Abstract
Neurologic complications for HIV-infected persons retain significant prevalence despite an increasingly global use of antiretroviral therapies. Such complications are often ascribed to advanced immunosuppression; however, the most common neurologic problems for HIV-infected persons, distal sensory polyneuropathy and HIV-associated neurocognitive disorders, affect a significant proportion of patients who have successfully achieved immunologic restoration with normal or near-normal CD4 count levels and undetectable HIV RNA in the periphery. Understanding specific considerations for HIV-associated complications, including the epidemiology, risk factors, medication-adverse effects, and benefits of appropriate management, is vital for all providers caring for those with HIV. This review will describe such considerations, as well as providing a more detailed review of the most common neurologic complications of HIV infection, and will highlight some of the challenges involved with diagnosis, management, and long-term effects.
Keywords: Neurologic, HIV, CNS, neuroHIV, HAND, HIV-associated neurocognitive disorder, HIV sensory neuropathy, Review
Introduction
Both the central and peripheral nervous systems are potential targets for HIV-associated injury and subsequent disease and disability. Despite the widespread availability of combination antiretroviral therapy (CART) throughout much of the developed world, these diseases persist for various reasons that are only partially understood. This review will focus on areas that have received considerable or increased research attention over the past several years: central nervous system (CNS) injury during early HIV infection, HIV-associated neurocognitive disorders (HANDs), and HIV-sensory neuropathy (HIVSN). These topics highlight the widespread neurologic injury associated with HIV infection, as well as the numerous unanswered questions that are key in learning to effectively manage and potentially repair such injury.
Biology of HIV Nervous System Disease
The blood–brain barrier (BBB) is a selectively permeable brain compartment that is typically resistant to infections of the periphery. Unfortunately, HIV-1 traverses the barrier within days of primary infection, likely through its tropism for CD4+ cells, including T-lymphocytes and macrophages that act as transporters across the barrier. This “Trojan horse” mechanism occurs in an uncompromised BBB, and so CNS invasion by HIV is essentially unavoidable [1]. Once in the CNS, the virus replicates within macrophages and perivascular microglia (also CD4+), although it can be found in other CNS cells, including astrocytes [2].
While HIV does not directly invade neurons, they can be damaged by indirect mechanisms, including the HIV neurotoxic proteins Tat and gp120, or through HIV-infected macrophage and microglia cells’ release of proinflammatory cytokines [3]. Multiple brain sites are susceptible to injury from HIV; however, the most prominently affected areas include the basal ganglia, frontal cortices, and subcortical white matter [4]. Furthermore, specific areas such as the caudate nucleus, amygdala, and corpus callosum appear to suffer volume loss despite the use of CART, suggesting either irreparable damage induced prior to initiation of CART or ongoing injury that is unmitigated in the presence of CART [5]. Thus, there can be broad damage to the nervous system by HIV, and this is increasingly recognized without the advanced immunosuppression that has historically been associated with CNS injury.
Disease Burden of CNS HIV Complications
CART has overall decreased CNS-related morbidity and mortality over the past 15 years in patients infected with HIV. However, neurologic disease remains a persistent burden for many patients. In a unique population-based study of prevalence of all registered HIV-infected patients in southern Alberta, Canada (n = 1,653), 24.5% had a diagnosed neurologic complication of HIV [6]. The highest prevalence rates were for distal-sensory polyneuropathy (10%) and HIV-associated neurocognitive disorders (6.2%, with asymptomatic forms excluded). Patients with at least one neurologic diagnosis had a mortality rate 3.1 times higher than for those without neurologic disease, and such patients had significantly lower nadir and baseline (at the time of neurologic diagnosis) CD4+ cell counts and higher baseline plasma HIV RNA viral loads.
Importantly, the rates of neurocognitive impairment and sensory neuropathy in this population were reported as significantly lower than in other recent studies of large cohorts infected with HIV. The differences likely extend from the exclusion of asymptomatic patients in the Canadian study, as well as potential referral bias in that patients with a confirmed neurologic diagnosis were included only if HIV practitioners had referred them to neurologists who had then made the diagnosis of HAND or neuropathy. The true incidence of such conditions is likely higher among general populations of HIV-infected persons; however, despite these limitations, this large epidemiologic study confirms a significant neurologic disease burden for HIV-infected patients that predicts mortality in the modern era.
