Abstract
Objective
Identifying the depression symptoms most closely associated with suicidal thoughts and which medications provide the fastest relief may help suicide prevention.
Method
Post hoc analysis of data from a randomized, double-blind, eight-week clinical trial of the serotonin reuptake inhibitor paroxetine (N=36) versus the norepinephrine-dopamine reuptake inhibitor bupropion (N=38) in patients with DSM-IV major depressive disorder and past suicide attempt or current suicidal thoughts. Treatment effects on Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory symptom clusters were compared. We hypothesized a superior effect of paroxetine on non-suicide, affective/cognitive depression symptom clusters that our prior work found to be associated with suicidal thoughts and attempts. Data were collected from February 2005 to January 2010.
Results
There was a treatment main effect on HDRS Psychic Depression (depressed mood, guilt, retardation, helpless, hopeless, worthless) (estimate = −2.2, 95% CI = −3.2 to −1.1, t = −4.01, df = 67.16, p < 0.001), one of the clusters most strongly correlated to suicidal ideation. The net drug effect was 2.2 points lower average Psychic Depression scores after one week of paroxetine, compared to bupropion, and was statistically significant until Week 4. Results for other depression scale factors were non-significant (p > 0.05).
Conclusion
The results require replication, but suggest a pathway by which SSRI treatment may exert a stronger effect compared with NDRI treatment on reduction of suicidal thoughts during initial weeks of pharmacotherapy in these higher risk patients.
Keywords: Suicide, depression, symptom cluster, antidepressant, clinical trial
Introduction
Suicide, most often associated with depressive disorders,1;2 and attempts cause substantial loss of life and suffering, and cost an estimated $33 billion yearly in the U.S..3;4 Little change in the suicide rate, from 10 deaths per 100,000 persons in 1955 to 11 per 100,000 in 2005,5 shows the lack of progress on prevention in the past half century.
Depression predicts suicide attempts via its effect on suicidal ideation.6 Ninety percent of unplanned and 60% of planned first attempts are reported to occur within a year of ideation onset.7 Clinicians assess suicidal ideation because it predicts attempts8 and suicide.9 Gibbons et al. reported that antidepressants reduce suicidal ideation in adults mainly by reducing depression severity.10 Better understanding of how depression leads to suicidal thoughts and which treatments provide the fastest relief may enhance suicide prevention.
The heterogeneity of depression and the suicidal phenotype is a challenge to research. Suicidal thoughts are a common depressive symptom and scales like the Hamilton Depression Rating Scale (HDRS)11 and Beck Depression Inventory (BDI)12 contain suicide items. However, global depression severity relates only modestly to suicidal behavior raising the question as to which aspects of depression psychopathology are most closely related to suicidal ideation and behavior.13–15
The HDRS and BDI are multi-dimensional scales comprised of up to seven symptom clusters or factors.16–19 Antidepressant clinical trials have investigated treatment effects on these factors.20–25 We found that depressed suicide attempters (N=296) exhibited greater severity on a BDI self-blame factor compared to non-attempters.26 Our study extracted five factors from the 24-item HDRS and three from the BDI.26 In agreement with other reports, both scales had a major affective/cognitive factor and minor factors representing somatic/vegetative or motivational symptoms.16–18;27–30
In a follow-up study of an overlapping but larger depressed sample (N=400), the factors most correlated to suicidal thoughts, measured with the Scale for Suicidal Ideation (SSI),31 were Psychic Depression (HDRS), Subjective Depression (BDI), and Self-Blame (BDI), while somatic symptoms had little or no association.32 Assessment of symptom clusters in antidepressant clinical trials may reveal treatment effects on depression features related to the severity of suicidal thoughts.
In our prior report on a randomized, double-blind, clinical trial of paroxetine (N = 36) vs. bupropion (N = 38) in depressed patients with past suicide attempt or current suicidal ideation, we found that patients with greater baseline suicidal ideation experienced greater acute improvement in suicidal thoughts, and independently total depression score, on paroxetine compared with bupropion.33 Serotonin reuptake inhibitors (SSRI), including paroxetine, primarily enhance serotonin neurotransmission, while bupropion is a norepinephrine-dopamine reuptake inhibitor (NDRI) with minimal (or no) direct effects on serotonin.34 Understanding the reasons for this differential drug effect on suicidal ideation could potentially enhance clinical care for suicidal patients.
In this study, we used these clinical trial data to investigate the relationship of treatment to change in the HDRS and BDI depression factors examined in our prior work.26;32 We hypothesized differential drug effects on the depression factors most strongly associated with suicidal ideation,32 i.e. that the HDRS Psychic Depression, BDI Subjective Depression, and BDI Self-Blame factors would improve more with paroxetine compared to bupropion.
Method
Patients
Detailed trial methods and primary results have been reported.33 Briefly, patients 18 to 75 years old with current DSM-IV major depressive disorder (MDD), scoring 16 or greater on the HDRS-17,11 and with either a past suicide attempt or current suicidal ideation or both were eligible. Minimum ideation for non-attempters was a score of 2 or greater on HDRS item 3 (suicide), “wishes to be dead or has any thoughts of possible death to self.”11 Exclusions were: bipolar disorder, psychosis, anorexia or bulimia nervosa, current SSRI or bupropion use for other indications (e.g. anxiety), drug or alcohol dependence within 6 months, unstable medical illness, contraindication to either drug, non-response to three other SSRIs, paroxetine, or bupropion in the last two years, pregnancy or lactation, and lack of capacity to consent.
