Introduction
The treatment of hematological malignancies by harnessing immune responses has long been pursued. It is well accepted that the success of allogeneic hematopoietic stem cell transplantation (HSCT) as a treatment for hematological malignancies is due primarily to immunologic recognition and elimination of recipient leukemia cells by donor T cells, the so-called graft versus leukemia (GVL) effect. Based on the discovery and identification of leukemia antigens, it is now possible to target leukemia either by specific vaccination or adoptive transfer of in vitro generated anti-leukemia cytotoxic lymphocytes (CTLs). Leukemia antigens can be categorized broadly into 3 classes: (1) ubiquitously expressed alloantigens, also known as minor histocompatibility antigens (mHags), widely expressed by normal tissues in the recipient as well as by leukemia cells, and capable of initiating both GVHD and GVL responses; (2) alloantigens expressed uniquely by cells of the hematopoietic system (tissue-restricted mHags) such as HA-1 and HA-2; and (3) leukemia antigens, including leukemia-specific antigens such as BCR-ABL in Philadelphia-chromosome–positive leukemia and over- or aberrantly expressed leukemia-associated antigens (LAAs) such as proteinase 3 (PR3), Wilms tumor 1 (WT1) and the preferentially expressed antigen of melanoma (PRAME). A number of studies have shown a temporal inverse relationship between circulating T cells directed against mHags or LAAs and minimal residual disease in patients with acute and chronic leukemia after allogeneic HSCT, supporting a role for these antigens in the GVL response.1,2
This review will encompass a bench to bedside approach evaluating strategies for active induction or passive transfer of tumor-specific T cells in patients with hematological malignancies.
Post-Transplant Vaccination in Leukemia
Several different strategies of vaccination against leukemia have been tried, including delivery of specific antigens with peptide, protein, DNA or RNA vaccines, or induction of non-specific antileukemic responses using leukemic dendritic cells (DCs), and leukemia cells engineered to secrete GM-CSF. These approaches, while eliciting convincing anti-leukemia immune responses, have only led to anecdotal clinical responses.3-6
A major limitation of the various vaccine approaches is related to the fact that most defined leukemia antigens are products of normal genes overexpressed or selectively expressed in leukemia cells. The immune system is finely balanced to distinguish foreign from self antigens. In effect, cancer vaccination aims to break tolerance to self and elicit an ‘autoimmune’ response. Thus, one of the major hurdles for effective vaccination is to overcome the central and peripheral tolerance to these self antigens. The existing T-cell repertoire specific for self-antigens is limited to low avidity T cells with limited recognition of endogenously processed leukemia antigens7. Nevertheless, vaccination can be effective even though the response is limited to low avidity CTLs8. Attempts have been made to create more immunogenic antigens by molecular manipulation. By inserting an amino acid change in the peptide epitope, it is possible to produce an antigen that binds more strongly to the relevant HLA molecule and therefore stands a higher chance of breaking tolerance against self-proteins9. Vaccination of patients with hematological malignancies with modified HLA class I and class II epitopes from the self antigen WT1 has been shown to induce immune responses associated with evidence of clinical response in some cases.4,5
Stem cell transplantation and adoptive immunotherapy
The intersection of SCT and more specific immunotherapy based on the knowledge of defined antigens offers exciting opportunities to develop novel therapeutic approaches. The profoundly lymphopenic environment immediately after transplantation provides a favorable milieu for rapid and extensive lymphocyte expansion and facilitates immune responses to weak self-antigens (reviewed in10). The lymphopenic environment allows strong expansion of antitumor T cells in the presence of cytokines responsible for thymic-independent homeostatic T-cell proliferation, such as IL-7, IL-15, and IL-21. In addition to eradicating cells that may suppress antitumor responses, such as regulatory T cells, myeloid derived suppressor cells (MDSCs) and tumor-associated macrophages, lymphoid reconstitution of either donor or host origin may overcome inherent defects in T-cell signaling, processing, or presentation and may strengthen the costimulatory functions of APCs. Because reconstitution of the T-cell compartment in lymphopenic hosts is regulated by peptides occupying MHC class I and II molecules, there may be an opportunity to skew the T-cell repertoire at the time of T-cell recovery by engaging the available MHC class I and class II molecules with antigens of particular interest. These observations imply that the first few months after transplantation offer a unique environment for delivering GVL directed against both leukemia-associated antigens and mHags expressed by vaccination.