Early HIV Infection and CNS Penetration
CNS invasion and injury have been difficult to document in the early seroconversion period because of the difficulty in identifying these patients early enough for valid research; however, recent studies have brought forth new data during this period. In a recently reported sample of 18 patients, CNS invasion by HIV was documented as early as 8 days after infection [7]. However, at a median of 14 days after exposure, 3 of the patients studied did not have measurable levels of HIV RNA in CSF, suggesting that such early infection is not universal and host and/or viral characteristics may affect the timing of such viral penetration. A similar study of 107 patients with infection occurring a median of 78 days after HIV transmission compared patients with a detectable versus undetectable CSF viral load, using a variety of characteristics, including neurocognitive performance and immune activation levels [8]. The patients with undetectable CSF viral loads had lower rates of plasma and CSF CD8+ T-cell activation; however, there was no such difference for monocyte activation levels. Furthermore, when a subset of patients were retested approximately 1 year later, there were no such differences found between the groups. It remains unknown why there is such variation in CNS susceptibility to HIV during the early postinfection period and how and whether such variability affects neurologic outcomes during longer-term infection.
The importance of early CART therapy in reducing neurologic disease effects was highlighted in a longitudinal cohort of acutely infected HIV persons with baseline results a median of 4 months from infection date [9]. Of these patients, 20% performed at least 1 SD below standardized norms on several neuropsychological measures of cognitive function. When patients were followed with serial testing, performance continued to decline for those not initiated on CART. For those patients initiated on CART after a median of 254 days after HIV exposure, cognitive function stabilized but did not improve. This suggests that early damage after HIV exposure may not improve after initiation of CART; however, further deficits are prevented.
HAND
A 2007 AAN consensus panel updated the definitions for cognitive impairments related to HIV that are now defined as HANDs [10••]. These include the milder form asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND), which have an especially high prevalence in the modern era of up to 45%, as well as HIV-associated dementia (HAD), which was more commonly seen in the pre-CART era and now affects fewer than 5% of patients with HIV [11••]. These diagnoses require a detailed neuropsychological battery, determinants of impairments of daily living activities, and an exclusion of any confounding factors, including advanced psychiatric illness, CNS opportunistic infections, or hepatitis C viremia. In sum, up to 50% of the general HIV population may be affected by such impairments. And even the milder forms have significant negative outcomes on patients’ quality of life and performance measures, such as driving, employment, and medication management [12••].
Research in neuro-HIV over the past decade has been heavily focused on HAND because of its continued high prevalence despite successful viral suppression and restoration of immune status with CART. This section will highlight some of the advances regarding screening tools, pathogenesis, and treatment trials for HAND.
Screening for HAND has been difficult in the recent era because of the often subtle forms of impairment that do not present as frank dementia. Validated screening tools that evaluate for specifically HIV dementia include the HIV Dementia Scale and the International HIV Dementia Scale, although both of these were developed before the 2007 AAN consensus guidelines [13, 14]. When both the HDS and IHDS were studied to screen for both MND and HAD, the sensitivities and specificities were lower than when originally studied to screen only for HIV dementia [15]. Newer measures that are commonly substituted for a more comprehensive neuropsychological (NP) battery are composites of several NP tests, including the NPZ-4 or NPZ-5, which offer a truncated version of the more extensive NP tests that are required for the diagnosis; however, these still typically require some familiarity with cognitive testing outside the realm of general practitioners [16–18]. A recently reported study of HIV-1 infected military personnel revealed that abbreviating an NP battery of greater than an hour to two, three, or four tests, all of which were under 20 min of total administration time, showed well-preserved sensitivities and specificities for detecting cognitive impairments [19].
The Montreal Cognitive Assessment (MOCA) is a 5- to 10-min bedside tool that covers a broad range of cognitive measures, including tests for subcortical abnormalities commonly encountered with HAND. An abbreviated version of the MOCA showed promise for early detection of neurocognitive impairments in a cohort of HIV-infected patients, although more detailed study is needed to further validate this widely-used screening test for patients with HIV [20]. Additionally, a 3-min screen that includes age, current CD4 cell count, history of CNS disease, and duration of CART therapy provided good sensitivity and specificity for detecting HAND; further studies are reportedly ongoing and may show significant promise for appropriately identifying patients at risk of HAND [21•]. A brief screening tool for practitioners is essential for early identification of those with HAND presenting to general medicine or HIV specialist clinics so that appropriate therapies can be instituted.