The trial was conducted at Columbia University Medical Center-New York State Psychiatric Institute. Data were collected from February 2005 to January 2010. Participants were recruited via media, internet, and clinician referral. After complete description of the study to subjects, written informed consent was obtained.
Treatment
Patients, treating psychiatrists, and clinical raters were blind to treatment assignment. Patients met weekly for eight weeks with a psychiatrist for pharmacotherapy and a psychologist for ratings. Daily dose was: (weeks 1–2) paroxetine 25mg or bupropion 150mg; (weeks 3–4) paroxetine 37.5mg or bupropion 300mg, (weeks 5–8) optional increase to paroxetine 50mg or bupropion 450mg, if clinically indicated. Concomitant benzodiazepine for anxiety or zolpidem for insomnia were allowed. Patients with inadequate response or intolerable side effects were switched to open treatment.
Outcome and measures
Raters were PhD or masters level psychologists. Axis I and II diagnoses were made using the Structured Clinical Interview for DSM-IV (SCID I and II).35;36 Suicide attempts were assessed with the Columbia Suicide History Form.37 Diagnostic and suicide attempt classifications were made in a weekly, interdisciplinary consensus conference. Depressive symptoms were assessed at baseline and weekly with the 24-item HDRS11 and the BDI.12 Suicidal ideation was assessed at baseline and weekly with the clinician-rated SSI.31
Main outcomes in this study were the five HDRS and three BDI factors analyzed in our prior study.26 In this paper we use the terms ‘factor’ and ‘symptom cluster’ interchangeably. Because our goal was to investigate non-suicide depression symptom clusters, we excluded the suicide item from the HDRS Psychic Depression factor where it loaded in our prior study.26 Factor compositions are as follows:
| HDRS Factors: | |
| Psychic depression | Depressed mood, guilt, retardation, helplessness, hopelessness, worthlessness |
| Loss of motivated behavior | Work and interests, appetite, libido, weight loss |
| Anxiety | Agitation, psychic anxiety, somatic anxiety, hypochondriasis |
| Disturbed thinking | Lack of insight, depersonalization, paranoia, obsessions/compulsions |
| Sleep disturbance | Early, middle, and late insomnia items |
| Unassigned items | Energy, diurnal variation |
| BDI Factors: | |
| Subjective depression | Sadness, pessimism, dissatisfaction, interest in others, indecisiveness, body image, work, energy, libido |
| Self-blame | Sense of failure, guilt, feeling punished, self-dislike, self-criticism |
| Somatic complaint | Early awakening, appetite, weight loss |
| Unassigned items | Suicidal thoughts, crying, irritability, somatic concern |
Statistical methods
We used SPSS version 18 (SPSS, Inc.). We created plots of mean score on each HDRS and BDI factor by time and treatment group (Figures 1 and 2). Since this analysis involves correlated longitudinal data with repeated measures, we used mixed-effects regression models38 to analyze each factor. Data were examined for influential or outlying data points. We adjusted for potential baseline differences in factor scores between treatment groups by including baseline severity of each factor as a predictor in the model of that factor. The time variable was the natural log of study week. Baseline inpatient and attempter status were terms in all models since randomization was stratified on those variables. The model for each factor included fixed effects for treatment, inpatient status at randomization (yes/no), history of past suicide attempt (yes/no), baseline severity of the factor, natural log(study week), and an interaction term of treatment × natural log(study week). The model tested for each factor was:
FactorDuring Treatment = Treatment + Inpatient + Attempt + FactorBaseline + Ln(Study Week) + Treatment × Ln(Study Week)
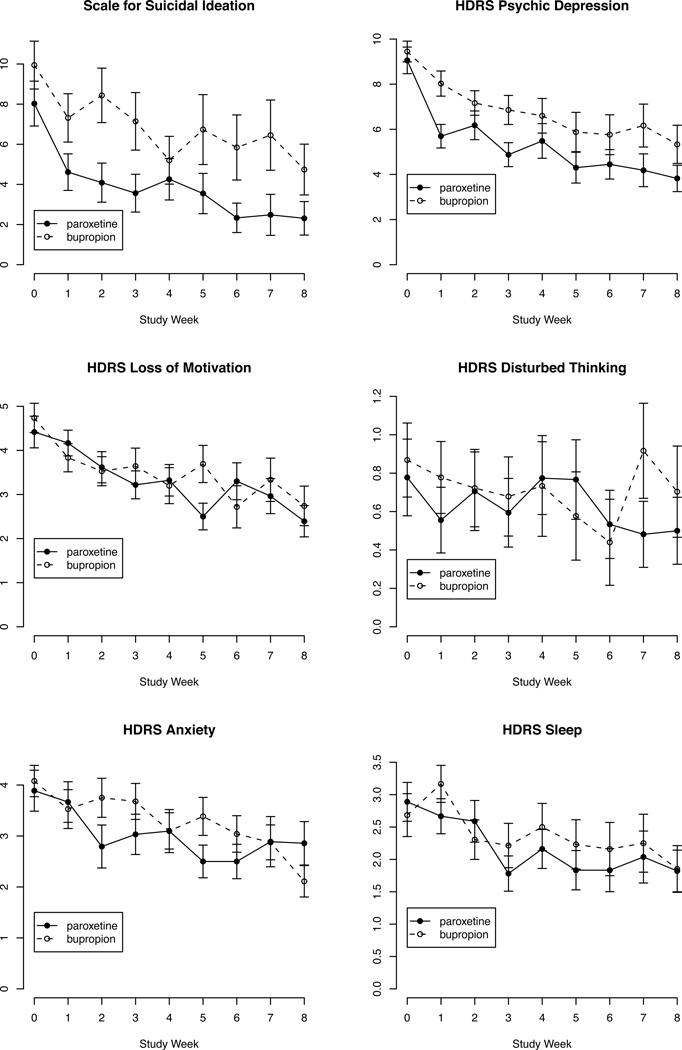
Figure 1.
