Abstract
Progressive renal fibrosis is common to all chronic kidney diseases (CKD). Suthanthiran and colleagues identified a positive association between transforming growth factor-β1 and several risk factors for CKD progression in African Americans but not in whites. This study offers a possible explanation for the higher prevalence of end-stage renal disease in African Americans and highlights the need for a better therapeutic strategy for this population.
Regardless of the underlying etiology, chronic kidney disease (CKD) is characterized by progressive fibrosis and sclerosis that ultimately affect all substructures of the kidney. Epidemiologic studies have identified several variables that can predict CKD outcomes and may therefore be considered risk factors for CKD progression. These factors include African-American race, proteinuria, high blood pressure, high dietary protein intake, obesity, anemia, dyslipidemia, and smoking. In comparison with whites, African Americans and Native Americans have 3.6 and 1.8 times higher incidences of end-stage renal disease (ESRD), respectively.1 Furthermore, African-American race was independently associated with a greater rate of decline in glomerular filtration rate in the modification of Diet in Renal Disease Study.2 The incident rate of ESRD in the Hispanic population is 1.5 times greater than that in non-Hispanics.1 Although mechanisms underlying such racial differences remain to be elucidated, possible explanations include an increased prevalence of diabetes mellitus, lower nephron number, increased susceptibility to salt-sensitive hypertension, yet- undiscovered genetic factors, and differences in environment, lifestyle, and socioeconomic status.3
Identifying treatable risk factors for CKD in African Americans has the potential to reduce the excess burden of ESRD in this population. Because renal fibrosis is associated with CKD progression and transforming growth factor- β1 (TGF- β1) likely facilitates this fibrosis, Suthanthiran and colleagues explored the hypothesis that TGF-β1 overexpression occurs more frequently in African Americans with ESRD than in whites suffering from the same disease. This group has previously reported that African Americans with hypertension and ESRD exhibit higher circulating levels of TGF-β1when compared with whites.4 – 6 In this issue of Kidney International,7 Suthanthiran and colleagues dig a little bit deeper, using a cross-sectional study to further examine the impact of TGF-β1 on ESRD.
TGF-β1 is a well-studied profibrogenic cytokine that likely plays an important role in the process of CKD progression, and blockade of this cytokine in experimental models prevents progression of CKD.8 – 10 The role of the renin – angiotensin – aldosterone system (RAAS) in the progression of CKD has also been the focus of much investigation. Many experimental studies have shown bidirectional activities of TGF-β and the RAAS: renin or angiotensin II induces TGF-β activation,11,12 and TGF-β1 also stimulates angiotensin II.13,14
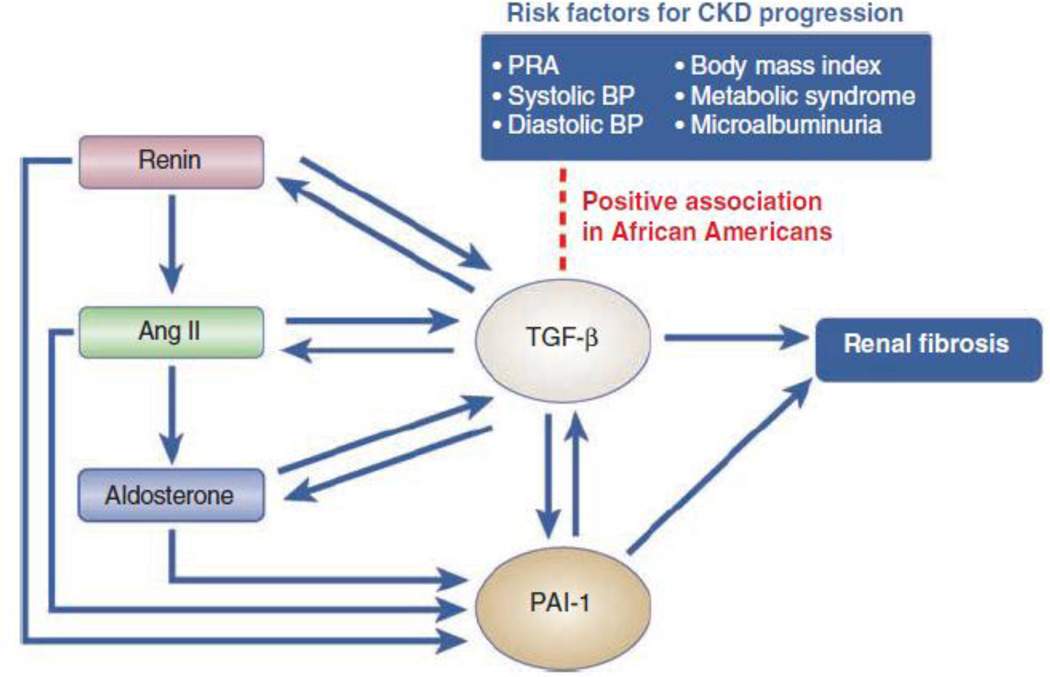
Suthanthiran and colleagues7 report a positive association between the circulating concentration of TGF-β1 and a variety of established risk factors for CKD progression (Figure 1). Peripheral blood TGF-β1 protein levels are positively associated with plasma renin activity (PRA), systolic blood pressure, diastolic blood pressure, body mass index (BMI), and the presence of metabolic syndrome in African Americans but not in whites. TGF-β1 protein levels are also predictive of microalbuminuria in African Americans when compared with whites. These results suggest that TGF-β1 may contribute to renal disease progression by modulating multiple risk factors more robustly in African Americans. Such different degrees of association between TGF-β1 and risk factors for CKD in African Americans and whites may explain the greater incidence of ESRD in African Americans.