Such therapies that have the aim of stabilizing or modifying HAND have also been a significant research interest over the past years. CART has been the mainstay therapy for HAND, since its impact on both CD4+ T-cell count and HIV RNA viral load addresses the underlying factors most strongly associated with the development of neurologic complications of HIV. However, a nadir CD4+ count, rather than current CD4+ count or current plasma viral load, may play a more important role, since nadir count has been a consistent risk factor associated with eventual development of HAND [11••, 22, 23]. Indeed, HAND may be somewhat refractory to initiating CART even if aviremic for an extended time period. After a mean of 63 months of CART treatment, 62.8% of an Italian cohort with neurocognitive impairment at baseline had persistent impairment, despite the majority of patients achieving virologic suppression (≤50 copies/ml of HIV RNA in plasma) while on therapy [24]. In contrast, in a large study of both HIV-infected and HIV-uninfected men without cognitive symptoms, there were no differences after 5 years of serial cognitive testing on measures of psychomotor speed and speed of mental processing, even among stratified groups of HIV-infected men, including those who had long-standing viral control on CART [25]. It is reassuring that previously unaffected individuals continue to preserve cognitive measures over time if the virus remains well controlled; however, it appears that prior cognitive impairment does not significantly benefit cognitive outcomes as much as might be expected. Further longitudinal analyses are needed to come to conclusions regarding the cognitive impact of CART for those with and without baseline neurocognitive impairment.
Adjunctive therapies for CART in patients with HAND have received considerable attention in several controlled clinical trials, but unfortunately, these have had unsuccessful results. Minocycline, an antibiotic derivative with neuroprotective and antiinflammatory in vitro effects, did not show significant improvements in cognitive performance after a 24-week trial [26]. Memantine, an N-methyl-D-aspartate NMDA-receptor blocker that is currently FDA-approved for the treatment of Alzheimer's disease, did not lead to neurocognitive improvement after an initial 20-week double-blind phase; however, after the first 12 weeks of a subsequent open-label phase, the group who had been randomly assigned to memantine in the blinded phase had significant improvements in cognitive performance not seen in the group previously assigned to the placebo group [27]. When this open-label study was extended to 48 weeks, the original memantine group had stable performance, and the original placebo group had improved performance. While the open-label results of memantine are promising, the lack of benefit from the double-blind phase suggests either that ascertainment bias accounted for the participants’ improvements in the open-label phase or that the time length of the blinded study was inadequate to detect meaningful neurocognitive benefit. Similar results were seen with transdermal selegeline, a monoamine oxidase (MAO)-B inhibitor with an unsuccessful 24-week double-blind phase result and improvements later seen in all treatment groups after an additional 24 weeks of an open-label phase [28]. Benefits from nontherapeutic interventions such as cognitive rehabilitation have shown some promise in early-phase studies [29, 30], although larger trials are needed to more adequately assess the impacts of such interventions.
Another potential treatment strategy currently under investigation for HAND involves the variation in each antiretroviral therapy to penetrate the CNS. Such quantification of a CNS penetration-effectiveness (CPE) score for different cART regimens has led many to consider whether changing or initiating a regimen with a higher CPE score clinically benefits appropriate patients. To date, results have not led to recommendations for such regimens, and research continues to evolve. Authors have demonstrated that CPE scores are associated with HIV-1 viral suppression in CSF [31, 32] and improved neuropsychological performance [33]. Some results have been conflicting [32], and a blinded and randomized trial is needed to further define whether higher CPE regimens are indicated for those affected with HAND.