Depressed patients' mean score of suicidal ideationa and HDRS-24 factorsb by study week and treatment with paroxetine vs bupropionc
footnotes:
aSuicidal ideation measured using the Beck Scale for Suicidal Ideation.
bHDRS-24 factor content:
Psychic Depression = Depressed mood + Guilt + Retardation + Helplessness + Hopelessness + Worthlessness.
Loss of Motivation = Work/activities+ Appetite + Libido + Weight loss.
Disturbed Thinking = Lack of insight + Depersonalization/derealization + Paranoia + Obsessions/compulsions.
Anxiety = Agitation + Psychic anxiety + Somatic anxiety + Hypochondriasis.
Sleep = Insomnia early + Insomnia middle + Insomnia late.
cError bars represent standard error of the mean. Paroxetine (N=36) and bupropion (N=38) sample sizes decreased over time due to drop-out (see Table 1).
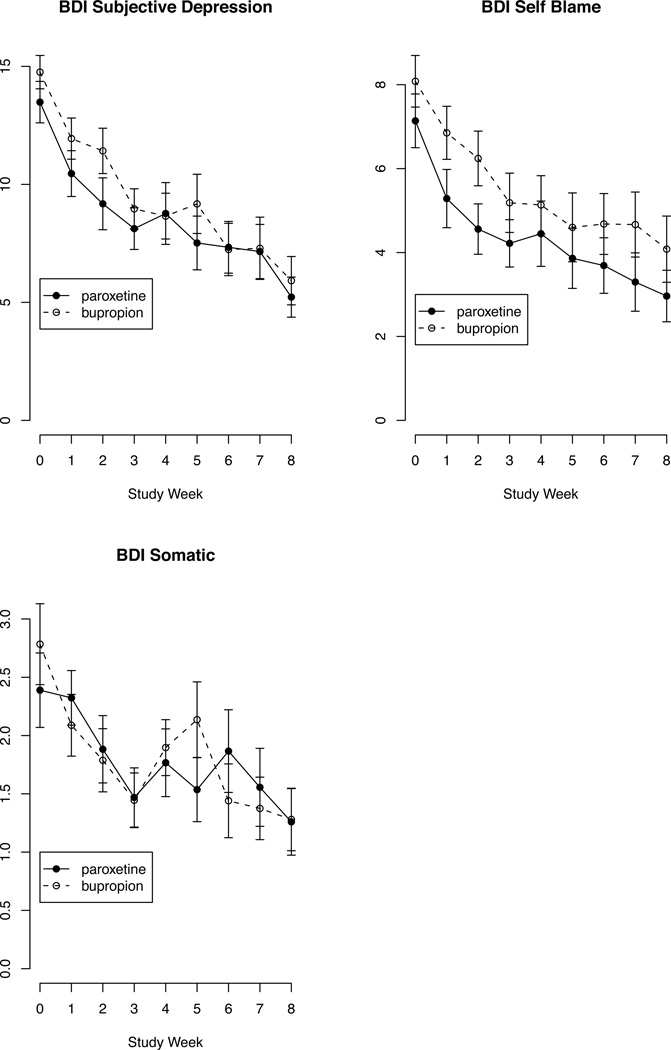
Figure 2.
Depressed patients' mean score on BDI factorsa by study week and treatment with paroxetine vs bupropionb
footnotes:
aBeck Depression Inventory factor content:
Subjective Depression = Sadness + Pessimism + Lack of satisfaction + Loss of interest + Indecisiveness + Appearance + Work + Tiredness + Libido.
Self-Blame = Failure + Guilt + Feel Punished + Disappointed in Self + Self-blame.
Somatic = Disturbed sleep + Appetite + Weight loss.
bError bars represent standard error of the mean. Paroxetine (N=36) and bupropion (N=38) sample sizes decreased over time due to drop-out (see Table 1).
To estimate the effect of treatment on each factor, we calculated predicted differences in factor scores between drug groups at each time point, assuming other variables were constant. These point estimates represent the net effect of drug on each depression factor. Differences in predicted scores between treatment groups were tested.
We next analyzed the entire sample (N=74), combining the drug groups, to explore whether change in suicidal ideation, irrespective of treatment, was associated with change in either the symptom clusters or the overall HDRS or BDI scores. We computed Spearman correlations between change (final minus baseline score) in suicidal ideation (SSI) and change in score on the depression factors or total HDRS and BDI scales.