Figure 1. Association between risk factors for CKD progression and circulating TGF-β1 levels in the context of the renin-angiotensin-aldosterone system and the plasminogen activator inhibitor-1 system.
This schematic highlights the positive association between TGF-β1 levels and plasma renin activity (PRA), systolic blood pressure (BP), diastolic BP, body mass index, metabolic syndrome, and microalbuminuria. The arrows denote stimulatory effects. Ang II, angiotensin II; PAI-1, plasminogen activator inhibitor-1.
The new report by Suthanthiran and colleagues7 is the first clinical study demonstrating a positive relationship between TGF-β1 and the RAAS in humans. It is now well established that inhibition of the RAAS with angiotensin-converting enzyme inhibitors (ACEIs) and / or AT1 receptor blockers (ARBs) has a beneficial effect on several disease categories, such as hypertension with or without end organ damage and progressive CKD, and that it is effective in preventing and treating diabetic nephropathy.
Although the cross-sectional nature of this study limits the ability to infer a causal relationship between TGF-β1 and the RAAS, the observed positive association and its limitation to African Americans are important. A well-designed cohort study could provide more clear evidence in the future and allow for further building on this provocative finding. Both RAAS inhibitors, ACEIs and ARBs, have been shown to reduce the production of TGF-β1 in experimental rodent models15,16 and in humans with CKD.17 In a prospective study of renal-transplant recipients, treatment with typical antihypertensive doses of the ARB losartan decreased plasma levels of TGF-b 1 within 2 weeks.17 Is it possible to infer from this evidence that inhibiting the RAAS in African Americans with CKD will prevent or slow the progression of their renal dysfunction by suppressing TGF-β1 production? The answer to this question is not a simple ‘yes.’ As Suthanthiran and colleagues7 point out, African Americans tend to exhibit salt volume – dependent hypertension with a lower level of PRA, and they show a lower sensitivity to RAAS inhibitors when compared with whites.18 Furthermore, a recent multicenter cohort study revealed that most African Americans with hypertensive CKD treated with RAAS inhibitors continued to exhibit disease progression in the long term.19 These findings suggest that RAAS inhibitors may not effectively reduce TGF-β1 in the African-American population. In this regard, it would be interesting to investigate whether there is an association between TGF-β1 and the various risk factors for CKD and how the effect of RAAS inhibitors on CKD progression is a function of PRA in the population with either lower or higher PRA, independent of race. Previous studies have shown that RAAS inhibitors can reduce TGF-β1 levels by a maximum of about 50 – 60% in experimental disease.14,17 Unfortunately, the data for human disease remain limited. The degree of TGF-β1 reduction in humans treated with an ACEI or an ARB remains undetermined. Can TGF-β1 levels be normalized to baseline, or are they only marginally affected by ACEI or ARB treatment? At present, clinical trials are investigating the utility of anti-TGF-β antibodies in idiopathic pulmonary fibrosis, focal segmental glomerulosclerosis, renal-cell carcinoma, and diabetic nephropathy.10 Anti-TGF-β therapy may combine well with ACEIs or ARBs in human kidney diseases such as diabetic nephropathy and focal segmental glomerulosclerosis, especially in the African-American population.
