Abstract
Acid-base homeostasis to a great extent relies on renal ammonia metabolism. In the past several years, seminal studies have generated important new insights into the mechanisms of renal ammonia transport. In particular, the theory that ammonia transport occurs almost exclusively through nonionic NH3 diffusion and NH4+ trapping has given way to a model postulating that a variety of proteins specifically transport NH3 and NH4+ and that this transport is critical for normal ammonia metabolism. Many of these proteins transport primarily H+ or K+ but also transport NH4+. Nonerythroid Rh glycoproteins transport ammonia and may represent critical facilitators of ammonia transport in the kidney. This review discusses the underlying aspects of renal ammonia transport as well as specific proteins with important roles in renal ammonia transport.
Keywords: acid-base homeostasis, aquaporin, Rh glycoproteins, Na+-K+-ATPase, Na+-K+-2Cl− cotransport, Na+/H+ exchange, H+-K+-ATPase
INTRODUCTION
Acid-base homeostasis depends on renal ammonia1 metabolism. Large amounts of bicarbonate are filtered by the glomerulus, but under normal circumstances renal tubules reabsorb essentially all filtered bicarbonate. The generation of new bicarbonate is necessary to replace the alkali consumed in the buffering of endogenous and exogenous acids. Under normal conditions 60–70% of the new bicarbonate formed by the kidney is due to renal ammonia metabolism, and in response to chronic metabolic acidosis or acid loads, an even larger proportion of new bicarbonate results from increased renal ammonia metabolism (1–3).
Recent studies have yielded important new insights into the mechanisms of renal ammonia transport. In particular, the theory that ammonia transport occurs almost exclusively through nonionic NH3 diffusion has been replaced by the observation that a variety of proteins specifically transport NH3 and NH4+ and that this transport is critical for normal ammonia metabolism. In this review, we discuss the underlying aspects of renal ammonia transport and then discuss in more detail the specific proteins that mediate important roles in renal ammonia transport.
AMMONIA CHEMISTRY
Ammonia exists in biological solutions in two molecular forms, NH3 and NH4+. The relative amounts of each are governed by the buffer reaction NH3 + H+ ↔ NH4+. This reaction occurs essentially instantaneously and has a pKa′ under biologically relevant conditions of ~9.1–9.3. Accordingly, in most biological fluids of pH 7.4 or less, most ammonia is present as NH4+. At pH 7.4 only ~1% of total ammonia is present as NH3. Moreover, because most biological fluids exist at a pH substantially below the pKa′ of this buffer reaction, small changes in pH result in exponential changes in relative NH3 concentration with almost no change in NH4+ concentration. Because NH3 is a relatively small, uncharged molecule, it can diffuse across most lipid bilayers. However, its membrane permeability is not infinite, and thus transepithelial NH3 gradients can occur in the presence of high rates of either NH4+ or H+ transport. NH4+ is a cation with very limited permeability across lipid bilayers in the absence of specific transport proteins. However, in aqueous solutions, NH4+ and K+ have nearly identical biophysical characteristics (Table 1), which enables NH4+ transport at the K+ transport site of many proteins.
Table 1.
Biophysical characteristics of common cationsa
| Cation | Atomic weight |
Ionic radius (126, 127) (Å) |
Hydrodynamic radius (126, 127) (Å) |
Mobility in H2O (126, 127) (10−4 cm2 s−1 V−1) |
TiH2O (126, 128) |
|---|---|---|---|---|---|
| Li+ | 6 | 0.060 | 1.73 | 4.01 | 0.33 |
| Na+ | 23 | 0.095 | 1.67 | 5.19 | 0.39 |
| NH4+ | 18 | 0.133 | 1.14 | 7.60 | 0.49 |
| K+ | 39 | 0.143 | 1.14 | 7.62 | 0.49 |
References, where applicable, are in parentheses in column headers.
RENAL AMMONIA METABOLISM
Renal ammonia metabolism and transport involve integrated responses of multiple portions of the kidney (Figure 1). Essentially none of urinary ammonia is filtered by the glomerulus, making urinary ammonia excretion unique among the major compounds present in the urine. Instead, ammonia is produced by the kidney and is then selectively transported into either the urine or into the renal vein. Ammonia excreted into the urine as NH4+ results in equimolar new bicarbonate formation, whereas ammonia that returns to the systemic circulation through the renal vein is metabolized by the liver to urea and glutamine (4). Under normal conditions, approximately two-thirds of hepatic ammonia metabolism result in urea formation; hepatic ureagenesis utilizes equimolar bicarbonate, thereby consuming bicarbonate produced in the proximal tubule in ammoniagenesis and resulting in no net new bicarbonate formation (5). The selective transport of ammonia into either the urine or the renal vein includes integrated transport mechanisms in the proximal tubule, thick ascending limb of the loop of Henle, and collecting duct. We do not review all data regarding the development of this model, as several excellent reviews can be consulted for more detail (1, 6–8).
Figure 1.
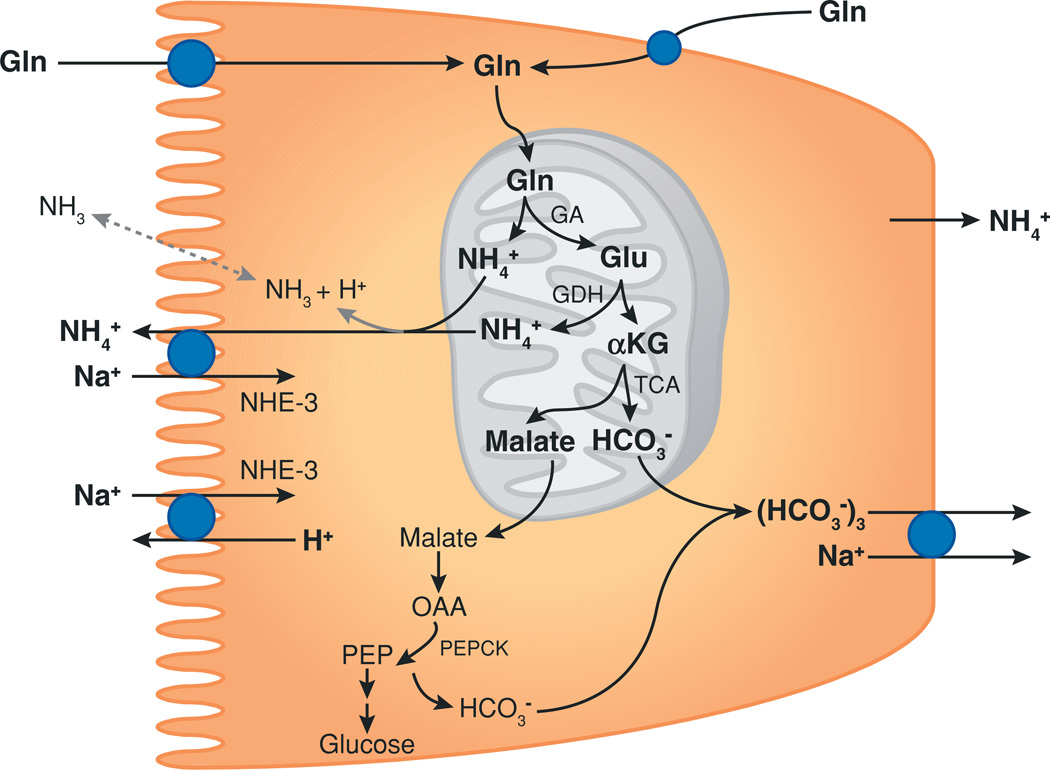
Proximal tubule ammoniagenesis. Ammonia is produced from glutamine (Gln) as a result of proximal tubule ammoniagenesis. Glutamine is transported across both the apical and basolateral plasma membranes and then transported into mitochondria. The enzyme glutaminase (GA) is the first step in ammoniagenesis, and glutamate dehydrogenase (GDH) results in the production of the second NH4+ molecule. Metabolism of α-ketoglutarate (αKG) leads to the production of the first of two HCO3− ions. Further metabolism in the cytoplasm results in the production of a second HCO3−. Thus, complete metabolism of each glutamine produces two NH4+ and two HCO3− ions. Blue circles denote transport proteins. Dotted gray line indicates the minor component of transport, and the solid gray line indicates the major component of transport. Other abbreviations used: NHE-3, Na+/H+ exchanger type 3; OAA, oxaloacetic acid; PEP, phosphoenolpyruvate; PEPCK, phosphoenolpyruvate carboxykinase; TCA, tricarboxylic acid cycle.