Results
As previously reported, the treatment groups did not differ in baseline socio-demographic, clinical or suicidal characteristics.33 The treatment groups did not differ in study attrition rate, medication adherence, side effects, or frequency of concomitant benzodiazepine or zolpidem, although benzodiazepine dose was higher in the bupropion treated group.33 Figures 1 and 2 illustrate the trajectories of SSI score and the HDRS and BDI depression factors by treatment during the trial.
HDRS Psychic Depression
Table 1 summarizes the sample size, mean score and SD for this factor at each time point. There was a treatment main effect on the Psychic depression factor (estimate = −2.2, 95% CI = −3.2 to −1.1, t = −4.01, df = 67.16, p < 0.001). Compared to bupropion, paroxetine treatment was on average associated with greater improvement in Psychic Depression symptoms (depressed mood, guilt, retardation, helpless, hopeless, worthless). There was a treatment by time interaction, but the effect size was two thirds smaller (estimate = 0.74, 95% CI = 0.005 to 1.5, t = 2.01, df = 61.01, p = 0.048). The net treatment effect (main effect plus interaction) on this symptom cluster showed greater improvement on paroxetine during the first month of treatment (Table 2). Predicted Psychic depression scores were lower for patients randomized to paroxetine, compared to bupropion, with the difference largest after one week (2.2 points) and statistically significant through week four (Table 2).
Table 1.
HDRS-24 Psychic depression factor score mean, standard deviation, and sample size (N) at baseline and clinical trial weeks 1–8.
| Paroxetine | Bupropion | |||||
|---|---|---|---|---|---|---|
| Study Week Completed | N | mean | SD | N | mean | SD |
| Baseline | 36 | 9.1 | 3.5 | 38 | 9.4 | 2.8 |
| Week 1 | 36 | 5.7 | 3.1 | 36 | 8.0 | 3.3 |
| Week 2 | 34 | 6.2 | 3.7 | 36 | 7.2 | 3.3 |
| Week 3 | 32 | 4.9 | 3.0 | 28 | 6.9 | 3.4 |
| Week 4 | 31 | 5.5 | 4.3 | 30 | 6.6 | 4.2 |
| Week 5 | 30 | 4.3 | 3.7 | 25 | 5.9 | 4.4 |
| Week 6 | 29 | 4.4 | 3.5 | 25 | 5.8 | 4.4 |
| Week 7 | 27 | 4.2 | 3.8 | 24 | 6.2 | 4.7 |
| Week 8 | 28 | 3.8 | 3.1 | 27 | 5.3 | 4.4 |
Table 2.
Predicted differencesa between treatment groupsb in HDRS Psychic depression factor score during clinical trial weeks 1–8c
| Study week completed |
Predicted difference in HDRS Psychic depression score |
95% CI | SE | z | p |
|---|---|---|---|---|---|
| Week 1 | −2.2 | −3.3 to −1.1 | 0.54 | −4.01 | <0.001 |
| Week 2 | −1.6 | −2.6 to −0.6 | 0.49 | −3.30 | <0.001 |
| Week 3 | −1.3 | −2.3 to −0.3 | 0.53 | −2.52 | 0.012 |
| Week 4 | −1.1 | −2.2 to 0.0 | 0.58 | −1.95 | 0.051 |
| Week 5 | −0.9 | −2.1 to 0.3 | 0.63 | −1.55 | 0.122 |
| Week 6 | −0.8 | −2.1 to 0.5 | 0.67 | −1.25 | 0.213 |
| Week 7 | −0.7 | −2.1 to 0.7 | 0.71 | −1.02 | 0.309 |
| Week 8 | −0.6 | −2.1 to 0.8 | 0.74 | −0.83 | 0.404 |
Predicted difference (paroxetine group − bupropion group) is based on the model equation: FactorDuring Treatment = Treatment + Inpatient + Attempt + FactorBaseline + Ln(Study Week) + Treatment × Ln(Study Week)
Paroxetine (N=36) vs. bupropion (N=38)
HDRS Psychic depression factor=depressed mood + guilt + retardation + helplessness + hopelessness + worthlessness item sum.
Other Depression Factors
The net treatment effect on the BDI Self-Blame factor (sense of failure, guilt, feeling punished, self-dislike, self-criticism/blame) was not significant at Week 1 (estimate = −1.1, 95% CI = −2.4 to 0.22, SE = 0.68, z = −1.64, p = 0.101), with larger p values at subsequent time points. For HDRS Loss of Motivation (work/interests, appetite, libido, weight loss), the treatment main effect was not significant (p = 0.37) and the treatment by time interaction showed a trend toward greater improvement on paroxetine compared to bupropion (estimate = −0.49, 95%CI = −0.98 to 0.001, t = −1.99, df = 62.13, p = 0.051). Models of the other factors (HDRS Anxiety, Disturbed thinking, or Sleep disturbance, and BDI Subjective depression or Somatic complaint) showed no differential treatment effects (p > 0.1).
