Abstract
Distributions of oxygen concentration (pO2) are a critical determinant of normal tissue health as well as tumor aggressiveness and response to therapy. A number of studies show the value of normal tissue and tumor tissue oxygenation images and some of these will be discussed here. A strong correlation between tumor hypoxic fraction as measured with electron paramagnetic resonance oxygen imaging and radiation treatment success or failure has been found in 2 separate cancer types. Oxygen images of the torso of wild type mice show initial reduction of lung, liver, visceral, and muscle pO2 with cyclic halving of fraction of inspired oxygen (FiO2), but variation is blunted over an hour. Spontaneous breast cancers in Mouse Mammary Tumor Viral (MMTV) promoted-polyoma middle T antigen (PyMT) mice with BNIP3, a major factor in promotion of mitochondrial autophagy, knocked out will be compared with wild type animals. Preliminary studies for the BNIP3 knock out animals show extremely low pO2. The wide variety of studies, in which oxygen images can play an integral role, serve to demonstrate the importance of oxygen images.
1 Introduction
In vivo oxygen concentration (pO2) has been found to be crucially important in determining normal tissue health as well as the aggressiveness of tumors and their response to various forms of treatment1–4. Due to the ubiquitous influence of tissue pO2 various methods for measuring and/or imaging pO2 have been developed5–8.
One such method is electron paramagnetic resonance (EPR) oxygen imaging (EPROI). EPROI is a particularly robust method of imaging in vivo tissue pO2 distributions for several reasons. EPROI provides full 3D images of pO2. These images have good spatial resolution (~1 mm3 voxels) as well as pO2 resolution (1–3 torr). The low electromagnetic wave excitation frequencies (e.g., 250 MHz) currently used in EPRI are comparable to those used in 6T whole body MRI and can penetrate deep in tissue (>7cm) in animals as large as humans. EPROI images are obtained non-invasively, which means they can be used to study in vivo pO2 distributions without perturbing the system. EPROI requires an intravenously injected, non-toxic spin probe, which distributes in the extracellular compartment of tumors, to report local pO29. The accuracy of EPROI oxygen measurements has been established by correlating with well-established optical fiber based oxygen measurement techniques10.
The information provided by EPROI can be applied to help study myriad interesting oxygen related biologic and physiologic topics. A number of studies demonstrating the array of interesting applications of 3D EPR oxygen images of normal tissue and/or tumor tissue will be presented here.
2 Methods
EPROI is used to non-invasively determine the effect of tumor pO2 on success of tumor treatment with radiation therapy. Two cancer models are used: fibrosarcoma (FSa) and murine mammary MCa4 carcinoma. Fraction of EPROI voxels with less than 10 torr pO2 (HF10) is used as a measure of tumor hypoxia. The variable HF10 is then correlated to success or failure of radiotherapy to see what role, if any, hypoxia as determined by EPROI plays in tumor resistance to treatment.
EPROI will be used to provide insight into the effect of fraction of inspired oxygen (FiO2) changes on the distribution of pO2 in various organs of mice. The result of cyclic FiO2 variation can also be observed in various organs using EPROI. This will enable simulation of important disorders such as sleep apnea so that their biologic effects may be studied.
To examine the role of BNIP3 in tumor progression and regulation of oxygenation, changes in pO2 levels and distributions in mouse mammary tumors are compared for BNIP3 null mice that have been crossed to the Mouse Mammary Tumor Viral (MMTV) promoted-polyoma middle T antigen (PyMT) mouse model of breast cancer and wild type mice. EPROI images are registered with anatomic CT images to analyse differences in oxygenation within these tumors.
