. J Neurosci. 1987;7:700–7. doi: 10.1523/JNEUROSCI.07-03-00700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