The proximal tubule segments metabolize the amino acid glutamine to produce equal numbers of NH4+ and HCO3− molecules (Figure 1) (see References 9 and 10 for a review of the biochemistry of ammonia production). The proximal tubule secretes ammonia into the tubule lumen, but there is also some transport across the basolateral membrane and ultimately into the renal veins (~24–45% of ammonia produced, depending on acid-base homeostasis) (11). Ammonia is secreted into the luminal fluid as NH4+ through a mechanism involving transport via the apical Na+/H+ exchanger NHE-3 (12, 13) and to a lesser degree through an apical Ba2+-sensitive K+ channel (13, 14). In addition, a significant component of NH3 diffusion across the apical membrane occurs, although the exact proportion is unresolved. Figure 2 summarizes proximal tubule ammonia metabolism.
Figure 2.
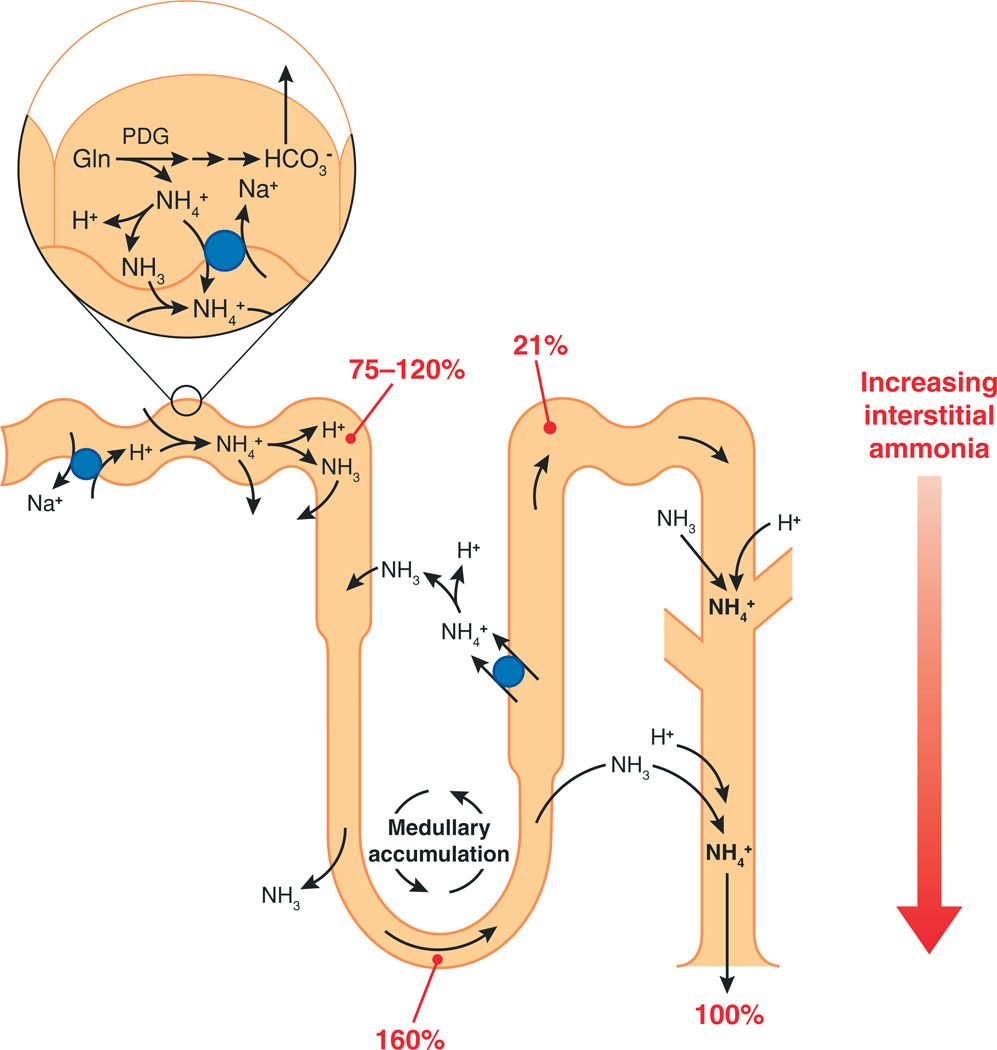
Summary of renal ammonia metabolism. Ammonia is produced in the proximal tubule as a result of metabolism of glutamine (Gln) to NH4+ and HCO3−; ammonia is preferentially secreted into the luminal fluid as either NH4+ or NH3. Bicarbonate produced from ammoniagenesis is preferentially secreted across the basolateral membrane. Ammonia is secreted by the proximal tubule into the luminal fluid and then undergoes recycling by the loop of Henle, resulting in medullary accumulation. Ammonia delivery to the bend of the loop of Henle is ~160% of that which is excreted in the final urine. Ammonia is reabsorbed in the thick ascending limb of the loop of Henle through multiple mechanisms and then is secreted in the collecting duct. Collecting duct ammonia secretion involves parallel NH3 and H+ secretion. Numbers in red represent delivery as a percentage of final urinary ammonia excretion. Blue circles denote transport proteins. PDG, phosphate-dependent glutaminase.
In the thick ascending limb of the loop of Henle, multiple proteins contribute to luminal ammonia reabsorption. The furosemide-sensitive apical Na+-K+-2Cl− cotransporter NKCC2 mediates the majority of NH4+ reabsorption; an apical K+/NH4+ antiporter and an amiloride-sensitive NH4+ conductance also contribute (15–17). Some of the ammonia absorbed by the medullary thick ascending limb of the loop of Henle undergoes recycling into the thin descending limb of the loop of Henle; this involves passive NH3 diffusion, with a small component of passive NH4+ transport (18, 19). Secondarily active ammonia absorption by the thick ascending limb of the loop of Henle and passive ammonia secretion into the thin descending limb of the loop of Henle result in the increasing axial ammonia concentrations in the medullary interstitium that parallel the hypertonicity gradient. Moreover, because of ammonia absorption by the thick ascending limb, ammonia delivery to the distal tubule is only ~20% of final urinary ammonia content (1, 2).
The secretion of ammonia by the collecting duct accounts for the majority (~80%) of urinary ammonia content. Several studies using in vitro–microperfused collecting duct segments have shown that collecting duct ammonia secretion involves parallel NH3 and H+ secretion, with little to no NH4+ permeability in collecting duct segments (1, 7). However, the findings of parallel NH3 and H+ secretion are also consistent with parallel NH4+/H+ exchange and H+ transport. In the absence of luminal carbonic anhydrase activity in most segments of the collecting duct, a luminal disequilibrium pH amplifies the NH3 gradient and increases the rate of transepithelial ammonia secretion (1, 7). Thus, the fundamental mechanisms of ammonia transport in the collecting duct differ from those in the proximal tubule and the thick ascending limb of the loop of Henle.
Recent studies have examined the mechanism of the NH3 transport observed in the collecting duct. NH3 is a small, neutral molecule that is relatively permeable across lipid bilayers (20). As a result, collecting duct NH3 transport may reflect either diffusive or transporter-mediated NH3 transport. One way to differentiate between these two possibilities is to examine the correlation between extracellular ammonia concentration and ammonia transport; with diffusive transport the transport rate parallels the concentration gradient, whereas transporter-mediated uptake exhibits saturable kinetics. Researchers addressed this question using a cultured renal collecting duct epithelial cell line, mouse inner medullary collecting duct (mIMCD)-3 cells, grown on permeable support membranes to enable separate study of apical and basolateral transport mechanisms. Methylammonia (MA) was used as an ammonia analog, enabling the use of the radiolabeled molecule analog [14C]-MA for quantification of transport.