3 Results
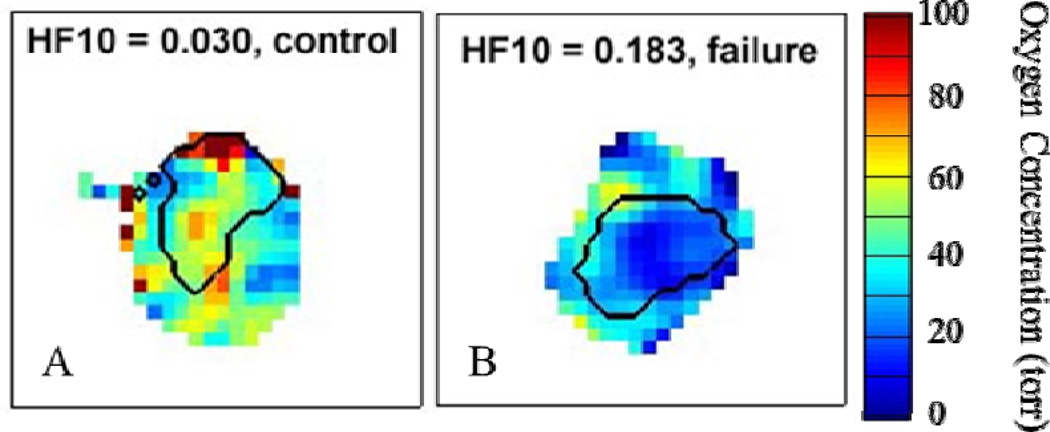
Using two cancer models (FSa and MCa4), we found that EPROI corroborates the theory that tumors exhibiting a higher degree of hypoxia tend to be more resistant to radiation therapy. A cohort of animals with either of the two tumor lines were treated to the previously determined 50% tumor control dose (TCD50) for each tumor type. The HF10 as determined by EPROI for each tumor was correlated with radiation therapy treatment outcome. For the FSa tumors, hypoxic tumors (HF10 > 10%) 37% were successfully controlled while for tumors with HF10 < 10%, 90% were successfully controlled (p=0.0138)11,12. For the MCa4 tumors, hypoxic tumors (HF10 > 10%) 23% were successfully controlled while for tumors with HF10 < 10%, 90% were successfully controlled (p=0.0072)12. An example of the dramatic oxygenation difference observed in animals whose tumors were successfully controlled with radiotherapy versus those for which radiotherapy failed is shown in Fig. 1.
Fig. 1.
