Abstract
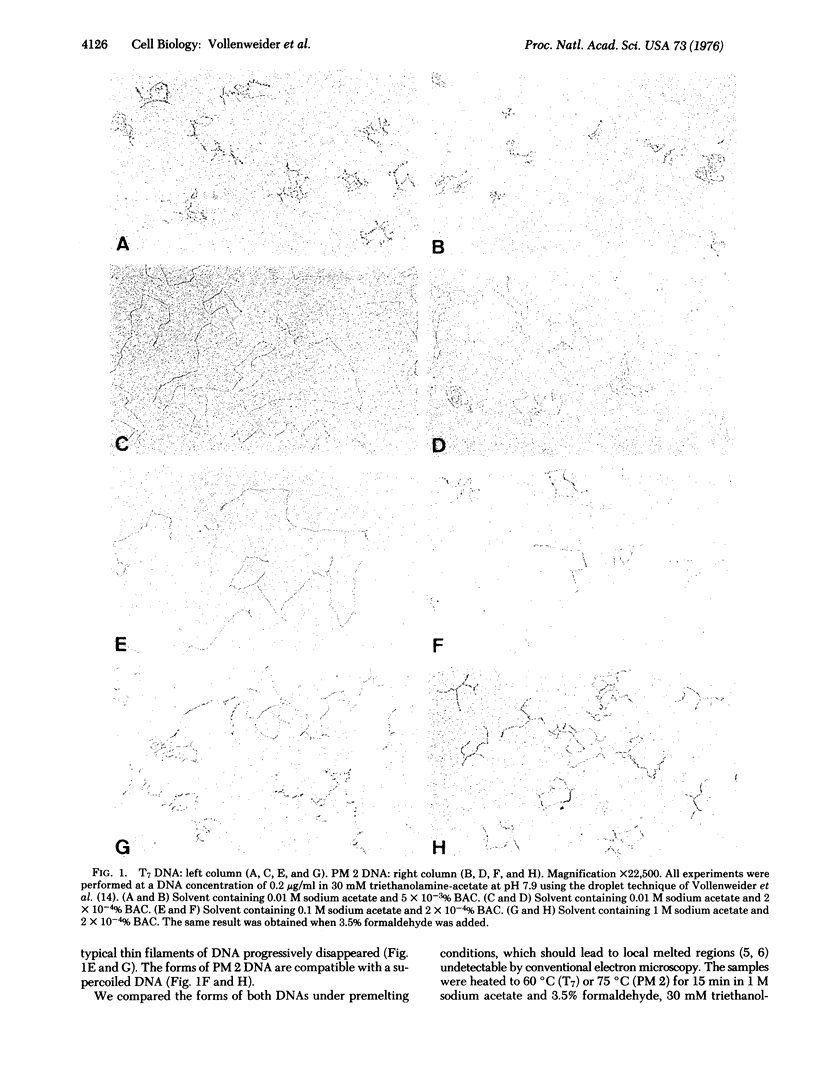
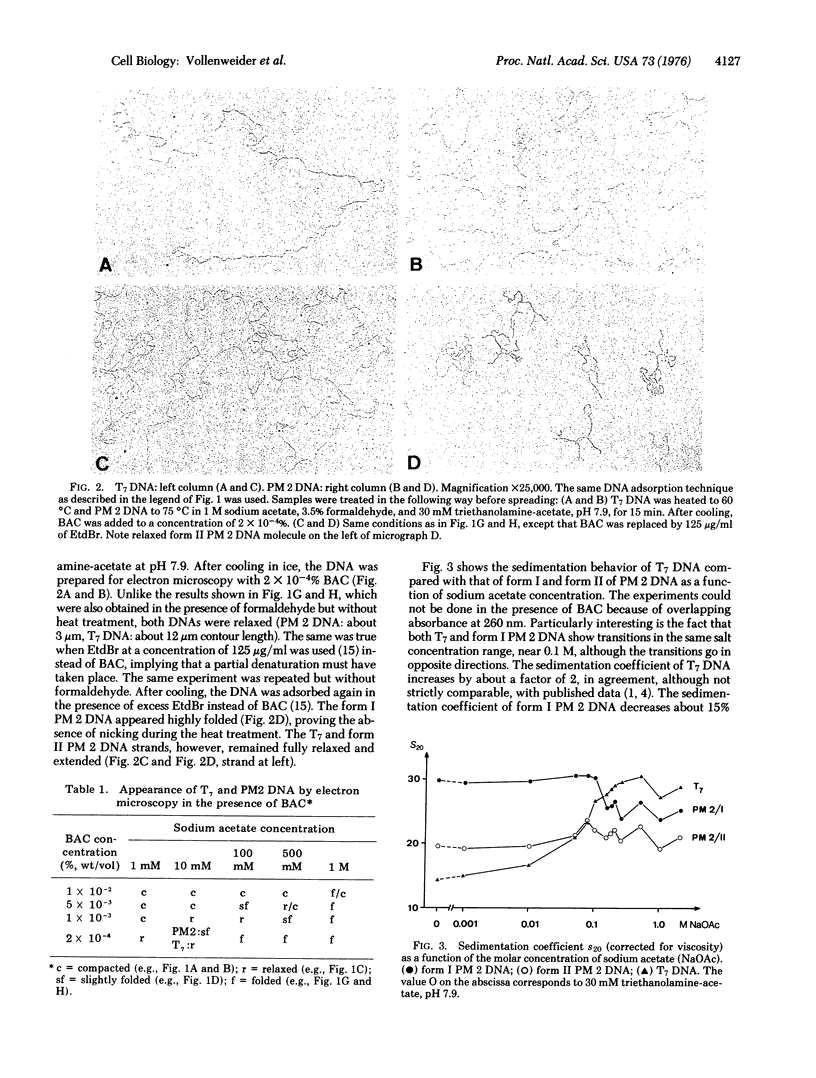
The superstructure of a covalently closed circular DNA (of bacteriophage PM 2) was compared by electron microscopy with that of a linear duplex DNA (of bacteriophage T7) when ionic strength and benzyldimethylalkylammonium chloride concentration were varied. In parallel studies the sedimentation behavior of these DNAs was studied by analytical ultracentrifugation, but for technical reasons these had to be without benzyldimethylalkylammonium chloride. By combining the information from the two methods one has to conclude that with increasing ionic strength the linear duplex T7 DNA spontaneously forms a structure similar to that of the superhelical structure of closed circular PM 2 DNA. The superstructure is destroyed under premelting conditions and in the presence of an excess of ethidium bromide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R. Premelting unwinding of the deoxyribonucleic acid duplex by aqueous magnesium perchlorate. Biochemistry. 1972 Jul 18;11(15):2915–2920. doi: 10.1021/bi00765a027. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Structure of DNA in denaturing solvents. I. Bacteriophage PM2 DNA in aqueous sodium perchlorate. J Mol Biol. 1972 Jun 20;67(2):183–198. doi: 10.1016/0022-2836(72)90235-5. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Campbell A. M. Conformational analysis of deoxyribonucleic acid from PM2 bacteriophage. The effect of size on supercoil shape. Biochem J. 1976 Apr 1;155(1):101–105. doi: 10.1042/bj1550101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century T. J., Fenichel I. R., Horowitz S. B. The concentrations of water, sodium and potassium in the nucleus and cytoplasm of amphibian oocytes. J Cell Sci. 1970 Jul;7(1):5–13. doi: 10.1242/jcs.7.1.5. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Gourévitch M., Puigdoménech P., Cavé A., Etienne G., Méry J., Parello J. Model studies in relation to the molecular structure of chromatin. Biochimie. 1974;56(6-7):967–985. doi: 10.1016/s0300-9084(74)80518-3. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Lang D. Regular superstructures of purified DNA in ethanolic solutions. J Mol Biol. 1973 Aug 5;78(2):247–254. doi: 10.1016/0022-2836(73)90113-7. [DOI] [PubMed] [Google Scholar]