An alternative approach to SCT may be the combination of adoptive cell transfer with vaccination. In this setting, patients can be treated with lymphodepleting therapies to eliminate immunosuppressive cells and other lymphoid cells that compete for T-cell growth factors, such as IL-7 and IL-15. The success of this approach was shown in seminal work by Dudley and colleagues where lymphodepletion was found to be critical to the success of tumor-infiltrating lymphocyte transfer in the treatment of melanoma.11 The adoptively transferred T cells could be primed against leukemia in vivo by vaccinating the patient (or the donor in the setting of allo-SCT) which could then be collected and infused following chemotherapy as described by June and colleagues12, or they could be genetically modified in vitro or expanded ex-vivo prior to adoptive transfer.
Conclusions
Allogeneic HSCT continues to play a unique role in achieving cure of hematological malignancies. The vigorous homeostatic proliferation of donor T cells after HSCT may represent a hitherto under-utilized window of opportunity for immunotherapy. It is likely that a multifaceted approach to immunotherapy involving HSCT, adoptive T-cell transfer, and vaccination will be the next step toward effective immunotherapy.
AUTO-IMMUNOTRANSPLANTATION FOR LYMPHOMA
Introduction
We have developed a strategy of therapeutic vaccination against lymphoma that we interdigitate with conventional autologous hematopoietic cell transplantation (autoHCT). Autologous tumor cells are activated ex vivo with a TLR9 ligand (CpG oligonucleotide), irradiated, and then used as a vaccine to induce a T cell immune response in the patient against the tumor. The immune T cells are collected and then re-infused into the patient immediately after autoHCT. The goal is to allow the T cells to expand in the patient during the period of immunologic recovery and to mediate immune rejection of residual tumor. We refer to this maneuver as immunotransplantation.
This strategy has a number of important features- Simplicity: No tumor antigens need be identified in advance; instead the whole tumor cell is the vaccine. The activation step employs an “off the shelf”, chemically defined substance (CpG oligonucleotide) with a track record of safety and effectiveness as an immune stimulant in lymphoma13-15. Feasibility: We have now performed the entire treatment strategy under our IND without adverse effects. Homeostatic T cell proliferation: We have shown in preclinical models that during the immediate post transplantation period adoptively transferred T cells expand and that T effector cells preferentially do so over T regulatory cells, resulting in powerful therapeutic effects.
Phase I/II study of immunotransplantation for patients with mantle cell lymphoma
We have initiated a clinical trial for patients newly diagnosed with mantle cell lymphoma and have accrued 35 patients during the first 3 years. Sixteen of these patients have completed the entire treatment program. We have a defined target of freedom from MRD at one year of greater than or equal to 85%. We developed a sequencing-based platform for MRD quantification in lymphoid malignancies. Using universal primer sets, we amplify rearranged IgH variable (V), diversity, and joining (J) gene segments from genomic DNA. To minimize the chance of somatic hypermutation interfering with detection of a cancer sequence, each IgH sequence is amplified by different sets of multiplex PCR primers in the three framework regions of the V segments and a common J segment primer. Amplified products can be sequenced to obtain >1 million reads and are analyzed using algorithms for clonotype determination. Tumor-specific clonotypes are identified for each patient based on their high frequency in the original tumor specimen. Quantitative MRD levels are then determined in serial samples of peripheral blood using spiked-in reference sequences. Our test has a sensitivity of 1 tumor cell per million leukocytes. This technology has also been extended to immunoglobulin light chain and T cell receptors. To quantify T cell responses to tumor vaccinations, the same samples are used for amplification, sequencing and analysis of the entire TCRB repertoire allowing for the assessment of T cell immune responses to the vaccine.