Sample slices from representative EPROI of mouse legs bearing tumors (black outline) demonstrating the difference in tumor o observed in animals where treatment with radiation therapy eventually (A) successfully controlled the tumor or (B) failed to control the tumor.
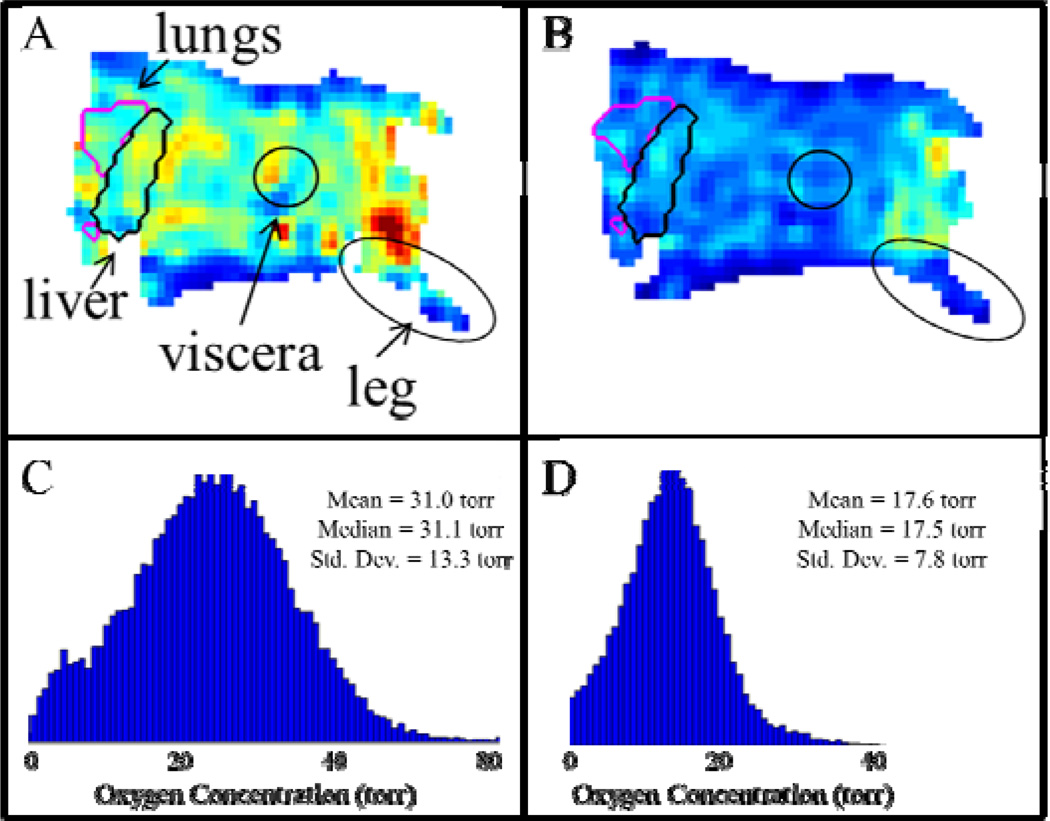
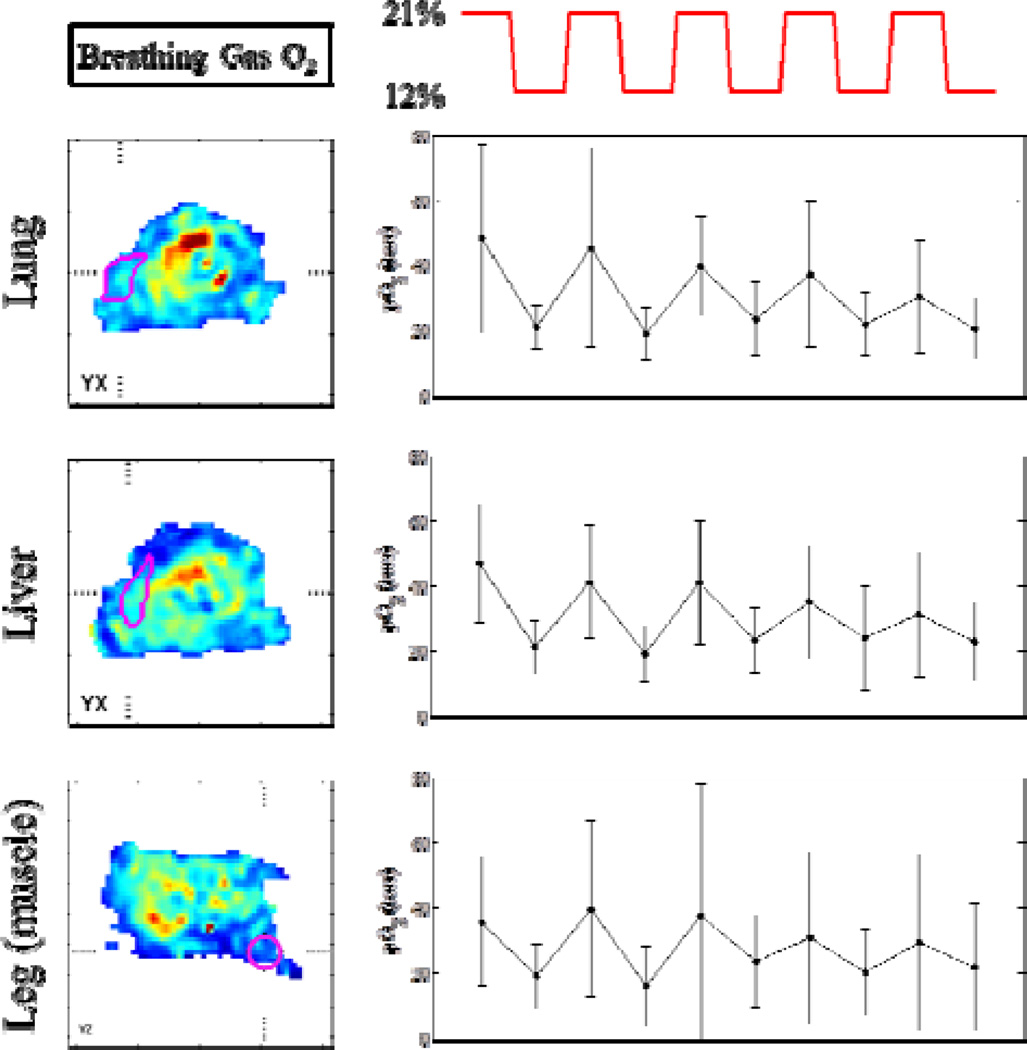
Significant differences in the overall oxygenation of a mouse breathing air versus a mouse breathing 12% O2 are seen in whole-body EPROI (Fig. 2). Such whole-body EPROI also allows for eventual analysis of oxygenation changes in response to FiO2 changes in specific organs. It is seen that significant variations in tissue pO2 are observed with oscillating FiO2, however the response becomes damped over time (Fig. 3).
Fig. 2.
Demonstration of the overall effect on the tissue pO2 of a mouse when the breathing gas oxygen content is changed from 21% O2 to 12% O2 as measured with EPROI. Whole body EPROI of a mouse breathing (A) 21% O2 and (B) 12% O2, with labeled regions of interest. The pO2 distributions for the whole body EPROI when the mouse is breathing (C) 21% O2 and (D) 12% O2.
Fig. 3.