Studies examining basolateral ammonia/MA uptake showed that the renal collecting duct cell line mIMCD-3 exhibited basolateral MA transport (21). Transport was due to a combination of both a saturable, transporter-mediated component and a nonsaturable, diffusive component; the transporter-mediated component predominated at concentrations below 7 mM. With a Ki of ~2 mM, ammonia competitively inhibited MA transport activity. Functional characterization of the saturable transport activity demonstrated that it did not involve extracellular Na+ or K+; was not inhibited by chemical inhibitors of Na+-K+-ATPase, Na+-K+-2Cl− cotransporters NKCC-1 or NKCC-2, K+ channels, or KCC proteins; and was not inhibited by changes in membrane voltage. Varying intracellular and extracellular pH demonstrated that transport activity paralleled the transmembrane H+ gradient. Thus, the renal collecting duct mIMCD-3 cell exhibits basolateral, electroneutral, facilitated NH3 transport activity, an ammonia transport activity not previously described in mammalian cells. The transport activity observed is also consistent with NH4+/H+ exchange activity. Because these cells expressed basolateral Rh B glycoprotein (Rhbg), this transport activity likely reflects Rhbg-mediated transport. However, Rhbg-specific inhibitors were unavailable, and manipulation of Rhbg protein expression was not performed, preventing definitive proof of the role of Rhbg in basolateral NH3 transport.
Similar studies examined apical ammonia transport mechanisms. Apical transport exhibited both a saturable, transporter-mediated component and a nonsaturable, diffusive component (22). The transporter-mediated component exhibited (a) Na+ and K+ independence, (b) modulation by extracellular and intracellular pH, and (c) lack of inhibition by changes in membrane voltage or extracellular K+. The apparent affinity of this transport activity for ammonia, measured as the Ki of ammonia, ~4 mM, was slightly less than that observed for the basolateral ammonia transport activity, consistent with the higher luminal than peritubular ammonia concentrations to which collecting duct cells are exposed. Inhibitors of apical Na+-K+-ATPase, H+-K+-ATPase, and Na+/H+ exchange did not alter transport. Thus, the collecting duct mIMCD-3 cell exhibits an apical electroneutral transport activity functionally consistent with either NH4+/H+ exchange or facilitated NH3 transport. These cells express apical Rh C glycoprotein (Rhcg), suggesting that Rhcg mediates this transport activity. Although these studies (21, 22) establish facilitated ammonia transport, the relative extents of lipid solubility diffusion of NH3 and protein-mediated facilitated diffusion of NH3 in the apical and basolateral membranes of collecting duct cells in vivo remain to be determined.
Metabolic acidosis, which increases renal net acid excretion and new bicarbonate formation predominantly through increases in ammonia production and excretion, is associated with the stimulation of the key components of ammonia metabolism and transport. These include augmented proximal tubule ammoniagenesis, which involves increases in the following: activity and expression of key ammoniagenic enzymes (9, 23); proximal tubule luminal NH4+ secretion (24, 25) due, at least in part, to greater NHE-3 expression (26); ammonia reabsorption in the thick ascending limb of the loop of Henle (27); and collecting duct ammonia secretion (2, 3).
SPECIFIC PROTEINS INVOLVED IN RENAL AMMONIA TRANSPORT
NHE-3
NH4+ produced in the proximal tubule is preferentially transported across the apical membrane into the luminal fluid, as opposed to transport across the basolateral plasma membrane into the peritubular space. Apical ammonia secretion is inhibited by the combination of low luminal Na+ and luminal amiloride, suggesting that this process may involve Na+/NH4+ exchange via apical NHE-3 (28). Consistent with this interpretation is that proximal tubule brush border vesicles exhibit Na+/NH4+ exchange activity (29). Although parallel Na+/H+ exchange and NH3 secretion may account for some of this transport, luminal acidification in the absence of apical Na+/H+ exchange activity does not result in equivalent rates of net ammonia secretion (28). These observations suggest that apical NHE-3 has an important role in the preferential secretion of cytosolic ammonia into the luminal fluid in the proximal tubule.
NHE-3 is also present in the apical membrane of the thick ascending limb of the loop of Henle. However, because this transporter secretes NH4+ and the thick ascending limb of the loop of Henle reabsorbs NH4+, NHE-3 is unlikely to have an important role in loop of Henle ammonia reabsorption.
The regulation of proximal tubule apical NHE-3 is important in the regulation of proximal tubule ammonia transport. Chronic metabolic acidosis, changes in extracellular K+, and angiotensin II (AII) regulate proximal tubule ammonia secretion, apparently through changes in apical Na+/H+ exchange activity (25, 30). In chronic metabolic acidosis and hypokalemia, parallel changes in NHE-3 expression were observed (26, 31). In the S3 segment, chronic metabolic acidosis increases AII-stimulated apical ammonia secretion, and the Na+/H+ exchange inhibitor amiloride in the presence of low luminal Na+ concentration blocks secretion (32). This suggests that chronic metabolic acidosis increases AII-stimulated, Na+/H+ exchange–mediated apical ammonia secretion in the S3 segment.
K+ Channels
At a molecular level, K+ and NH4+ have nearly identical biophysical characteristics, including bare and hydrated radii (Table 1). This molecular mimicry enables NH4+ to substitute for K+ at the K+-binding site of most K+ transporters, including K+ channels (Table 2). K+ channel–mediated transport of NH4+ has been shown for various K+ channel families, including strong and weak inward rectifying, voltage-gated, Ca2+-activated, delayed rectifier, and L-type transient K+ channels (summarized in Reference 33). In general, the relative conductance of K+ channels for NH4+ is 10–20% of that observed for K+ (33). This decreased affinity probably reflects a lower affinity for NH4+ than for K+ at binding sites within the channel’s pore (33).
Table 2.
Comparison of selectivity properties of several K+ channelsa
| Channel | γ, pS | PX/PK, bi-ionic | ||||||
|---|---|---|---|---|---|---|---|---|
| K+ | Rb+ | NH4+ | Tl+ | Rb+ | NH4+ | Tl+ | ||
| KcsA (129) | 55.6 ± 0.6 | 23.2 ± 0.8 | 24.0 ± 0.3 | 16.4 ± 0.1 | 0.78 ± 0.07 | 0.20 ± 0.01 | 3.2 ± 0.1 | |
| Shaker(130) | 18 | 9 | 14 | — | 0.66 | 0.09 | — | |
| BK (131) | 230 | 20 | 41 | 111 | 0.7 | 0.1 | 1.3 | |
| Kir2.1 (33) | 29 | 3 | 17 | 24 | 0.49 | 0.1 | 1.8 | |
References are in parentheses under the first column.
In vitro microperfusion studies show that barium, a nonspecific K+ channel inhibitor, blocks a component of proximal tubule ammonia transport (13). Several K+ channels, including KCNA10, TWIK-1, and KCNQ1, are present in the apical membrane of the proximal tubule (34–38); which of these mediate ammonia transport is currently not known. Because the negative intracellular electrical potential favors NH4+ uptake, apical K+ channels are more likely to contribute to NH4+ absorption that can occur in the proximal straight tubule than to NH4+ secretion that predominates in the proximal convoluted tubule. Basolateral barium-sensitive transport is also present in the proximal tubule (39).
In the medullary thick ascending limb of the loop of Henle, apical K+ channels can contribute to NH4+ transport, particularly when apical Na+-K+-2Cl− cotransport is inhibited (17). The primary apical K+ channels in the medullary thick ascending limb of the loop of Henle are ATP sensitive. These proteins appear primarily to enable recycling of K+ reabsorbed by the apical Na+-K+-2Cl− co-transporter and do not seem to have important roles in ammonia transport in the absence of inhibition of apical Na+-K+-2Cl− cotransport (40).