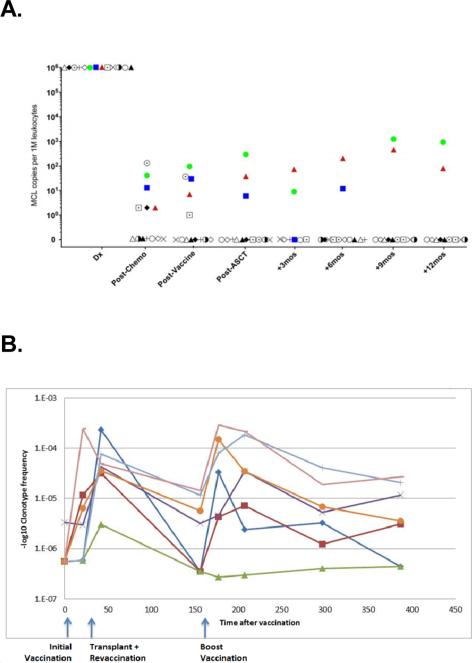
To date we have analyzed serial samples from 13 MCL patients who received the protocol of induction chemotherapy, vaccination, and HCT followed by vaccine primed T cell infusions and booster vaccinations. MRD was assayed in blood samples immediately post-transplant in 3 patients, 2 of whom ultimately relapsed (Fig 1A). In these two cases, disease was detected by sequencing 14 months and 4.5 months prior to detection by radiologic techniques. Clinical relapse in the second patient was originally restricted to the CNS; however, at a later point lymph node relapse occurred. The third patient has not shown signs of clinical relapse. The remaining 10 patients were MRD negative at 1 year following transplant, and these patients have not shown signs of clinical relapse with a median follow up of >24 months. To identify T cells specific to the vaccination, we searched for clonotypes that were highly enriched (>10X) in an in vitro tumor-stimulated culture. In 2 of 3 patients assayed, we identified such clonotypes. The enrichment of these same clonotypes was also seen in the patients after vaccinations and boosters, adding to the evidence that they are directed against the vaccine. An example for one such patient is shown in Fig. 1B. These clonotypes increased in frequency significantly after the boost by comparison to a set of frequency-matched unrelated clonotypes (p<2.5× 10−6). Figure 1B also shows the dynamics of these clonotypes through the course of treatment demonstrating frequency increases upon initial and booster vaccinations.
Fig 1. A. Assessment of vaccine response by molecular techniques.
LymphoSIGHT MRD monitoring in MCL patients. (B) Frequency and dynamics of vaccine-specific clonotypes over the course of treatment.
Conclusions
We conclude that our auto-immunotransplant procedure shows promise in MCL patients. Using a high throughput sequencing method for MRD, 77% of patients (10/13) were negative at the landmark of 1 year post-transplant. Continued follow-up for molecular and clinical relapse is ongoing. T cell repertoire analysis identified clonotypes responding to the vaccination in some patients and follow up analyses will determine whether the presence of these clonotypes correlates with clinical outcomes in MCL patients.
T CELL ADOPTIVE IMMUNOTHERAPY OF HEMATOLOGICAL MALIGNANCY
Introduction
The impetus to develop T cell based cell therapy for treatment of hematologic malignancies comes from the well-grounded observation that allogeneic T-lymphocytes are capable of potent graft-versus-leukemia (GVL) effects in stem cell transplant (SCT) recipients (reviewed16). Although murine studies demonstrated in the 1950's that a GVL effect could be induced after bone marrow transplantation the reality of a potent GVL effect in man first achieved general acceptance after two critical publications. The first from the IBMTR showed in a large patient population that relapse after SCT was lowest in recipients of T replete as opposed to T cell depleted SCT and was least when T replete SCT were accompanied by both acute and chronic GVHD. The second observation from Kolb and colleagues showed that infusions of donor lymphocytes achieved durable remissions in CML patients who had relapsed after SCT. In the 1990s attempts were made to refine T cell therapy for the treatment and prevention of relapse. Studies showed some separation of GVHD and GVL when CD4 T cells were used as DLI. Combining DLI with interferon-alpha appeared to provide some advantage in relapse of chronic myelogenous leukemia (CML). Other investigators reported some efficacy of using DLI with GMCSF as a means of upregulating antigen presentation by leukemia cells. In Milan, Bonini and colleagues inserted a suicide gene into T cells destined for DLI in order to eliminate GVHD17. Other groups explored the preemptive depletion of alloreacting T cells from the graft inoculum18. Falkenburg was the first to demonstrate that CTL generated by repeatedly stimulating donor lymphocytes with CML cells could be used to treat a patient with relapsing leukemia after SCT. A patients relapsing after transplant who had residual chronic myelogenous leukemia disease standard DLI received three infusions of T cell lines generated in vitro using patients leukemia with prompt and persisting eradication of leukemia 19. In recent years the technology to select and expand leukemia antigen specific T cells has improved to a level where clinical grade CD4 and CD8 T cells can be created recognizing either minor histocompatibility antigens20 or leukemia associated antigens (LAA)21. To enhance cytotoxicity of leukemia specific T cells three strategies have been developed to redirect T cells using genetically inserted high avidity leukemia-specific T cell receptors22, or targeting the malignant cell with a monoclonal antibody attached either to a CD3 molecule (bispecific antibodies)23 or as a chimeric molecule triggering T cell activation24 (Table 1). This review summarizes the essential biology of T cell-leukemia interactions, the techniques used to generate leukemia reactive T cells for the clinic and the main challenges facing T cell therapy for hematological malignancies.
Table 1.