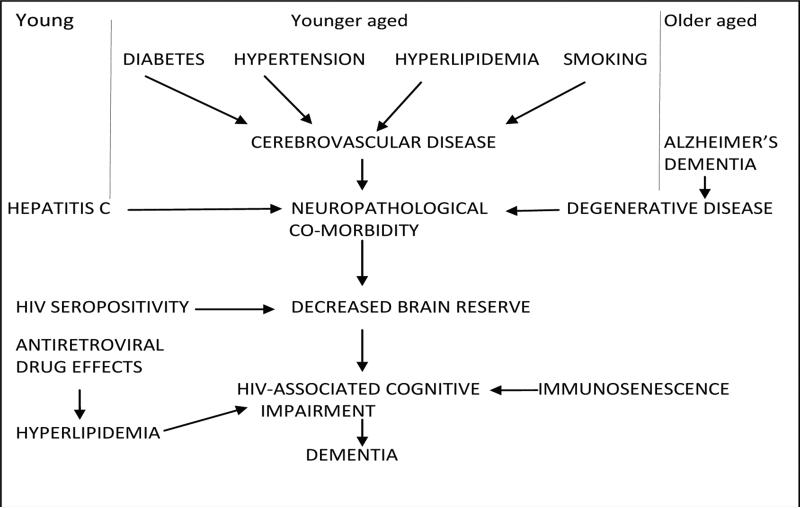
Additional research developments in our understanding of HAND have focused on determining potential etiologies. While the direct and indirect roles of HIV-associated CNS injury have been described, newer associations with both vascular disease and amyloid deposition likely contribute to HAND pathogenesis, especially in older HIV-infected individuals. The cumulative effects of cerebrovascular disease from risk factors such as cardiovascular disease [16], hypertension [16], carotid intima media thickness [34], glomerular filtration rate [34], diabetes [35], and obesity [35] are associated with an increased rate of cognitive impairments in those with HIV. And extracellular amyloid deposition, similar to that seen with normal aging and admittedly different from the pattern of amyloid deposition seen in Alzheimer's disease, has been shown to occur in HIV-infected individuals at significantly younger ages than in HIV-uninfected patients [36, 37, 38•, 39]. The understanding of contributing factors other than HIV itself has led to a new framework for viewing the mechanisms behind the development of HAND, as can be seen in Fig. 1. This model is especially notable because both cerebrovascular disease and amyloid deposition are additional targets for intervention that could improve the clinical course of HAND beyond that of CART alone.
Fig. 1.
Different mechanisms (cerebrovascular disease, amyloid deposition, and comorbidities) for varied age groups may contribute to decreased brain reserve that promulgates HIV-associated neurocognitive disorders. Courtesy of Ned Sacktor, MD
HIV-Associated Sensory Neuropathy (HIV-SN)
HIV-SN is another neurologic complication of HIV that continues to have a persistent effect on patients despite CART availability and viremic control, at least in developed countries. In the CHARTER cohort of HIV-infected patients, with the majority of patients meeting criteria for an AIDS diagnosis with a median nadir CD4 count of 175 cells/μl although with a current median CD4 count of 419 cells/μl, 57.2% had at least one abnormal sensory sign bilaterally on a standardized examination consistent with SN [40••]. Slightly more than one third of the participants had prior exposure to an antiretroviral associated with the development of SN (stavudine, didanosine, or zalcitabine, the “D-drugs” that are all nucleoside reverse transcriptase inhibitors), while about 13% had current exposure to the D-drugs. Risk factors other than neurotoxic drug exposure and low nadir CD4+ cell count in the current era include current CART use and older age. And for patients with currently suppressed plasma viral loads, low nadir CD4+ cell count was the best predictor of HIV-SN, as is seen in HAND. The presence of HIVSN was associated with reduced employment rates and with impairments in daily living activities, again highlighting the disability encountered with various forms of HIV-related neurologic disease. And of the patients with HIV-SN, 60.8% had associated neuropathic pain that increased the odds of unemployment or impairments with daily living activities even further.
The overall prevalence and risk factors for HIV-SN are similar to those reported in the pre-CART era [41, 42], with a notable exception being that prior, rather than current, D-drug therapy is a risk factor for HIV-SN in the modern era. Additionally, just the use of CART is now a risk factor for HIV-SN that could possibly reflect the continued use of stavudine in many CART regimens, or there may be other contributing factors such as less well recognized neurotoxic therapies [40••]. Other potential considerations for sources of HIV-SN include current substance use disorders [43], mood and other neurologic disorders [44], and, interestingly, neurocognitive performance on tests of psychomotor speed [45].
Recent research developments in HIV-SN include the use of skin punch biopsy for measuring epidermal nerve fiber density (ENFD), as well as the development of a rodent model of HIV-associated painful neuropathy. ENFD correlates strongly with both clinical and electrophysiologic measures of HIV-SN [46]. This is especially important because ENFD provides a measurement that correlates with disease severity; thus, it can be followed longitudinally for treatment trials to quantitatively evaluate potential changes in HIV-SN. Similarly, the rodent model has allowed more extensive research possibilities, since it has been used in studies examining the mechanisms behind HIV-induced peripheral nerve injury [47, 48]. Finding specific disease-related targets such as N-type voltage-gated calcium channels [49, 50] is crucial in developing treatments that could effectively treat neuropathic pain or repair peripheral nerve injury. Unfortunately, there are currently no FDA-approved therapies specifically for HIV-SN; however, trials of potential neuroregenerative treatments are ongoing. Current treatment is focused on relieving neuropathic pain, typically with agents that include gabapentin, pregabalin, or duloxetine, although controlled trials of these medications for HIV-SN have typically been limited by small sample sizes that may limit conclusions regarding efficacy [51•].