The electrochemical gradient for NH4+ transport by K+ channels normally favors NH4+ uptake from the extracellular into the intracellular compartment, primarily owing to the negative intracellular electrical potential. Thus, basolateral K+ channels in the collecting duct may contribute to peritubular NH4+ uptake. At present, no studies have examined this possibility. However, the observation that prolonged ammonia incubation acidifies CCD intercalated cells (41) suggests that significant NH4+ transport, possibly due to K+ channels, may occur in the cortical collecting duct (CCD).
Na+-K+-2Cl− Cotransport
Researchers have identified two isoforms of the Na+-K+-2Cl− transporter, termed NKCC-1 and NKCC-2 (42). These isoforms exist in a wide variety of tissues, where they mediate multiple physiological functions, including ion transport, cell volume regulation, blood pressure regulation, and saliva and endolymph formation (42–44). NKCC-1, also known as BSC-2 and as the secretory isoform, is expressed at the basolateral membrane of many cells involved in secretory functions. In the kidney, NKCC-1 is present almost exclusively in the basolateral membrane of A-type intercalated cells in the outer and inner medullary collecting ducts (OMCD and IMCD, respectively) (45). However, when bumetanide, a highly specific inhibitor, is added to the peritubular solution, it does not alter ammonia secretion by in vitro–microperfused rat OMCD (46). Thus, it is unlikely that NKCC-1 contributes to OMCD ammonia secretion. Studies using cultured IMCD cells suggest that basolateral Na+-K+-2Cl− cotransport in the IMCD, presumably involving NKCC-1,contributes to basolateral NH4+ uptake (47, 48). However, other studies using in vitro–microperfused rat terminal IMCD segments have not confirmed this observation (49).
NKCC-2 is a kidney-specific Na+-K+-2Cl− cotransporter isoform specifically expressed in the apical plasma membrane of the thick ascending limb of the loop of Henle (50, 51), where it is the major mechanism for ammonia reabsorption (8, 40). The affinity of NKCC-2 for ammonia is ~2 mM, enabling effective competition of NH4+ with K+ at the K+-binding site for transport (52). Hyperkalemia suppresses urinary ammonia excretion. This likely occurs, at least in part, because higher luminal K+ inhibits NH4+ reabsorption by NKCC-2, thereby inhibiting development of the medullary ammonia concentration gradient (40).
Changes in NKCC-2 protein expression are a major component of the increase in MTAL ammonia absorption that occurs with chronic metabolic acidosis (27, 53). Increased mRNA expression precedes increased protein expression (53); the elevation in systemic glucocorticoids that occurs with chronic metabolic acidosis may mediate the increases in mRNA and protein expression (54). Changes in cell-surface expression of NKCC-2 can regulate NKCC-2 ion transport in response to other stimuli such as cAMP (55); whether this mechanism is important for the regulation of ammonia transport is unknown.
Na+-K+-ATPase
Na+-K+-ATPase is a family of heterodimeric proteins present in essentially all nucleated cells. The alpha subunit comprises the catalytic and ion transport domain. The function of the beta subunit is not completely clear but may include directing membrane localization (56). Na+-K+-ATPase is present in the basolateral plasma membrane of essentially all kidney cells, but its expression appears greatest in the medullary thick ascending limb of the loop of Henle, with lesser expression in the cortical thick ascending limb, distal convoluted tubule (DCT), CCD, medullary collecting duct (MCD), and the proximal tubule (57).
NH4+ competes with K+ at the K+-binding site of Na+-K+-ATPase, thereby enabling net Na+-NH4+ exchange (58, 59). The affinity of the K+-binding site of Na+-K+-ATPase for NH4+ is similar to that for K+ (58, 59), but at least in the renal cortex, interstitial ammonia concentrations are lower than K+ concentrations. Moreover, in vitro–microperfused tubule studies show that, even in the presence of elevated peritubular ammonia concentrations, ouabain, a Na+-K+-ATPase inhibitor, does not alter transepithelial ammonia secretion in the CCD (60). Accordingly, basolateral Na+-K+-ATPase is not likely to transport NH4+ to a significant extent in the CCD (58).
In contrast, interstitial ammonia concentrations are substantially higher in the inner medulla, enabling Na+-K+-ATPase-mediated basolateral ammonia uptake to contribute significantly to transepithelial ammonia secretion by the IMCD (58, 59). Direct studies show that NH4+ competes with K+ as a substrate for hydrolytic activity and that basolateral Na+-K+-ATPase mediates NH4+ uptake, increases apical proton secretion, and is important for transepithelial ammonia secretion (49, 59, 61). Using measured values for Na+-K+-ATPase Km and Vmax and assuming that vasa recta K+ and NH4+ concentrations are similar to interstitial concentrations, Wall and colleagues (62) have shown that rates of NH4+ uptake via Na+-K+-ATPase are remarkably similar to measured rates of NH4+ secretion. Furthermore, physiological changes in interstitial K+ concentrations associated with hypokalemia predict a two- to threefold increase in NH4+ uptake (62). Direct studies examining the effects of hypokalemia on Na+-K+-ATPase-mediated ammonia transport have confirmed this model (63). Moreover, the increase in ammonia uptake is almost totally attributable to changes in interstitial K+ concentration and does not involve changes in Na+-K+-ATPase hydrolytic activity (63). Thus, the increased rate of ammonia secretion that occurs during hypokalemia may be due, at least in part, to the decreased interstitial K+ concentration, which results in more effective NH4+ transport by basolateral Na+-K+-ATPase.
K+/NH4+ (H+) Exchanger
An apical K+/NH4+ (H+) transporter that is present in the thick ascending limb of the loop of Henle mediates most of the ammonia reabsorption in this nephron segment that is not mediated by NKCC-2 (8). This transport activity is electroneutral and inhibited by barium and verapamil (16, 64). Neither the gene product nor the protein that correlates with this transport activity has been identified.
H+-K+-ATPase
NH4+ also is a substrate for nongastric (or colonic) H+-K+-ATPase in the collecting duct and colon. This has been demonstrated in heterologous expression systems, colonic apical membranes, and IMCDs perfused in vitro (65–68). Although the exact substrates for colonic H+-K+-ATPase are still not completely resolved or reconciled with in vivo transport, we know that NH4+ can substitute for K+. However, recent data suggest that NH4+ cannot substitute for the H+ site (68). Because colonic H+-K+-ATPase in vivo is induced during K+ deficiency, it may facilitate increased ammonia excretion (67). Ammonia also stimulates H+ secretion and bicarbonate reabsorption in the CCD, possibly via several mechanisms (69), including stimulation of, or coupling with, H+-K+-ATPase (41).
Aquaporins
Aquaporins are an extended family of proteins that mediate facilitated transmembrane water transport (70, 71). Because H2O and NH3 have similar molecular sizes and charge distribution, an increasing number of studies are examining the role of aquaporins in transmembrane NH3 transport, in particular ammonia transport by the aquaporins AQP1, AQP3, AQP8, and AQP9.
The first study investigating the possible role of aquaporins as ammonia transporters examined AQP1 (72). There, the addition of extracellular ammonia to control, H2O-injected oocytes that did not express AQP1 resulted in intracellular acidification and cellular depolarization. These results indicate NH4+ entry coupled with a relatively low NH3 permeability (72). In contrast, significantly less intracellular acidification occurred in AQP1-expressing oocytes. Thus, AQP1 expression either inhibited endogenous NH4+ permeability or increased NH3 permeability. Because AQP1 expression did not alter ammonia-dependent membrane depolarization, AQP1 expression is unlikely to alter NH4+ permeability (72). Moreover, increasing extracellular pH from 7.5 to 8.0 in the continuous presence of extracellular ammonia, which increases extracellular NH3 but does not substantially change extracellular NH4+, caused more rapid intracellular alkalinization in AQP1-expressing oocytes than in control oocytes. Thus, AQP1 appeared to enable facilitated NH3 transport (72). Although it is tempting to speculate that AQP1 may contribute to NH3 permeability in the thin descending limb of the loop of Henle, it is unknown whether AQP1 knockout mice show altered ammonia transport there. However, not all studies have confirmed that AQP1 can transport NH3 (73, 74).