Acknowledgements
The work in the authors’ laboratory is funded by grants from the US National Institutes of Health (DK55001, DK62987, DK13193, DK61688), the PKD Foundation, and the research fund of the Division of Matrix Biology at the Beth Israel Deaconess Medical Center. Soo Bong Lee is funded by Pusan National University and Pusan National University Hospital, Pusan, Korea. We thank Sarah Flier for her critical reading and input on this manuscript.
Footnotes
Disclosure
The authors declared no competing interests.
References
- 1.United States Renal Data System. 2008 Annual Data Report. Bethesda, Maryland, USA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 2.Hunsicker L, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 3.Taal M, Brenner B. Renal risk scores: progress and prospects. Kidney Int. 2008;73:1216–1219. doi: 10.1038/ki.2008.36. [DOI] [PubMed] [Google Scholar]
- 4.Suthanthiran M, Khanna A, Cukran D, et al. Transforming growth factor-beta 1 hyperexpression in African American end-stage renal disease patients. Kidney Int. 1998;53:639–644. doi: 10.1046/j.1523-1755.1998.00858.x. [DOI] [PubMed] [Google Scholar]
- 5.Suthanthiran M, Li B, Song J, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: a novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci USA. 2000;97:3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.August P, Suthanthiran M. Transforming growth factor beta and progression of renal disease. Kidney Int Suppl. 2003:S99–S104. doi: 10.1046/j.1523-1755.64.s87.15.x. [DOI] [PubMed] [Google Scholar]
- 7.Suthanthiran M, Gerber LM, Schwartz JE, et al. Circulating transforming growth factor- β1 levels and the risk for kidney disease in African Americans. Kidney Int. 2009;76:72–80. doi: 10.1038/ki.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisberg M, H J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelialto-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 9.Böttinger E. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Deelman L, Sharma K. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr Opin Nephrol Hypertens. 2009;18:85–90. doi: 10.1097/MNH.0b013e32831c50a1. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Noble N, Zhang J, et al. Renin-stimulated TGF-beta1 expression is regulated by a mitogenactivated protein kinase in mesangial cells. Kidney Int. 2007;72:45–52. doi: 10.1038/sj.ki.5002243. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Border W, Anderson I, et al. Combining TGF-beta inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int. 2004;66:1774–1784. doi: 10.1111/j.1523-1755.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 13.Gaedeke J, Peters H, Noble N, et al. Angiotensin II, TGF-beta and renal fibrosis. Contrib Nephrol. 2001;135:153–160. doi: 10.1159/000060162. [DOI] [PubMed] [Google Scholar]
- 14.Border W, Noble N. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 15.Lavoie P, Robitaille G, Agharazii M, et al. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23:1895–1903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
- 16.Lim D, Lutucuta S, Bachireddy P, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campistol J, Iñigo P, Jimenez W, et al. Losartan decreases plasma levels of TGF-beta1 in transplant patients with chronic allograft nephropathy. Kidney Int. 1999;56:714–719. doi: 10.1046/j.1523-1755.1999.00597.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson R, Herrera-Acosta J, Schreiner G, et al. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 19.Appel L, Wright JJ, Greene T, et al. Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med. 2008;168:832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]