Baseline Inpatient and Past Attempter Status
Inpatient status at baseline was not significant in any model (p > 0.05). Past suicide attempt was not significant in any model except for BDI Somatic Complaint (early awakening, appetite loss, weight loss) where past attempters, on average, had a 0.71 point higher (worse) score during treatment (estimate = −0.71, 95% CI = −1.2 to −0.3, t = −3.13, df = 61.65, p = 0.003).
Suicidal Ideation Change: Correlations with Change in Depression Scales and Factors
Irrespective of treatment, change in suicidal ideation (SSI score) correlated comparably with change in total score on the 24-item HDRS (Spearman rho = 0.44, p < 0.001) and BDI (Spearman rho = 0.43, p < 0.001). Change in suicidal ideation correlated with change in factor scores in decreasing order of strength as follows: BDI Subjective depression (rho = 0.42, p < 0.001), BDI Self-blame (rho = 0.36, p = 0.002), HDRS Psychic depression (rho = 0.29, p = 0.012), BDI Somatic complaint (rho = 0.28, p = 0.02), HDRS Disturbed thinking (rho = 0.27, p = 0.02) and HDRS Sleep (rho 0.26, p = 0.03). Suicidal ideation change did not correlate with change in the HDRS Loss of motivation (rho = 0.15, p = 0.19) or Anxiety factors (rho = 0.17, p = 0.16).
Discussion
Depressed patients with past suicide attempt or current suicidal thoughts treated with paroxetine had a superior response compared to bupropion on the HDRS Psychic depression factor during the first month of treatment. Response of other HDRS and BDI factors did not differ by treatment.
The results partially support our hypothesis that an SSRI, which appears potentially advantageous compared to bupropion for reducing suicidal ideation among those most suicidal at baseline,33 would also be superior for non-suicide depression symptoms that are more closely associated with suicidal ideation.32 If confirmed, this would help guide treatment choice for more suicidal patients where faster reduction of suicidal risk would be a major clinical advantage.10
Our prior studies suggest that suicide attempts and ideation are more strongly associated with core affective/cognitive depression symptoms than with somatic/neuro-vegetative symptoms.26;32 The results from this independent clinical trial sample, irrespective of treatment, are consistent with our prior work. Beck et al reported that suicidal thoughts are more strongly associated with psychological compared to somatic symptoms in inpatients with a recent suicide attempt (N=247)39 and inpatients without an attempt but with suicidal thoughts (N=188).27 They found similar factor structures for the BDI in both samples, suggesting that the association of suicidal thoughts with non-suicide depressive symptoms is independent of past attempt history.27
While the above observational studies26;27;32;39 and this clinical trial suggest a stronger association of suicidal thoughts with affective/cognitive relative to somatic symptoms of depression, a simple dichotomy would be overstated. The HDRS Psychic depression factor contains five affective/cognitive items plus retardation, a psychomotor feature.26 It is noteworthy that along with ‘guilt’, two of these six items are features of melancholia, which we reported is associated with more lethal past suicide attempts and greater prospective attempt risk.40 In the present study, attempters had higher scores for BDI Somatic complaint, comprised of three melancholic features (early awakening, appetite loss, weight loss), but there was no differential drug effect on this cluster. The treatment effect for the HDRS Sleep factor, including early awakening, appeared to favor paroxetine, but was not statistically significant (p = 0.103), possibly due to insufficient power. Sleep disturbance, especially insomnia, is a risk factor for suicidal ideation and suicide.9;41–44
Symptom clusters within depression rating scales may be useful in the identification and treatment of depressed patients at higher risk for suicidal behavior. Evidence that severity of these symptom clusters is associated with relative regional activity in discrete brain regions45 may explain why only some factors are related to suicidal behavior in depressed patients. Our results suggest superior response of affective/cognitive depression symptoms with SSRI as compared to NDRI treatment. The findings require replication, but suggest a possible mechanism for the reported advantage of SSRI for suicidal ideation.33
We did not find evidence to support our hypotheses that the BDI Subjective depression and Self-blame factors would improve more with paroxetine relative to bupropion. The lack of a differential treatment effect for these factors may be partly explained by the self-report nature of the BDI, whereas the HDRS was rated by clinicians blind to treatment group. Several studies found the HDRS to be more sensitive than the BDI to change during treatment (reviewed in17). It is noteworthy that the HDRS Psychic depression factor contains the ‘hopelessness’ and ‘guilt’ items, which overlap, respectively, with the BDI Subjective depression and Self-blame factors.
The lack of a differential treatment effect on HDRS Anxiety is surprising given the common use of SSRIs to treat anxiety disorders. The relationship of anxiety to suicidal ideation and behavior is complex. Anxiety predicted suicidal behavior in some studies.6;9;46;47 Others, including ours, failed to find such an association or found a protective effect, after adjusting for other psychiatric disorders.26;48–54
Our results are strongest for the first month of treatment. However, Trivedi et al. noted the “crucial early stage (first four weeks)” of MDD treatment in a 12-week nefazodone trial.24 They demonstrated that the pattern of change in 17-item HDRS symptom clusters during the first month discriminated late responders from non-responders better than total HDRS score.24 This has particular relevance for suicidal patients where it is important to relieve suicidal ideation quickly and early signs of treatment efficacy would aid clinicians.