In the basolateral membrane of collecting duct principal cells, AQP3 plays an important role in H2O transport (75).When expressed in Xenopus oocytes, AQP3 increases membrane NH3 permeability, suggesting that AQP3 can function as an NH3 transporter (73). Whether AQP3 contributes to renal principal cell basolateral NH3 transport has not been reported.
AQP8, similar to AQP3, increases NH3 permeability in Xenopus oocytes; it also increases permeability to the ammonia analogs formamide and MA (73). In the kidney, AQP8 is weakly expressed in the cytoplasm of the proximal tubule, CCD, and OMCD, with no evidence of plasma membrane expression (76). Acid-base homeostasis, urine ammonia concentration, and urine pH are similar in AQP8 knockout and wild-type mice under both basal- and acid-loaded conditions, suggesting that AQP8 deletion does not alter basal- or acid-stimulated net acid excretion (77, 78). However, AQP8 knockout mice do exhibit minor changes in hepatic ammonia accumulation, renal excretion of infused ammonia, and intrarenal ammonia concentrations (78).
AQP9 expression, similar to AQP8, increases apparent NH3 permeability and permeability to the analogs formamide and MA in Xenopus oocytes (73). However, AQP9 does not appear to be expressed in the kidney.
The molecular specificity of ammonia transport by aquaporins may relate to specific amino acids in the aromatic/arginine constriction region of these proteins. This region is located below the channel mouth and may be narrower than the NPA constriction (79). Site-directed mutagenesis shows that specific amino acids, including Phe-56, His-180, and Arg-195 of AQP1, are important for ammonia permeability but not for water permeability (74). The central NPA constriction, although important for aquaporin water permeability, does not appear critical for aquaporin ammonia permeability (74).
Rh Glycoproteins
The identification that the Rh glycoprotein family proteins can transport ammonia has been the most recent addition to our understanding of the molecular mechanisms of renal ammonia transport. These proteins are mammalian orthologs of Mep/AMT proteins, the ammonia transporter families present in yeast, plants, bacteria, and many other organisms (80, 81). Three mammalian Rh glycoproteins—Rh A glycoprotein (RhAG/Rhag), Rh B glycoprotein (RhBG/Rhbg), and Rh C glycoprotein (RhCG/Rhcg)—have been identified to date (82, 83). By convention, Rh A glycoprotein in human tissue is termed RhAG, and in nonhumans it is termed Rhag; similar terminologies apply for RhBG/Rhbg and RhCG/Rhcg.
RhAG/Rhag
The Rh complex in erythrocytes consists of RhAG/Rhag in association with the nonglycosylated Rh proteins RhD and RhCE in humans or with Rh30 in non-human mammals. Marini et al. (84) predicted in 1997 that Rh proteins would have structural similarity to Mep and AMT proteins and might function as ammonia transporters. Subsequent studies established that RhAG and RhCG expression restored growth defects of yeast deficient in endogenous ammonia transporters and that RhAG mediated efflux of the ammonia analog MA (85), suggesting that RhAG functioned as a mammalian ammonia transporter.
Various studies have examined RhAG/Rhag-mediated ammonia transport characteristics. Heterologous expression studies show that RhAG transports the ammonia analog MA, with an EC50 of ~1.6 mM, and that transport is electroneutral and coupled to H+ gradients (86). Transport is not coupled to either Na+ or K+, nor is it affected by a wide variety of inhibitors of other transporter families. Moreover, RhAG-mediated transport is bidirectional (85, 87). Studies comparing ammonia transport in erythrocytes from Rhnull individuals, who do not express erythroid RhAG, with transport in erythrocytes with normal RhAG expression show that NH3 transport parallels RhAG expression (88). These characteristics functionally identify that RhAG mediates either facilitated NH3 transport or NH4+/H+ exchange activity. However, at least one study has suggested that RhAG, when expressed in HeLa cells, transports both NH3 and NH4+ (89).
RhAG/Rhag is an erythrocyte- and erythroid-precursor-specific protein (90, 91). At present, there is no evidence that RhAG/Rhag contributes to renal ammonia metabolism and/or transport.
The nonglycosylated Rh protein RhCE apparently transports neither ammonia nor its analog MA, nor does it alter transport by RhAG (86). Moreover, structural models, using the Escherichia coli AmtB structure as a template, suggest that the arrangement of key amino acids is sufficiently different in RhCE and RhD that RhCE and RhD either do not transport ammonia or do so by mechanisms that differ from those used by AmtB, RhAG, RhBG, and RhCG (92).
RhBG/Rhbg
RhBG/Rhbg is the second member of the mammalian Rh glycoprotein family. RhBG/Rhbg is expressed in a wide variety of organs involved in ammonia metabolism, including the kidneys, liver, skin, stomach, and gastrointestinal tract (82, 93). The kidneys express basolateral Rhbg immunoreactivity in the distal convoluted tubule (DCT), connecting segment (CNT), initial collecting tubule (ICT), CCD, OMCD, and IMCD (94, 95). Both intercalated and principal cells in the DCT, CNT, ICT, and CCD express Rhbg, and expression is greater in intercalated cells than in principal cells; this is consistent with intercalated cells having a greater role in acid-base homeostasis than do principal cells. In the IMCD, only intercalated cells express Rhbg. The only exception to this pattern is that the CCD B-type intercalated cell does not express Rhbg immunoreactivity detectable by light microscopy (95). This is consistent with a primary role of the B-type intercalated cell in bicarbonate secretion and Cl− reabsorption. The basolateral expression of Rhbg is stabilized through specific interactions of the cytoplasmic carboxy terminus with ankyrin-G (96).
RhBG/Rhbg is expressed in multiple extrarenal sites involved in ammonia transport and metabolism. In the liver, Rhbg is expressed in the sinusoidal membrane of perivenous hepatocytes (97), which is the basolateral membrane. Perivenous hepatocytes have an affinity for ammonia of ~110 µM and are responsible for high-affinity hepatic ammonia metabolism (98). In the skin Rhbg mRNA is expressed in hair follicles and sweat glands (82). Although the role of Rhbg in sweat formation is undefined at present, sweat ammonia concentrations average ~3 mM and can exceed 11 mM (99, 100); these concentrations are greater than in all other body fluids except for urine. The gastrointestinal tract is another major site of ammonia transport and metabolism, and villous epithelial cells, the major site of ion transport, from the duodenum through the colon express basolateral Rhbg immunoreactivity (93).
Multiple heterologous expression studies demonstrate that RhBG/Rhbg can transport ammonia and the ammonia analog MA. However, studies differ as to the exact molecular species, NH3 or NH4+, transported. Some studies show that ammonia and MA transport is electroneutral, coupled to proton gradients, independent of Na+ or K+, and unaffected by changes in membrane voltage, indicating electroneutral, cation-independent NH3 transport (101–103). However, other data suggest that Rhbg mediates electrogenic NH4+ transport (104). In all these studies, the affinity of Rhbg for ammonia is ~2–4 mM. Why different studies identify seemingly differing molecular transport mechanisms is currently a mystery.
Several studies have examined the physiological role of Rhbg in renal ammonia metabolism. Seshardri et al. (105) induced chronic metabolic acidosis, which increases renal ammonia excretion, in normal Sprague-Dawley rats. These researchers observed no detectable changes in Rhbg protein expression, either by immunoblot analysis or by immunohistochemistry. Two possibilities thus exist. First, Rhbg is not involved in the increased renal ammonia excretion in response to chronic metabolic acidosis. Alternatively, if Rhbg contributes to increased collecting duct ammonia transport, then the increased transport occurs through mechanisms independent of changes in protein expression detectable by immunoblot analysis or by light microscopy (105).
In another study, genetic deletion of pendrin, an apical Cl−/HCO3− exchanger present in B-type intercalated cells and non-A, non-B cells, decreased Rhbg expression (106). This adaptation may reflect increased urinary acidification in response to pendrin deletion, which increases the gradient for NH3 transport, thus decreasing the necessity for ammonia transport via Rhbg.