Approaches used to generate leukemia-specific cytotoxic T cells in SCT patients
| Strategy | Technique |
|---|---|
| Non-antigen defined | |
| Negative selection of GVHD reacting T cells | Suicide gene insertion |
| Selective allodepletion | |
| Generation of leukemia reactive T cells | Leukemic APC for culture expansion |
| Marrow infiltrating lymphocytes | |
| Antigen defined | |
| Expansion of minor antigen-specific T cells | MHC class I mHA |
| MHC class II mHA | |
| Expansion of leukemia antigen specific T cells | LAA peptides |
| Gene insertion into DC | |
| Antigen modified T cells | |
| Gene modified | CD19 Chimeric antigen receptors |
| TCR gene insertion | gene insertion into carrier T cell |
| Bispecific antibodies | CD19/CD3 or CD22 /CD3 antibody |
Challenges to the effectiveness of adoptive T cell transfer
Persistence and function in vivo
In recent years we have gained a much better understanding of the dynamics and functional segregation of the post-thymic T cell compartment which for therapeutic purposes can be considered as segregated into “early” T cells with great self-renewal potential and longevity and “late” end effector T cells which are cytotoxic but have a very limited survival. T cell cultures tend to senesce into end stage effectors rendering T cell infusions short-lived ineffective. By selecting “early” cells for expansion (CD45 RA naïve cells, or CD62+ CD27+ CD57- central memory cells), modifying culture conditions and reducing the length of in vitro expansion it is possible to create much longer lived cell products. Another important principle learned from the transfer of virus-specific T cells is the benefit of generating both CD4 and CD8 T cell lines to provide CD4 help which increases the persistence of CD8 cells and provide additional cytotoxicity from cytotoxic CD4 cells including the highly tumor reactive Th17 phenotype. Much remains to be learned about the conditions governing survival distribution and effector function of adoptively transferred CTL in vivo.
The recipient milieu
Of equal importance is the milieu into which therapeutic T cells are transferred. Numerous studies demonstrate that delivery of T cells into a lymphopenic environment enhances their in vivo expansion and persistence through the release of lymphokines which include IL-15. Ideally T cells should be transferred into a non-immunosuppressed recipient. Steroid therapy carries the risk of ablating infused T cells, however it is not clear how much immune function is blunted by calcineurin inhibitors. Regulatory T cells can also be anticipated to reduce the potency of adoptively transferred T cells. Although antigen-specific T cells appear to home to the bone marrow little is known about the distribution of CTL to extramedullary sites which are notable sites of relapse after allogeneic SCT. In tumor immunology and in lymphomas and myeloma much progress has been made in characterizing features of the tumor microenvironment which nurture malignant cells and protect them from immune attack, however less is known about the role of the marrow microenvironment in sheltering leukemia from immune attack. More studies are required in this area.
Immune escape and strategies to overcome it
Tumor cells have developed various mechanisms to escape from the host's immune system and are also able to overcome chemo- or immunotherapeutic therapies making them ineffective in the treatment and eradication of leukemia cells. A major obstacle for adoptive T cell therapy is the ability of malignant cells to change expression of antigens by down-regulation mechanisms due to selective pressure or selective elimination of cells which express the targeted antigen. AML cells can down-regulate MHC expression notably after haploidentical SCT when the relapsed leukemia loses expression of the entire mismatched MHC haplotype25. Leukemia can also evade T cell attack by becoming resistant to perforin and granzyme, preventing immune synapse formation and downregulating tumor antigen expression. Function of adoptively transferred T cells may be improved by gene modification strategies (reviewed in 22,26).
Moving from the ivory tower into the marketplace26
Cell therapies in general and adoptive T cell therapy in particular present unique challenges that discourage up corporate efforts to develop clinical grade cell products. The main blocks to the wider application of adoptive T cell therapy and some of the solutions to the impasse are summarized below:
Implementation
One of the immediate challenges is to move forward with T cell therapy beyond small case series illustrating proof of principle to larger phase II and phase III trials. If clinical trials with leukemia specific T cells can show similar success to the use of adoptive T cell therapy for virus infections after SCT of the striking success of CAR cells in CLL and show cost effectiveness over existing treatments, we can anticipate greater enthusiasm for T cell therapy. In turn this would do much to bridge the gap (the “valley of death” described by Malcolm Brenner) between the initial development of sophisticated cell therapies in ivory tower academic institutions to the uptake of the concept by manufacturers capable of widely disseminating the cell product.