Slices from an EPROI of a mouse are shown with regions of interest outlined in magenta. The breathing gas oxygenation for the mouse alternates from 21% O2 to 12% O2 (breathing gas O2 changes shown in red line). Next to each image with a particular region of interest (lung, liver, or leg muscle) the change in mean oxygenation within that region of interest in response to the breathing gas change is shown.
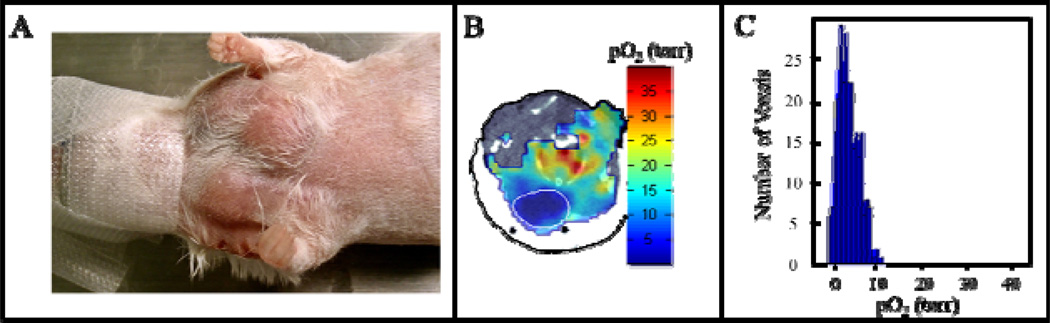
Preliminary studies of BNIP3 knock out (KO) mice show extremely hypoxic breast tumors (Fig.4). This is consistent with the fact that BNIP3 null tumor cells show increased invasive properties, suggesting that BNIP3 is a metastasis suppressor required to maintain mitochondrial integrity and mitigate against the metastasis promoting activities of reactive oxygen and hypoxia13.
Fig. 4.
(A) Photograph of MMTV-PyMT, BNIP3 null breast cancer tumors grown in the breast of a mouse. (B) Registered EPROI overlaid on an anatomical CT of the mouse shown, with the tumor outlined in white. (C) Distribution of pO2 found within the BNIP3 null tumor demonstrating that the tumor is extremely hypoxic.