The main study limitation is a relatively small sample. However, depressed suicide attempters and ideators are a selected population often excluded from clinical trials. The study is a post hoc analysis in that the regression model was not pre-specified, although investigation of depression scale factors was a pre-planned exploratory aim. The eight-week treatment is typical for a short-term study and more than covered the differential drug effect for HDRS Psychic depression, which was modest and statistically significant until Week 4.
An asymmetrical drug titration schedule may have contributed to the results. paroxetine CR, the formulation used in our study, showed antidepressant efficacy for some patients at 12.5 mg daily in one 8-week trial (N=447), though the customary dose range is 25–62.5 mg daily.55 Many clinicians do not consider bupropion 150 mg daily to be a minimally effective dose, though an 8-week RCT found bupropion SR 150 mg (N=121) versus 300mg (N=120) daily to have equal antidepressant efficacy in moderate to severely depressed patients and both were superior to placebo (N=121).56 A meta-analysis by Papakostas and Fava57 suggests that placebo-like effects, a proxy for expectation of improvement, may be greater in trials without a placebo arm. While non-specific effects could partly explain our findings, their meta-analysis suggests it is more likely these would have biased our results toward the null, making the differences that were found more convincing.
Antidepressant treatment effect sizes appear to be smaller in routine clinical practice than in efficacy trials.58 Our study may be considered an effectiveness trial in that the sample was selected for increased suicidal risk, and co-morbidities such as substance abuse and anxiety disorders were permitted, making it more applicable to community practice. Given the lack of evidence-based treatments to reduce suicidal risk, even a modestly greater reduction of suicidal ideation,33 and of the depression symptoms most strongly associated to it, even for one month, would be meaningful for suicidal depressed patients. It also raises hope for other treatments that may outperform those in the current study in reducing key components of depression related to suicidal ideation.
In summary, the results suggest that, in depressed patients with suicidal thoughts or past attempt, paroxetine treatment, more than bupropion, reduced the depression symptoms most strongly associated with suicidal ideation for up to one month of follow-up. The findings require replication, but may point to a mediating pathway for the potential advantage of SSRI over NDRI therapy in more suicidal depressed patients.33 Our analysis also suggests possible symptom targets - namely the subjective mood symptoms of depression - for the development of additional treatments that are advantageous for reducing suicidal thoughts.
Clinical Points.
In higher suicidal risk patients with major depressive disorder (suicidal ideation or past attempt), change in suicidal ideation is more strongly associated with change in affective/cognitive as compared to somatic/neuro-vegetative symptoms.
Controlled-release paroxetine appears to have a modest advantage, compared to extended-release bupropion, in reducing affective/cognitive depression symptoms, which are the most closely associated with suicidal ideation.
The results require replication, but suggest that SSRI therapy may have a modest advantage over NDRI therapy for reducing suicidal ideation via faster reduction of core affective/cognitive depression symptoms in the early weeks of treatment.
Acknowledgments
Support: NARSAD Young Investigator grant (Grunebaum) and NIMH K23 MH076049 (Grunebaum). Other support from grants MH48514 and MH62185. Paroxetine CR and bupropion XL pills were donated by GSK in the study’s first 3 years and then purchased with grant funds. Dr. Grunebaum received no personal direct support from GSK for this study. NARSAD, NIMH and GSK had no role in the design and conduct of the study, the collection, analysis, and interpretation of the data, or preparation, review, or approval of the manuscript.
Dr. Mann received past research grants for unrelated brain imaging studies from GSK and Novartis, and receives royalties from the Research Foundation for Mental Hygiene for use of the Columbia Suicide Severity Rating Scale (C-SSRS), which was not used in this study. Dr. Oquendo receives royalties for use of the C-SSRS and received financial compensation from Pfizer for the safety evaluation of a clinical facility, unrelated to this study. She received a grant from Eli Lilly to support a year’s salary for a Lilly Suicide Scholar. She has received unrestricted educational grants and/or lecture fees from Astra-Zeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire. Her family owns stock in Bristol Myers Squibb. Dr. Burke receives royalties for use of the C-SSRS. No pharmaceutical company was involved in the design or conduct of the study, had access to the data or any role in its analysis or presentation.
Footnotes
Conflict of Interest: The other authors have no conflicts of interest related to this study.