Finally, Chambrey et al. (107) have studied an Rhbg knockout mouse. Their extensive physiological studies show normal basal acid-base balance, a normal response to chronic acid loading, and normal basolateral NH3 and NH4+ transport in in vitro–microperfused CCD segments (107). These results suggest either that Rhbg is not involved in renal ammonia metabolism or that certain mechanisms activated in response to Rhbg deletion fully compensate for the lack of Rhbg (107). However, there were no differences in Rhcg, H+-ATPase, or AE1 expression, likely candidate proteins if compensatory adaptations were present (107).
At present, the specific role of RhBG/Rhbg in renal ammonia metabolism is unclear. It does not appear to be required for renal ammonia metabolism, at least under basal conditions or in response to metabolic acidosis. Its specific role in acid-base homeostasis will undoubtedly be an important area of investigation.
RhCG/Rhcg
RhCG/Rhcg is the third member of the mammalian Rh glycoprotein family. It is expressed in multiple ammonia-transporting/metabolizing organs, including the kidneys, central nervous system, testes, skeletal muscle and liver, and gastrointestinal tract (83, 85, 93, 97, 108). In the kidney, Rhcg expression parallels that of Rhbg; i.e., it occurs in the DCT, CNT, ICT, CCD, OMCD, and IMCD (95, 109). The cellular expression of Rhcg is similar to that of Rhbg, with intercalated cell expression exceeding principal cell expression in the DCT, CNT, ICT, CCD, and OMCD and expression only in intercalated cells in the IMCD (95, 109).
However, the subcellular location of Rhcg differs from the exclusive basolateral location observed for Rhbg. In the mouse kidney, Rhcg immunoreactivity is apical (95, 109). One study in the rat showed that Rhcg immunoreactivity was exclusively apical (109) whereas other rat studies show both apical and basolateral expression (105, 110). Recent studies in the human kidney show both apical and basolateral RhCG expression (111). Quantitative analysis of rat Rhcg localization, using immunogold electron microscopy to identify the specific cellular location of RhCG, shows that basolateral plasma membrane Rhcg expression exceeds apical plasma membrane expression, at least in the OMCD in the inner stripe (110). In addition, rat renal Rhcg is also present in intracellular sites, including cytoplasmic vesicles, suggesting that vesicular trafficking regulates plasma membrane Rhcg expression (Figure 3a) (110). The presence of both apical and basolateral plasma membrane RhCG/Rhcg expression in the human and rat kidneys raises the possibility that Rhcg contributes to both apical and basolateral plasma membrane ammonia transport.
Figure 3.
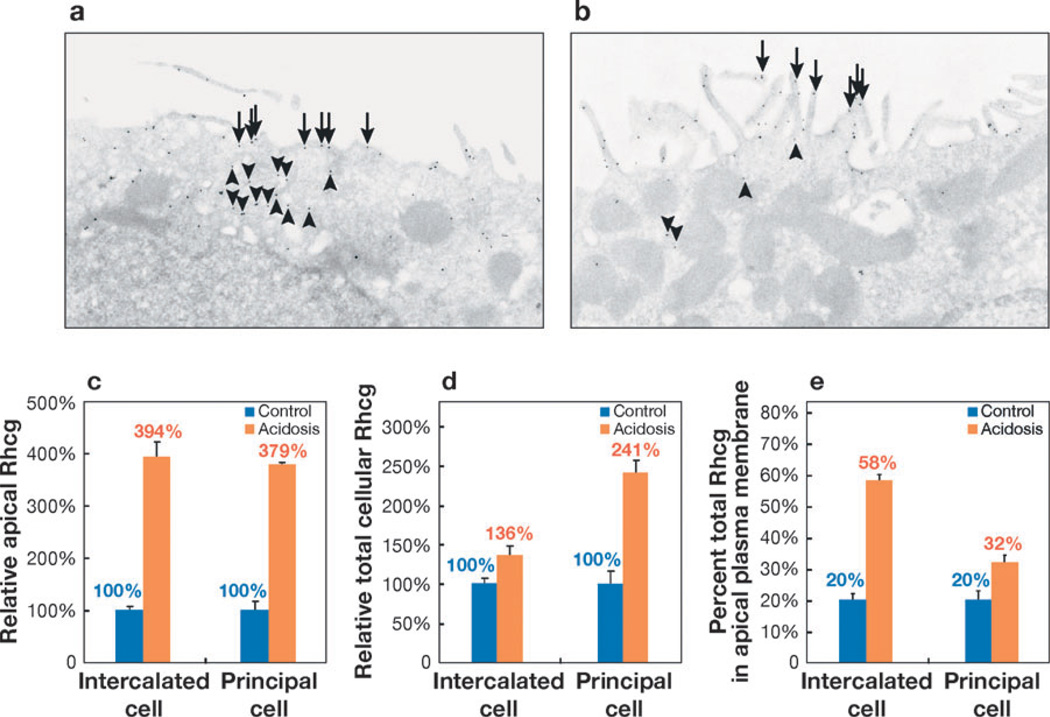
Rhcg expression in the OMCD in response to chronic metabolic acidosis. (a) Under basal conditions Rhcg is present both in the apical plasma membrane (arrows) and in intracellular compartments (arrowheads) in intercalated cells in the OMCD. (b) Chronic metabolic acidosis increases the proportion of Rhcg present in the apical plasma membrane (arrows) and decreases Rhcg present in the intracellular compartment (arrowheads). (c) Chronic metabolic acidosis increases the amount of Rhcg in the apical plasma membrane in both the OMCD intercalated cell and the principal cell as compared with control conditions (100%). The relative increase is similar in intercalated cells and principal cells. Absolute expression (not shown) is greater in the intercalated cell than in the principal cell under both control and acidosis conditions (110). (d) Total cellular Rhcg in intercalated and principal cells in response to chronic metabolic acidosis, expressed relative to total cellular Rhcg in control conditions (100%). Although Rhcg expression increases in both the intercalated cell and principal cell, the relative increase is greater in the principal cell than in the intercalated cell. (e) The proportions of total cellular Rhcg present in the apical plasma membrane in the intercalated cell and principal cell under control conditions and in response to chronic metabolic acidosis. The increase in response to metabolic acidosis is greater in the intercalated cell than in the principal cell. a and b are reprinted from Reference 110, with permission. c, d, and e use data published previously in Reference 110.
As for Rhbg, multiple studies have addressed and differed as to the molecular ammonia species transported by RhCG/Rhcg. Some studies suggest that RhCG/Rhcg mediates electroneutral NH3 transport (101–103), whereas others report both NH3 and NH4+ transport (112) or only electrogenic NH4+ transport (113). The explanation for these differing observations is currently unknown. However, only electroneutral NH3 transport likely contributes to apical membrane ammonia secretion in the collecting duct. The negative intracellular electrical potential and high luminal NH4+ concentrations would favor luminal NH4+ reabsorption via an electrogenic NH4+ transport mechanism (22, 114).
Studies examining the regulation of Rhcg in response to chronic metabolic acidosis have yielded important observations. Chronic metabolic acidosis increases Rhcg protein expression in the OMCD and the IMCD but not in the cortex (105). The OMCD and IMCD changes appear to occur through posttranscriptional mechanisms because Rhcg steady-state mRNA expression is not detectably altered. In the IMCD, the increase in expression is specific to the IMCD intercalated cell.
In the OMCD in the inner stripe, at least two mechanisms are involved in the response of Rhcg to chronic metabolic acidosis (110). Quantitative analysis of the subcellular location of Rhcg, via immunoelectron microscopy and morphometric analysis, shows that chronic metabolic acidosis increases Rhcg expression in both the intercalated cell and the principal cell (Figure 3). Although the absolute levels of Rhcg expression are greater in the intercalated cell in the principal cell, the relative increase in cellular expression was substantially greater in the principal cell (↑~240%) than in the intercalated cell (↑~35%).
A second regulatory mechanism operates through changes in the subcellular location of Rhcg (110). Under basal conditions, Rhcg is located in both the apical and basolateral plasma membrane and in subapical sites in both the principal cell and intercalated cell. In response to chronic metabolic acidosis, particularly in the intercalated cell, there is a dramatic increase in apical plasma membrane expression and a decrease in cytoplasmic Rhcg expression (110). These results suggest translocation of Rhcg from cytoplasmic to apical plasma membrane sites. A similar response, although one of substantially less magnitude, occurs in the principal cell (110).