- Rinehart F. P., Hearst J. E. The ionic strength dependence of s o 20 w of phage DNA in NH 4 AC. Arch Biochem Biophys. 1972 Oct;152(2):712–722. doi: 10.1016/0003-9861(72)90267-6. [DOI] [PubMed] [Google Scholar]
- Rinehart F. P., Hearst J. E. The ionic strength dependence of the coil dimensions of viral DNA in NH 4 AC solutions. Arch Biochem Biophys. 1972 Oct;152(2):723–732. doi: 10.1016/0003-9861(72)90268-8. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Scruggs R. L. Viscosity study of DNA. II. The effect of simple salt concentration on the viscosity of high molecular weight DNA and application of viscometry to the study of DNA isolated from T4 and T5 bacteriophage mutants. Biopolymers. 1968;6(8):1005–1018. doi: 10.1002/bip.1968.360060802. [DOI] [PubMed] [Google Scholar]
- Révet B. M., Schmir M., Vinograd J. Direct determination of the superhelix density of closed circular DNA by viscometric titration. Nat New Biol. 1971 Jan 6;229(1):10–13. doi: 10.1038/newbio229010a0. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Portmann R., Kaufmann P., Koller T. Adsorption of DNA molecules to different support films. J Microsc. 1975 Jul;104(2):187–198. doi: 10.1111/j.1365-2818.1975.tb04016.x. [DOI] [PubMed] [Google Scholar]
- Triebel H., Reinert K. E. Sedimentation analysis of the ionic strength dependence of the tertiary structure of native DNA in solution. Biopolymers. 1971;10(5):827–837. doi: 10.1002/bip.360100507. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Gray H. B., Jr, Vinograd J. Sedimentation velocity behavior of closed circular SV40 DNA as a function of superhelix density, ionic strength, counterion and temperature. J Mol Biol. 1971 Nov 28;62(1):21–38. doi: 10.1016/0022-2836(71)90128-8. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARING M. J. COMPLEX FORMATION WITH DNA AND INHIBITION OF ESCHERICHIA COLI RNA POLYMERASE BY ETHIDIUM BROMIDE. Biochim Biophys Acta. 1964 Jun 22;87:358–361. doi: 10.1016/0926-6550(64)90238-5. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Variation of the average rotation angle of the DNA helix and the superhelical turns of covalently closed cyclic lambda DNA. J Mol Biol. 1969 Jul 14;43(1):25–39. doi: 10.1016/0022-2836(69)90076-x. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Chisholm J. W. Uncoiling of bacteriophage PM2 DNA by binding of steroidal diamines. Biochim Biophys Acta. 1972 Feb 23;262(1):18–23. doi: 10.1016/0005-2787(72)90214-6. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]