Reference List
- 1.Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. J Am Med Assoc. 2005;294:2064–2074. doi: 10.1001/jama.294.16.2064. [DOI] [PubMed] [Google Scholar]
- 2.Knox KL, Caine ED. Establishing Priorities for Reducing Suicide and Its Antecedents in the United States. Am J Public Health. 2005;95:1898–1903. doi: 10.2105/AJPH.2004.047217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The cost of violence in the United States. Centers for Disease Control and Prevention; 2011. [Accessed May 4, 2012]. Available at: www.cdc.gov. [Google Scholar]
- 4.Corso PS, Mercy JA, Simon TR, et al. Medical costs and productivity losses due to interpersonal and self-directed violence in the United States. Am J Prev Med. 2007;32:474–482. doi: 10.1016/j.amepre.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization; 2012. [Accessed May 4, 2012]. Mental health: suicide prevention and special programmes; country reports and charts available. Available at: www.who.int. [Google Scholar]
- 6.Nock MK, Hwang I, Sampson NA, et al. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Molecular Psychiatry. 2009:1–9. doi: 10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617–626. doi: 10.1001/archpsyc.56.7.617. [DOI] [PubMed] [Google Scholar]
- 8.Oquendo MA, Galfalvy HC, Russo S, et al. Prospective Study of Clinical Predictors of Suicidal Acts After a Major Depressive Episode in Patients With Major Depressive Disorder or Bipolar Disorder. Am J Psychiatry. 2004:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147:1189–1194. doi: 10.1176/ajp.147.9.1189. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RD, Brown CH, Hur K, et al. Suicidal thoughts and behavior with antidepressant treatment: reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012 doi: 10.1001/archgenpsychiatry.2011.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Mann JJ, Waternaux C, Haas GL, et al. Towards a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 14.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 15.Van Gastel A, Schotte C, Maes M. The prediction of suicidal intent in depressed patients. Acta Psychiatr Scand. 1997;96:254–259. doi: 10.1111/j.1600-0447.1997.tb10160.x. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons RD, Clark DC, Kupfer DJ. Exactly what does the Hamilton Depression Rating Scale measure? J Psychiat Res. 1993;27:259–273. doi: 10.1016/0022-3956(93)90037-3. [DOI] [PubMed] [Google Scholar]
- 17.Bagby RM, Ryder AG, Schuller DR, et al. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am J Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- 18.Steer RA, Beck AT, Riskind JH, et al. Relationships between the Beck Depression Inventory and the Hamilton Psychiatric Rating Scale for Depression in depressed outpatients. Journal of Psychopathology and Behavioral Assessment. 1987;9:327–339. [Google Scholar]
- 19.Gullion CM, Rush AJ. Toward a generalizable model of symptoms in major depressive disorder. Biol Psychiatry. 1998;44:959–972. doi: 10.1016/s0006-3223(98)00235-2. [DOI] [PubMed] [Google Scholar]
- 20.Dimascio A, Weissman MM, Prusoff BA, et al. Differential Symptom Reduction by Drugs and Psychotherapy in Acute Depression. Arch Gen Psychiatry. 1979;36:1450–1456. doi: 10.1001/archpsyc.1979.01780130068008. [DOI] [PubMed] [Google Scholar]
- 21.Bruijn JA, Moleman P, Mulder PGH, et al. Depressed in-patients respond differently to imipramine and mirtazapine. Pharmacopsychiat. 1999;32:87–92. doi: 10.1055/s-2007-979200. [DOI] [PubMed] [Google Scholar]
- 22.Feighner JP, Overo K. Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. J Clin Psychiatry. 1999;60:824–830. doi: 10.4088/jcp.v60n1204. [DOI] [PubMed] [Google Scholar]
- 23.Jamerson BD, Krishnan KR, Roberts J, et al. Effect of bupropion SR on specific symptom clusters of depression: analysis of the 31-item Hamilton Rating Scale for depression. Psychopharmacol Bull. 2003;37:67–78. [PubMed] [Google Scholar]
- 24.Trivedi MH, Morris DW, Grannemann BD, et al. Symptom clusters as predictors of late response to antidepressant treatment. J Clin Psychiatry. 2005;66:1064–1070. doi: 10.4088/jcp.v66n0816. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JG, Harkness KL. Symptom specificity in the acute treatment of Major Depressive Disorder: A re-analysis of the treatment of depression collaborative research program. J Affect Disord. 2012;137:87–97. doi: 10.1016/j.jad.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Grunebaum MF, Keilp J, Li S, et al. Symptom components of standard depression scales and past suicidal behavior. J Affect Disord. 2005;87:73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Lester D, Beck AT. Suicidal wishes and depression in suicidal ideators: a comparison with attempted suicides. Journal of Clinical Psychology. 1977;33:92–94. doi: 10.1002/1097-4679(197701)33:1+<92::aid-jclp2270330118>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Brown C, Schulberg HC, Madonia MJ. Assessing depression in primary care practice with the Beck Depression Inventory and the Hamilton Rating Scale for Depression. Psychological Assessment. 1995;7:59–65. [Google Scholar]
- 29.Sayer NA, Sackeim HA, Moeller JR, et al. The relations between observer-rating and self-report of depressive symptomatology. Psychological Assessment. 1993;5:350–360. [Google Scholar]
- 30.Rhoades HM, Overall JE. The Hamilton depression scale: factor scoring and profile classification. Psychopharmacol Bull. 1983;19:91–96. [Google Scholar]