Chronic metabolic acidosis also increases basolateral plasma membrane Rhcg expression (110). This increase parallels the change in total cellular expression and does not appear to involve redistribution from cytoplasmic sites to the basolateral plasma membrane.
These observations suggest that at least two mechanisms regulate Rhcg expression: changes in total cellular expression and changes in the subcellular distribution of Rhcg (110). Moreover, the relative roles of these two mechanisms differ in adjacent cell types, the principal cell and the intercalated cell. These changes are also membrane-specific, as the increase in basolateral plasma membrane Rhcg expression in the principal cell parallels changes in total cellular expression and does not involve changes in the relative subcellular distribution of Rhcg. The mechanisms involved in the cell- and membrane-specific differences in Rhcg expression in response to specific physiological stimuli are unknown at present.
Tertiary structure of Rh glycoproteins
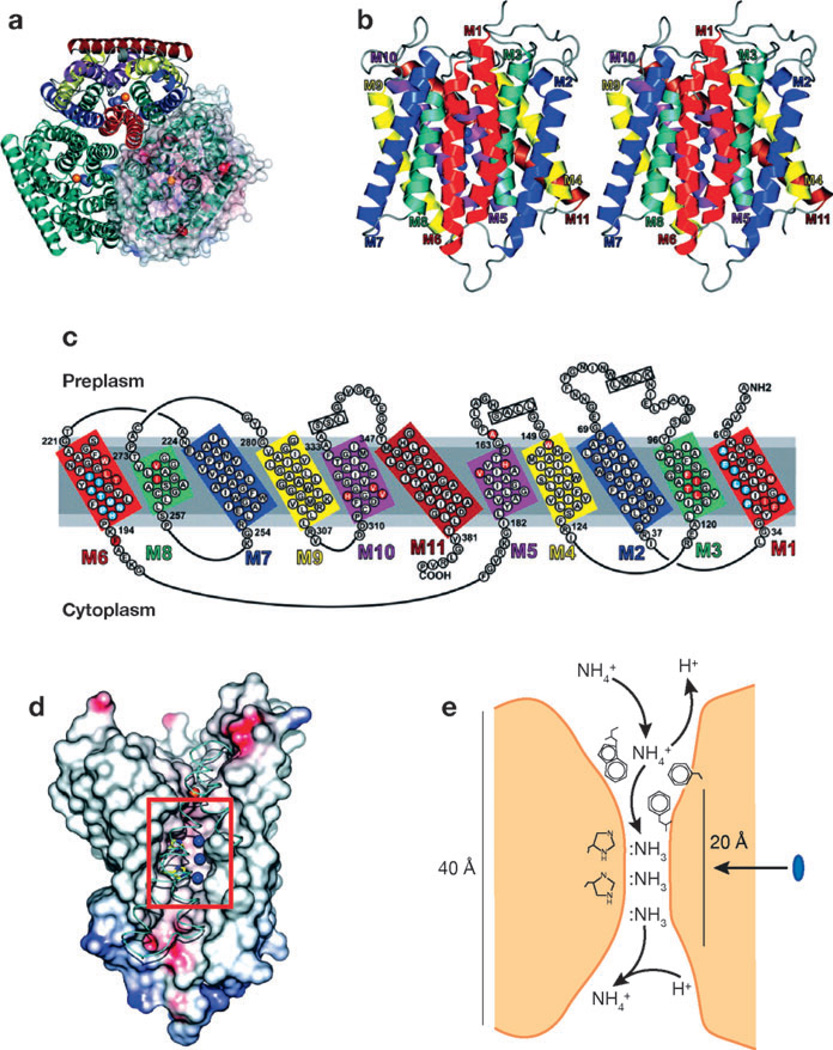
Researchers are beginning to unravel the molecular mechanisms by which Rh glycoproteins transport ammonia and its analog MA. The related E. coli protein AmtB has been crystallized, and its tertiary structure has been identified (Figure 4) (115–117). AmtB is expressed as a trimeric complex. The individual subunits of the trimeric complex span the plasma membrane 11 times and have two structurally related halves with opposite polarity. A vestibule, present in both the extracellular and intracellular regions of the protein, recruits NH4+. In AmtB, NH4+ and MA+ appear to be stabilized in the vestibule through interactions with Trp-148, Phe-103, and Ser-219. Although mutation of Asp-160 abolishes transport activity (118), this amino acid does not directly interact with ammonia or MA; instead, it orients the carbonyl groups of Asp-160, Phe-161, and Ala-162 (116). Transport then occurs through a 20-Å hydrophobic channel that spans the membrane. Trp-148, Phe-103, Phe-161, and Tyr-140 form the first hydrophobic barrier to transport through this channel; the diameter of this pore is 1.2 Å, suggesting that transport requires movement of the side chains of Phe-107 and Phe-215 (116). In the channel pore, two conserved histidines, His-168 and His-318, appear to interact with and stabilize NH3 at specific locations, Am2, Am3, and Am4 (116, 117). The unprotonated Nδ1s of His-168 and His-H318 are fixed by hydrogen bonds to each other, thereby providing two Cε1-Hs to the nitrogens of Am2 and Am3 and one Nε acceptor that interact with the N-H bond of Am4. Because the Cε1-Hs can donate, but cannot accept, N–H bonds, specificity for NH3 transport rather than NH4+ transport is generated. As a result, NH4+ is concentrated at the Am1 site, progressively desolvated as it progresses through the channel pore to Am2, and deprotonated at Am3. NH3 and the methyl derivative CH3NH2, but not NH4+ and CH3NH3+, are transported through the pore of AmtB (116). The tertiary structure of AmtB is a novel structural motif without significant similarity to other known proteins (116, 117).
Figure 4.
Tertiary structure of E. coli AmtB. (a) A ribbon representation of AmtB viewed from the extracellular surface demonstrates the trimeric structure of AmtB. In the top monomer, homologous transmembrane segments are shown in the same color. In the right monomer, a solvent-accessible transparent surface is colored according to the electrostatic potential (red for negative and blue for positive). Potential ammonia-binding sites are shown as the blue sphere for NH3 and red sphere for NH4+. (b) A stereoview of the monomeric structure. The extracellular surface is uppermost. NH3-binding sites are shown as blue spheres. (c) The amino acid sequences of the transmembrane extracellular and intracellular loops. Homologous sequences are shown in similar colors. (d) The ammonia-conducting portion of AmtB after removal of portions of helices M8, M9, and M10. The lumen surface is colored according to the electrostatic potential (red for negative and blue for positive). The histidines near the three NH3 sites (blue spheres) are shown in yellow. Narrow hydrophobic regions through the conducting channel lie above and below the NH3 sites. (e) The deduced mechanism of ammonia transport through AmtB. The blue oval indicates the 20-Å hydrophobic channel of the protein. Figure from Reference 116, with permission from AAAS.
The degree to which mammalian Rh glycoproteins mimic the structure of AmtB is an active area of investigation. Homology models suggest that Rhag, Rhbg, and Rhcg have a channel architecture similar to AmtB (92). Preliminary studies suggest that mutations at Phe-74, Val-137, and Phe-235 of RhCG, equivalent to Ile-28, Leu-114, and Phe-215, respectively, in AmtB, inhibit NH3 transport (119). This suggests that the molecular mechanism of ammonia transport by Rhcg is similar to those used by AmtB. Finally, the reported Kms of mammalian glycoproteins for ammonia and MA, >1 mM, are > 100-fold greater than those reported for AmtB. This is associated with differences in the π-cation-stabilizing rings; changes in Trp-148 to leucine or valine and Phe/Tyr-103 to isoleucine occur in the Rh glycoproteins (118).