Conclusion
The toxic effects of HIV on the neurologic system were known even before the identification of HIV as the causative agent. Despite this, there are numerous unanswered questions that remain. As the population living with HIV ages and becomes susceptible to more age-related comorbidities, these questions will continue. Potentially, with the development of additional animal models, the introduction of effective screening tools, and the availability of cohort studies with greater longitudinal data, these unknowns will become answerable so that effective therapies targeting the disease mechanisms can reduce the significant disability and mortality associated with neurologic complications of HIV.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
• Of interest
•• Of significant interest
- 1.Peluso R, Haase A, Stowring L, et al. A Trojan horse mechanism for the spread of visna virus in monocytes. Virology. 1985;14:231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- 2.Yoshioka M, Bradley M, Shapshak P, et al. Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases. Adv Neuroimmunol. 1995;5:335–358. doi: 10.1016/0960-5428(95)00012-q. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Scarano F, Martin-Garcia J. The Neuropathogensis of AIDS. Nat Rev Immunol. 2005;19:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 4.Martin A. HIV, cognition, and the basal ganglia. In: Grant I, Martin A, editors. Neuropsychology of HIV Infection. Oxford University Press; New York: 1994. pp. 234–259. [Google Scholar]
- 5.Ances BM, Ortega M, Vaida F, et al. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–77. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: A population based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson J, Lee E, Hecht F, et al. Changes in neurocognitive performance from early HIV-1 infection to initiation of ART [Abstract 80].. Presented at the 19th Annual Conference on Retroviruses and Opportunistic Infections; Seattle, WA. March 5-8, 2012. [Google Scholar]
- 9.Lee E, Gisslen M, Cinque P, et al. Early follow up of undetectable cerebrospinal fluid HIV-1 RNA during primary infection in the absence of ART [Abstract 457].. Presented at the 19th Annual Conference on Retroviruses and Opportunistic Infections; Seattle, WA. March 5-8, 2012. [Google Scholar]
- 10••.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [These consensus guidelines for diagnosing forms of HAND not only are important for researchers, but also are valuable for clinicians, especially with the understanding of how the different forms vary by level of testing performance and level of daily impairments. Additionally, the article includes descriptions of how cognitive impairments in HIV-infected persons have changed over the years, and it is important to clinically recognize the often subtler symptoms that are prevalent now.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Heaton RK, Clifford DB, Franklin DR, JR., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [This large multicenter cohort study with strong methodology highlights the overwhelming prevalence of HAND in the HIV population.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Gorman AA, Foley JM, Ettenhofer ML, et al. Functional consequences of HIV-associated neurocognitive impairment. Neuropsychol Rev. 2009;19:186–203. doi: 10.1007/s11065-009-9095-0. [This extensive and well-organized review of outcomes for patients affected with HAND emphasizes the role that even subtle disease can have major impacts on activities of daily living.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sacktor NC, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- 15.Skinner S, Adewale AJ, DeBlock L, et al. Neurocognitive screening tools in HIV/AIDS: comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009;10:246–252. doi: 10.1111/j.1468-1293.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 16.Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75:864–873. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra CM, Lockhart D, Zunt JR, et al. Changes in CSF and plasma HIV-1and cognition after starting potent antiretroviral therapy. Neurology. 2003;60:1388–1390. doi: 10.1212/01.wnl.0000058768.73358.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore DJ, Roediger M, Eberly L. Clinical Evaluation of Neurocognitive and Neuropsychiatric Dysfunction [Abstract 499].. Presented at the 19th Annual Conference on Retroviruses and Opportunistic Infections; Seattle, WA. March 5-8, 2012. [Google Scholar]
- 20.Koski L, Brouillette MJ, Lalonde R, et al. Computerized testing augments pencil-and-paper tasks in measuring HIV-associated mild cognitive impairment. HIV Med. 2011;12:472–80. doi: 10.1111/j.1468-1293.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 21•.Cysique LA, Murray JM, Dunbar M, et al. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11:642–649. doi: 10.1111/j.1468-1293.2010.00834.x. [This study describes a quick bedside questionnaire for screening patients for HAND with good sensitivity and specificity.] [DOI] [PubMed] [Google Scholar]
