Abstract
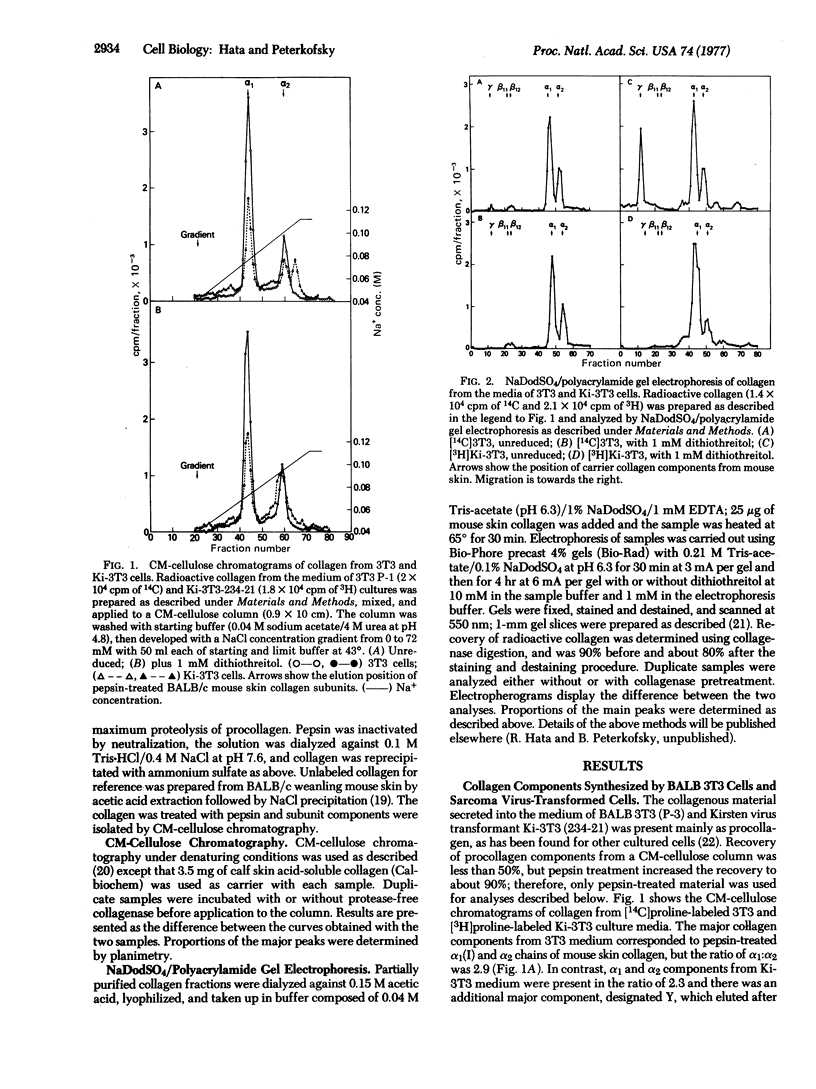
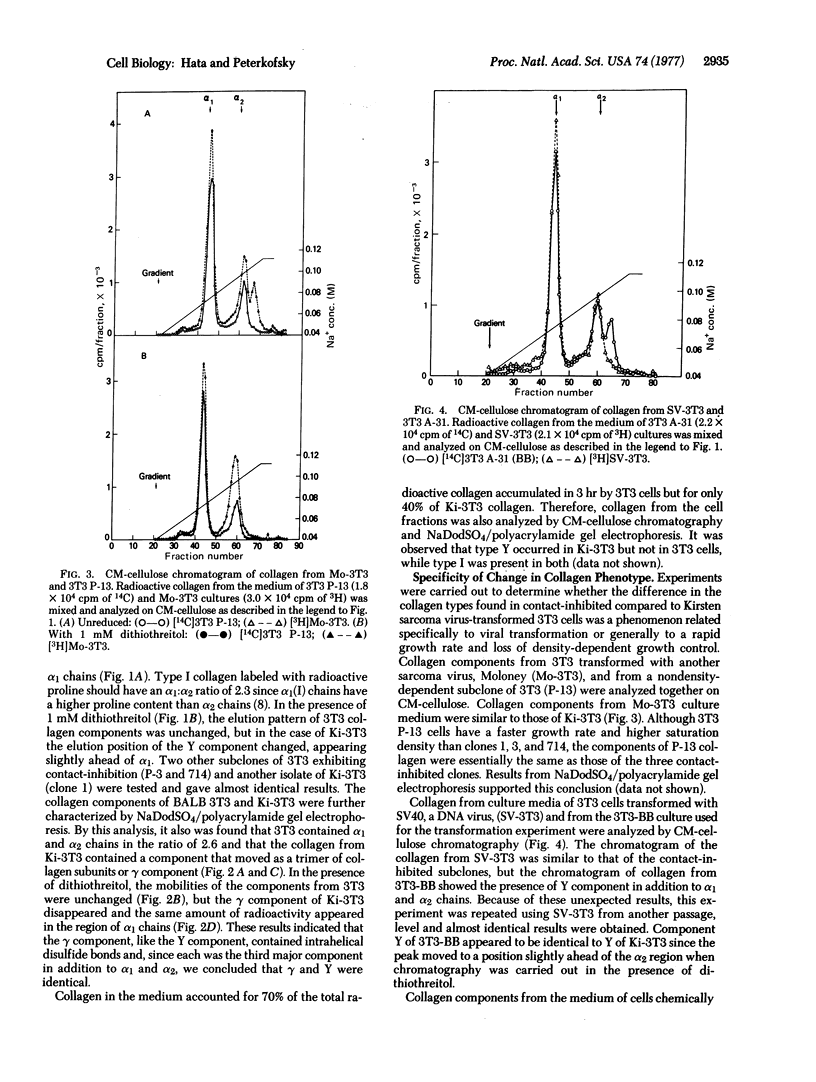
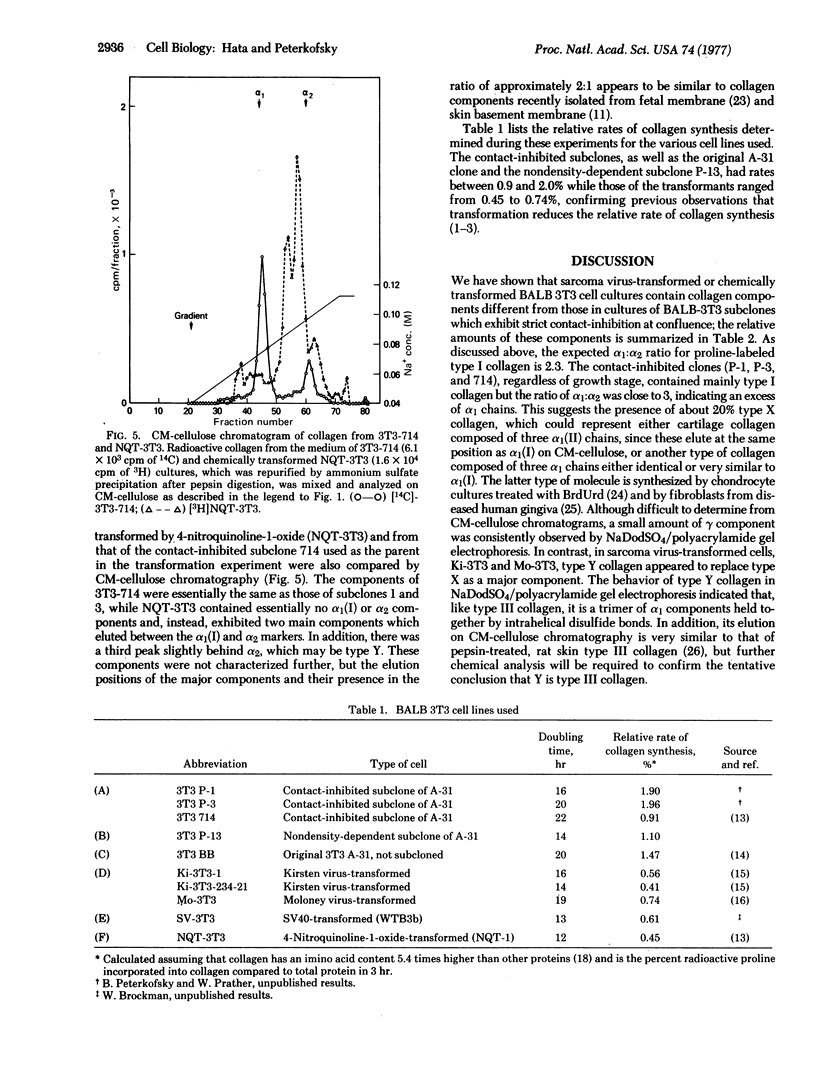
Radioactive proline-labeled procollagen, accumulated during a 3-hr incubation of normal and transformed BALB 3T3 cultures, was treated with pepsin and the resulting collagen components were analyzed by carboxymethyl-cellulose chromatography and sodium dodecyl sulfate/polyacrylamide gel electrophoresis in the presence or absence of reducing agent. Collagen in the medium of three subclones of BALB 3T3 A-31 that exhibited contact-inhibition of growth at confluence, as well as in the medium of one that did not, consisted of α1 and α2 subunits in the ratio of 3:1, suggesting that 3T3 cells synthesize type I collagen, [α1(I)]2α2, and another type, which we have designated X, composed of α1 chains, which may or may not be identical to α1(I). Culture medium from 3T3 transformed by Kirsten or Moloney sarcoma virus contained type I collagen and another type differing from I and X and designated as type Y. The latter appeared to be similar to type III collagen [α1(III)]3, since it contained intrahelical disulfide bonds. Analysis of intracellular collagen also demonstrated the presence of type III in Ki-3T3 and its absence from 3T3 cells. Collagen components from the medium of a simian virus 40 transformant were identical to those of the contact-inhibited clones, while the collagen from a 4-nitroquinoline-1-oxide-induced transformant was composed mainly of two components differing from α1(I), α2, or α1(III). These results suggest that the type of collagen accumulated in transformed cell cultures may be specifically related to the transforming agent.