- 31.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: The scale for suicide ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 32.Keilp JG, Grunebaum MF, Gorlyn M, et al. Suicidal ideation and the subjective aspects of depression. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunebaum MF, Ellis SP, Duan N, et al. Pilot randomized clinical trial of an SSRI vs bupropion: effects on suicidal behavior, ideation, and mood in major depression. Neuropsychopharmacology. 2012;37:697–706. doi: 10.1038/npp.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldessarini RJ. Drug Therapy of Depression and Anxiety Disorders. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11 ed. New York: McGraw-Hill; 2006. pp. 429–459. [Google Scholar]
- 35.Spitzer RL, Williams JBW, Gibbon M, et al. Structured Clinical Interview for DSM-III-R/DSM-IV Patient edition (SCID-P) Washington, D.C.: American Psychiatric Press; 1990. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), (Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 37.Oquendo MA, Halberstam B, Mann JJ. Risk Factors for Suicidal Behavior: utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice. Vol. 22. Washington, D.C.: APPI Press; 2003. pp. 103–130. [Google Scholar]
- 38.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 39.Beck AT, Lester D, Albert N. Suicidal wishes and symptoms of depression. Psychological Reports. 1973;33:770. doi: 10.2466/pr0.1973.33.3.770. [DOI] [PubMed] [Google Scholar]
- 40.Grunebaum MF, Galfalvy HC, Oquendo MA, et al. Melancholia and the probability and lethality of suicide attempts. Br J Psychiatry. 2004;184:534–535. doi: 10.1192/bjp.184.6.534. [DOI] [PubMed] [Google Scholar]
- 41.McCall WV, Blocker JN, D'Agostino J, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Medicine. 2010;11:822–827. doi: 10.1016/j.sleep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein TR, Bridge JA, Brent DA. Sleep disturbance preceding completed suicide in adolescents. J Consult Clin Psychol. 2008;76:84–91. doi: 10.1037/0022-006X.76.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudd MD, Berman AL, Joiner TEJ, et al. Warning Signs for Suicide: Theory, Research, and Clinical Applications. Suicide & Life - Threatening Behavior. 2006;36:255–262. doi: 10.1521/suli.2006.36.3.255. [DOI] [PubMed] [Google Scholar]
- 44.Bjorngaard JH, Bjerkeset O, Romundstad P, et al. Sleeping problems and suicide in 75,000 Norwegian adults: a 20 year follow-up of the HUNT 1 study. Sleep. 2011;34:1155–1159. doi: 10.5665/SLEEP.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milak MS, Parsey RV, Keilp J, et al. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- 46.Weissman MM, Klerman GL, Markowitz JS, et al. Suicidal ideation and suicide attempts in panic disorder and attacks. N Engl J Med. 1989;321:1209–1214. doi: 10.1056/NEJM198911023211801. [DOI] [PubMed] [Google Scholar]
- 47.Johnson J, Weissman MM, Klerman GL. Panic disorder, comorbidity, and suicide attempts. Arch Gen Psychiatry. 1990;47:805–808. doi: 10.1001/archpsyc.1990.01810210013002. [DOI] [PubMed] [Google Scholar]
- 48.Friedman S, Jones JC, Chernen L, et al. Suicidal ideation and suicide attempts among patients with panic disorder: A survey of two outpatient clinics. Am J Psychiatry. 1992;149:680–685. doi: 10.1176/ajp.149.5.680. [DOI] [PubMed] [Google Scholar]
- 49.Lepine JP, Chignon JM, Teherani M. Suicide attempts in patients with panic disorder. Arch Gen Psychiatry. 1993;50:144–149. doi: 10.1001/archpsyc.1993.01820140070008. [DOI] [PubMed] [Google Scholar]
- 50.Hornig CD, McNally RJ. Panic disorder and suicide attempt. A reanalysis of data from the Epidemiologic Catchment Area Study. Br J Psychiatry. 1995;167:76–79. doi: 10.1192/bjp.167.1.76. [DOI] [PubMed] [Google Scholar]
- 51.Roy-Byrne PP, Stang P, Wittchen HU, et al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, course and help-seeking. Br J Psychiatry. 2000;176:229–235. doi: 10.1192/bjp.176.3.229. [DOI] [PubMed] [Google Scholar]
- 52.Apter A, Horesh N, Gothelf D, et al. Depression and suicidal behavior in adolescent inpatients with obsessive compulsive disorder. J Affect Disord. 2003;75:181–189. doi: 10.1016/s0165-0327(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 53.Vickers K, McNally RJ. Panic disorder and suicide attempt in the National Comorbidity Survey. J Abnorm Psychol. 2004;113:582–591. doi: 10.1037/0021-843X.113.4.582. [DOI] [PubMed] [Google Scholar]
- 54.Placidi GPA, Oquendo MA, Malone KM, et al. Anxiety in major depression: Relationship to suicide attempts. Am J Psychiatry. 2000;157:1614–1618. doi: 10.1176/appi.ajp.157.10.1614. [DOI] [PubMed] [Google Scholar]
- 55.Trivedi MH, Pigott TA, Perera P, et al. Effectiveness of low doses of paroxetine controlled release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1356–1364. doi: 10.4088/jcp.v65n1010. [DOI] [PubMed] [Google Scholar]
- 56.Reimherr FW, Cunningham LA, Batey SR, et al. A multicenter evaluation of the efficacy and safety of 150 and 300 mg/d sustained-release bupropion tablets versus placebo in depressed outpatients. Clinical Therapeutics. 1998;20:505–516. doi: 10.1016/s0149-2918(98)80060-x. [DOI] [PubMed] [Google Scholar]
- 57.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19:34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 58.van der Lem R, van der Wee NJA, van Veen T, et al. Efficacy versus Effectiveness: A Direct Comparison of the Outcome of Treatment for Mild to Moderate Depression in Randomized Controlled Trials and Daily Practice. Psychotherapy and Psychosomatics. 2012;81:226–234. doi: 10.1159/000330890. [DOI] [PubMed] [Google Scholar]