- 22.Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tozzi V, Balestra P, Lorenzini P, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 2005;11:265–73. doi: 10.1080/13550280590952790. [DOI] [PubMed] [Google Scholar]
- 24.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 25.Cole MA, Margolick JB, Cox C, et al. Longitudinally preserved psychomotor performance in long-term asymptomatic HIV-infected individuals. Neurology. 2007;69:2213–2220. doi: 10.1212/01.WNL.0000277520.94788.82. [DOI] [PubMed] [Google Scholar]
- 26.Sacktor N, Miyahara S, Deng L, et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology. 2011;77:1135–1142. doi: 10.1212/WNL.0b013e31822f0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Navia BA, Marra CM, et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV Clin Trials. 2010;11:59–67. doi: 10.1310/hct1101-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans SR, Yeh TM, Sacktor N, et al. Selegiline transdermal system (STS) for HIV-associated cognitive impairment: open-label report of ACTG 5090. HIV Clin Trials. 2007;8:437–446. doi: 10.1310/hct0806-437. [DOI] [PubMed] [Google Scholar]
- 29.Iudicello JE, Kellogg EJ, Weber E, et al. Semantic cueing improves category verbal fluency in persons living with HIV infection. J Neuropsychiatry Clin Neurosci. 2012;24:183–190. doi: 10.1176/appi.neuropsych.11100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber E, Woods SP, Kellogg E, Grant I, Basso MR. Self-generation enhances verbal recall in individuals infected with HIV. J Int Neuropsychol Soc. 2012;18:128–133. doi: 10.1017/S135561771100124X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusini A, Vernazza PL, Yerly S, et al. Higher CNS Penetration-Effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr. 2012 doi: 10.1097/QAI.0b013e318274e2b0. In press. [DOI] [PubMed] [Google Scholar]
- 32.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78:485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Achim CL, Adame A, Dumaop W, et al. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green DA, Masliah E, Vinters HV, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 38•.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [The authors report on how the HIV-1 protein inhibits amyloid clearance but also describe how this inhibition actually leads to CNS amyloid deposition. The discussion concisely summarizes the clinical implications of amyloid deposition in HIV-infected patients.] [DOI] [PubMed] [Google Scholar]
- 39.Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [The authors report on HIV-SN within a large cohort of HIV-positive patients and describe very high prevalence despite the use of CART and the numerous risk factors that are associated with HIV-SN.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Childs EA, Lyles RH, Selnes OA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 42.Tagliati M, Grinnell J, Godbold J, et al. Peripheral nerve function in HIV infection: clinical, electrophysiologic, and laboratory findings. Arch Neurol. 1999;56:84–89. doi: 10.1001/archneur.56.1.84. [DOI] [PubMed] [Google Scholar]
- 43.Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank. Arch Neurol. 2004;61:546–551. doi: 10.1001/archneur.61.4.546. [DOI] [PubMed] [Google Scholar]
- 44.Schifitto G, McDermott MP, McArthur JC, et al. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 45.Fellows RP, Byrd DA, Elliott K, et al. Distal sensory polyneuropathy is associated with neuropsychological test performance among persons with HIV. J Int Neuropsychol Soc. 2012;18:898–907. doi: 10.1017/S1355617712000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Kitch DW, Evans SR, et al. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 2007;68:2113–2119. doi: 10.1212/01.wnl.0000264888.87918.a1. [DOI] [PubMed] [Google Scholar]
- 47.Keswani SC, Jack C, Zhou C, et al. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace VC, Blackbeard J, Segerdahl AR, et al. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130:2688–702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brittain JM, Duarte DB, Wilson SM, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat Med. 2011;17:822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ripsch MS, Ballard CJ, Khanna M, et al. A peptide uncoupling CRMP-2 from the presynaptic CA(2+) channel complex demonstrates efficacy in animal models of migraine and AIDS therapy-induced neuropathy. Transl Neurosci. 2012;3:1–8. doi: 10.2478/s13380-012-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Phillips TJ, Cherry CL, Cox S, et al. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PloS One. 2010;5:e14433. doi: 10.1371/journal.pone.0014433. [The meta-analysis section of this paper describes the few published clinical trials of medications that are commonly prescribed for HIV-associated painful neuropathies.] [DOI] [PMC free article] [PubMed] [Google Scholar]