Keywords: collagen types, 4-nitroquinoline-1-oxide, simian virus 40
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., El Adli F. A., Kaitila I. I., Hollister D. W. Fetal membrane collagens: identification of two new collagen alpha chains. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2579–2583. doi: 10.1073/pnas.73.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., McKenney K. H., Lichtenstein J. R., Martin G. R. Preparation of type III procollagen and collagen from rat skin. Biochemistry. 1974 Dec 3;13(25):5243–5248. doi: 10.1021/bi00722a030. [DOI] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Daniels J. R., Chu G. H. Basement membrane collagen of renal glomerulus. J Biol Chem. 1975 May 10;250(9):3531–3537. [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Todaro G. J., Green H. The production of hyaluronate by spontaneously established cell lines and viral transformed lines of fibroblastic origin. Biochim Biophys Acta. 1965 Nov 1;101(3):343–351. doi: 10.1016/0926-6534(65)90013-8. [DOI] [PubMed] [Google Scholar]
- Ishimoto N., Temin H. M., Strominger J. L. Studies of carcinogenesis by avian sarcoma viruses. II. Virus-induced increase in hyaluronic acid synthetase in chicken fibroblasts. J Biol Chem. 1966 May 10;241(9):2052–2057. [PubMed] [Google Scholar]
- Kakunaga T. A quantitative system for assay of malignant transformation by chemical carcinogens using a clone derived from BALB-3T3. Int J Cancer. 1973 Sep 15;12(2):463–473. doi: 10.1002/ijc.2910120217. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Levinson W., Bhatnagar R. S., Liu T. Z. Loss of ability to synthesize collagen in fibroblasts transformed by rous sarcoma virus. J Natl Cancer Inst. 1975 Oct;55(4):807–810. doi: 10.1093/jnci/55.4.807. [DOI] [PubMed] [Google Scholar]
- Marciani D. J., Kuff E. L. Isolation and partial characterization of the internal structural proteins from murine intracisternal A particles. Biochemistry. 1973 Dec 4;12(25):5075–5083. doi: 10.1021/bi00749a008. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4511–4515. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Biochemical characterization of collagens synthesized by fibroblasts derived from normal and diseased human gingiva. J Biol Chem. 1976 Sep 25;251(18):5464–5471. [PubMed] [Google Scholar]
- Ooira A., Kimata K., Suzuki S., Takata K., Suzuki I. A correlation between synthetic activities for matrix macromolecules and specific stages of cyto-differentiation in developing cartilage. J Biol Chem. 1974 Mar 10;249(5):1637–1645. [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Renger H. C. Retransformation of ts SV40 transformants by murine sarcoma virus at non-permissive temperature. Nat New Biol. 1972 Nov 1;240(96):19–21. doi: 10.1038/newbio240019a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Uzman B. G. Production and secretion of chondroitin sulfates and dermatan sulfate by established mammalian cell lines. Biochem Biophys Res Commun. 1971 May 21;43(4):723–728. doi: 10.1016/0006-291x(71)90675-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The mechanism of carcinogenesis by avian sarcoma viruses. 1. Cell multiplication and differentiation. J Natl Cancer Inst. 1965 Oct;35(4):679–693. [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Uitto J., Lichtenstein J. R. Defects in the biochemistry of collagen in diseases of connective tissue. J Invest Dermatol. 1976 Feb;66(02):59–79. doi: 10.1111/1523-1747.ep12481404. [DOI] [PubMed] [Google Scholar]