“Boutique” therapy
many of the strategies being developed require the creation of a unique product from a donor for a specific patient. generalizability is limited by HLA restriction and the specific array of antigens present in the malignancy. Approaches that may overcome these constraints are the development of multiantigen specific T cells targeting common LAA and conferring broad applicability across many malignancies; use of “off the shelf” third party t cells partially HLA matched with the recipient similar to 3rd party virus specific T cells which have proven efficacy. The insertion of a TCR into the donor T cell and the redirection of T cells through TCR insertion, use of bispecific antibodies or chimeric antigen receptors also represent useful ways to generalize T cell therapy.
Quicker simpler safer cell production
Culture approaches have improved considerably in recent years because conditions have been optimized (see above). However cells are typically manufactured in GMP facilities, expensive to construct and operate and restricting the on site production of antigen-specific or modified T cells to a few centers in the country. One successful commercial model is to set up a central cell-factory which takes leukapheresis collections from patients in the hospital and ships the manufactured T cell product back to the users (cf the dendritic cell approach used in the product sipuleucil- T) Alternatively in the case of 3rd party T cells the product can be made commercially and delivered “off the shelf” to the users. Ultimately the greatest flexibility would come from the creation of complete benchtop cell factories, where donor cells are infused into “black boxes” capable of cell selection, antigen-stimulation, culture expansion and product delivery, whose GMP grade internal workings are separated from the environment, replacing the need for a classical GMP unit. While working devices that could be operated in any blood bank are some way off a number of commercial manufacturers are moving towards this goal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezvani K, Yong AS, Savani BN, Mielke S, Keyvanfar K, Gostick E, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood. 2007;110:1924–1932. doi: 10.1182/blood-2007-03-076844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslak PG, Dao T, Krug LM, Chanel S, Korontsvit T, Zakhaleva V, et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116:171–179. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113:6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 7.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 8.Rezvani K, Yong AS, Mielke S, Jafarpour B, Savani BN, Le RQ, et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96:432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett J, Rezvani K. Immunotherapy: Can we include vaccines with stem-cell transplantation? Nat Rev Clin Oncol. 2009;6:503–505. doi: 10.1038/nrclinonc.2009.115. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat. Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 13.Brody JD, Goldstein MJ, Czerwinski DK, Levy R. Immunotransplantation preferentially expands T-effector cells over T-regulatory cells and cures large lymphoma tumors. Blood. 2009;113:85–94. doi: 10.1182/blood-2008-05-155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J. Clin. Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein MJ, Varghese B, Brody JD, Rajapaksa R, Kohrt H, Czerwinski DK, et al. A CpG-loaded tumor cell vaccine induces antitumor CD4+ T cells that are effective in adoptive therapy for large and established tumors. Blood. 2011;117:118–127. doi: 10.1182/blood-2010-06-288456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett AJ. Understanding and harnessing the graft-versus-leukaemia effect. Br. J. Haematol. 2008;142:877–888. doi: 10.1111/j.1365-2141.2008.07260.x. [DOI] [PubMed] [Google Scholar]
- 17.Lupo-Stanghellini MT, Provasi E, Bondanza A, Ciceri F, Bordignon C, Bonini C. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum. Gene Ther. 2010;21:241–250. doi: 10.1089/hum.2010.014. [DOI] [PubMed] [Google Scholar]
- 18.Mielke S, Solomon SR, Barrett AJ. Selective depletion strategies in allogeneic stem cell transplantation. Cytotherapy. 2005;7:109–115. doi: 10.1080/14653240510018172. [DOI] [PubMed] [Google Scholar]
- 19.Falkenburg JH, Wafelman AR, Joosten P, Smit WM, van Bergen CA, Bongaerts R, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94:1201–1208. [PubMed] [Google Scholar]
- 20.Bleakley M, Riddell SR. Exploiting T cells specific for human minor histocompatibility antigens for therapy of leukemia. Immunol. Cell Biol. 2011;89:396–407. doi: 10.1038/icb.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdemann U, Katari U, Christin AS, Cruz CR, Tripic T, Rousseau A, et al. Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma. Mol. Ther. 2011;19:2258–2268. doi: 10.1038/mt.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwu P, Rosenberg SA. The genetic modification of T cells for cancer therapy: an overview of laboratory and clinical trials. Cancer Detect. Prev. 1994;18:43–50. [PubMed] [Google Scholar]
- 23.Nagorsen D, Bargou R, Ruttinger D, Kufer P, Baeuerle PA, Zugmaier G. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk. Lymphoma. 2009;50:886–891. doi: 10.1080/10428190902943077. [DOI] [PubMed] [Google Scholar]
- 24.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert. Opin. Biol. Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 26.Bonini C, Brenner MK, Heslop HE, Morgan RA. Genetic modification of T cells. Biol. Blood Marrow Transplant. 2011;17:S15–S20. doi: 10.1016/j.bbmt.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]