4 Discussion and Conclusions
There are many areas of biology in which oxygenation plays a crucial role. Therefore we can learn a great deal from the oxygen images provided by EPROI. This is evidenced by the successful application of EPROI for studying interesting biology related to both cancer and general oxygen homeostasis.
The hypoxic fraction of a tumor, as determined by EPROI, has been found to be quite a powerful determinant in the eventual outcome of treatment with radiation. This validates EPROI as a tool to analyze spatial distributions of pO2 in vivo.
In preliminary studies EPROI has also proven to be a valuable tool for tracking tissue pO2 response to changes in FiO2. EPROI therefore has the potential to enhance studies investigating the biologic consequences of temporally fluctuating tissue oxygenations (e.g., sleep apnea conditions) by allowing noninvasive tracking the response to FiO2 changes of whole body pO2 as well as pO2 of individual organs and how this response changes over time.
EPR oxygen images can also help investigate the complicated effects of hypoxia on several aspects of tumor and tissue development. This is evidenced by preliminary results from a study investigating the effect of the BNIP3 protein, which limits production of reactive oxygen species by promoting mitochondrial degradation at the autophagosome, on tumor oxygenation. Initial results show that tumors in BNIP3 KO mice are extremely hypoxic, which may be due to dysfunctional mitochondria. EPROI will help in further studies to investigate the effect of BNIP3 on oxygenation as well as radiation resistance.
In general, oxygen images from EPROI provide an important tool in understanding the relationship between microenvironment oxygenation and a wide variety of crucial physiologic functions.
Acknowledgments
Supported by NIH grants P41 EB002034 and R01 CA98575.
References
- 1.Hockel M, Schlenger K, Aral B, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 2.Shannon AM, Bouchier-Hayes DJ, Condron CM, et al. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 4.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 5.Dewhirst MW, Klitzman B, Braun RD, et al. Review of methods used to study oxygen transport at the microcirculatory level. Int J Cancer. 2000;90:237–255. [PubMed] [Google Scholar]
- 6.Zhao DW, Jiang L, Mason RP. Measuring changes in tumor oxygenation. Methods Enzymol. 2004;386:378–418. doi: 10.1016/S0076-6879(04)86018-X. [DOI] [PubMed] [Google Scholar]
- 7.Tatum JL. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Rad Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 8.Bayer C, Vaupel P. Acute versus chronic hypoxia in tumors: controversial data concerning time frames and biological consequences. Strahlenther Onkol. 2012;188:616–627. doi: 10.1007/s00066-012-0085-4. [DOI] [PubMed] [Google Scholar]
- 9.Golman K, Petersson JS, Ardenkjaer-Larsen JH, et al. Dynamic in vivo oxymetry using overhauser enhanced MR imaging. J Magn Reson Imaging. 2000;12:929–938. doi: 10.1002/1522-2586(200012)12:6<929::aid-jmri17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Elas M, Ahn KH, Parasca A, et al. Electron paramagnetic resonance oxygen images correlate spatially and quantitatively with Oxylite oxygen measurements. Clin Cancer Res. 2006;12:4209–4217. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]
- 11.Elas M, Bell R, Hleihel D, et al. Electron paramagnetic resonance oxygen image hypoxic fraction plus radiation dose strongly correlates with tumor cure in FSa fibrosarcomas. Int J Radiat Oncol Biol Phys. 2008;71:542–549. doi: 10.1016/j.ijrobp.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elas M, Magwood JM, Butler B, et al. EPR oxygen images predict tumor control by a 50 percent tumor control radiation dose. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0069. (on line and in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracy K, Macleod KF. Regulation of mitochondrial integrity, autophagy and cell survival by BNIP3. Autophagy. 2007;3(6):616–619. doi: 10.4161/auto.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]