Alternative functions of Rh glycoproteins
There are important controversies regarding the functions of the mammalian Rh glycoproteins. As noted above, genetic deletion of Rhbg does not detectably alter renal ammonia metabolism under basal conditions or in response to chronic metabolic acidosis, nor does it alter basal plasma ammonia levels, suggesting that Rhbg deletion does not impact hepatic ammonia metabolism (107). Thus, either Rhbg does not function as an ammonia transporter or other transport mechanisms can compensate in the absence of Rhbg.
Recent studies suggest that Rh glycoproteins transport CO2. The green algae Chlamydomonas reinhardtii express both Amt proteins homologous to Mep/Amt proteins and Rh proteins that are relatively more homologous to mammalian Rh glycoproteins (120). Inhibiting expression of the Rh glycoprotein homolog Rh1 does not alter extracellular ammonia uptake (121). Interestingly, elevating media CO2 content increases Rh1 expression and inhibiting Rh1 expression suppresses the normal increase in growth rates that occur in response to elevated CO2 (121, 122). One possibility, as yet unproven, is that Rh1 is involved in CO2 uptake; the CO2 is then used for carbon fixation, increased nutrient utilization, and increased growth rates and is not involved in extracellular ammonia uptake.
Evolutionary analysis of AMT proteins and Rh glycoproteins suggests that Rh glycoprotein genes arose after the development of AMT proteins and that the development of Rh glycoproteins was associated with the loss of AMT proteins (123). In organisms expressing both AMT and Rh proteins, Rh proteins appeared to initially diverge rapidly away from Amt and then, over a longer period, to evolve more slowly. Furthermore, functionally divergent amino acid sites exist in the transmembrane segments surrounding the NH3-conducting lumen identified in AmtB, suggesting the presence of differing substrate specificity (123).
Two recent studies have examined whether mammalian RhAG/Rhag transports CO2. These studies used erythrocytes from normal individuals and Rhnull individuals, who do not express erythrocyte RhAG, and from wild-type and Rhag knockout mice. In one study, erythrocyte CO2 permeability was decreased in erythrocytes lacking RhAG/Rhag (124), whereas in the other, RhAG/Rhag absence did not alter CO2 permeability (125). The reason for these differing results is not known but may reflect use of differing analytical techniques—mass spectrometric measurement of C18O16O permeability versus fluorometric analysis of CO2-induced intracellular pH changes— and in their use of intact erythrocytes versus erythrocyte ghosts (124, 125).
CONCLUSIONS
Fundamental changes in our understanding of the molecular mechanisms of renal ammonia transport have occurred in the past several years. The specific transport of NH3 and NH4+ by specific proteins appears critical for normal acid-base homeostasis.
SUMMARY POINTS.
Ammonia metabolism is a primary component of acid-base homeostasis through its role in new bicarbonate generation.
Models of ammonia transport solely involving passive lipid-phase diffusion of NH3 and diffusion trapping of NH4+ cannot explain many aspects of ammonia metabolism.
Specific proteins that have important roles in transepithelial ammonia transport are expressed in different regions of the kidney, including the proximal tubule, thick ascending limb of the loop of Henle, distal convoluted tubule, connecting segment, initial collecting tubule, and collecting duct.
FUTURE ISSUES.
The role of NHE-3 and other transporters in proximal tubule ammonia secretion should be investigated.
The role(s) of aquaporin family members in renal ammonia transport deserves further research.
The transport characteristics of mammalian Rh glycoproteins, i.e., facilitated NH3 transport versus NH4+/H+ exchange versus electrogenic NH4+ transport, should be elucidated.
The relative roles of Rhbg and Rhcg in distal ammonia secretion should be explored.
ACKNOWLEDGMENTS
The preparation of this review was supported by funds from the NIH (grants DK-45788 and NS-47624).
Glossary
- NHE-3
Na+/H+ exchanger type 3
- KCC proteins
K+-Cl− cotransporting proteins
- Rhbg
Rh B glycoprotein
- Rhcg
Rh C glycoprotein
- AII
angiotensin II
- CCD
cortical collecting duct
- OMCD
outer medullary collecting duct
- IMCD
inner medullary collecting duct
- AQP1, 3, 8, 9
aquaporins 1, 3, 8, 9
- Rhag
Rh A glycoprotein
Footnotes
Ammonia exists in two molecular forms, NH3 and NH4+. In this review, we use the terms “ammonia” or “total ammonia” to refer to the sum of these two molecular species. When referring to either of these molecular species, we use specifically either “NH3” or “NH4+.”
Contributor Information
I. David Weiner, Email: weineid@ufl.edu.
L. Lee Hamm, Email: lhamm@tulane.edu.
LITERATURE CITED
- 1.DuBose TD, Jr, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J. Am. Soc. Nephrol. 1991;1:1193–1203. doi: 10.1681/ASN.V1111193. [DOI] [PubMed] [Google Scholar]
- 2.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am. J. Physiol. Renal Physiol. 1987;253:F595–F605. doi: 10.1152/ajprenal.1987.253.4.F595. [DOI] [PubMed] [Google Scholar]
- 3.Sajo IM, Goldstein MB, Sonnenberg H, Stinebaugh BJ, Wilson DR, Halperin ML. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1982;20:353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- 4.Haussinger D, Lamers WH, Moorman AF. Hepatocyte heterogeneity in the metabolism of amino acids and ammonia. Enzyme. 1992;46:72–93. doi: 10.1159/000468779. [DOI] [PubMed] [Google Scholar]
- 5.Haussinger D. Nitrogen metabolism in liver: structural and functional organization and physiological relevance. Biochem. J. 1990;267:281–290. doi: 10.1042/bj2670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Good DW, Knepper MA. Ammonia transport in the mammalian kidney. Am. J. Physiol. Renal Physiol. 1985;248:F459–F471. doi: 10.1152/ajprenal.1985.248.4.F459. [DOI] [PubMed] [Google Scholar]
- 7.Knepper MA. NH4+ transport in the kidney. Kidney Int. 1991;40:S95–S102. [PubMed] [Google Scholar]
- 8.Karim Z, Attmane-Elakeb A, Bichara M. Renal handling of NH4+ in relation to the control of acid-base balance by the kidney. J. Nephrol. 2002;15(Suppl. 5):S128–S134. [PubMed] [Google Scholar]
- 9.Tannen RL, Sahai A. Biochemical pathways and modulators of renal ammoniagenesis. Miner. Electrolyte Metab. 1990;16:249–258. [PubMed] [Google Scholar]
- 10.Tannen RL. Ammonia metabolism. Am. J. Physiol. Renal Physiol. 1978;235:F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- 11.Tizianello A, Deferrari G, Garibotto G, Robaudo C, Acquarone N, Ghiggeri GM. Renal ammoniagenesis in an early stage of metabolic acidosis in man. J. Clin. Invest. 1982;69:240–250. doi: 10.1172/JCI110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagami GT. Ammonia production and secretion by the proximal tubule. Am. J. Kidney Dis. 1989;14:258–261. doi: 10.1016/s0272-6386(89)80198-2. [DOI] [PubMed] [Google Scholar]
- 13.Simon EE, Merli C, Herndon J, Cragoe EJ, Jr, Hamm LL. Effects of barium and 5-(N-ethyl-N-isopropyl)-amiloride on proximal tubule ammonia transport. Am. J. Physiol. Renal Physiol. 1992;262:F36–F39. doi: 10.1152/ajprenal.1992.262.1.F36. [DOI] [PubMed] [Google Scholar]
- 14.Hamm LL, Simon EE. Ammonia transport in the proximal tubule. Miner. Electrolyte Metab. 1990;16:283–290. [PubMed] [Google Scholar]
- 15.Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature. 1989;339:478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- 16.Amlal H, Paillard M, Bichara M. NH4+ transport pathways in cells of medullary thick ascending limb of rat kidney. NH4+ conductance and K+/NH4+ (H+) antiport. J. Biol. Chem. 1994;269:21962–21971. [PubMed] [Google Scholar]
- 17.Attmane-Elakeb A, Amlal H, Bichara M. Ammonium carriers in medullary thick ascending limb. Am. J. Physiol. Renal Physiol. 2001;280:F1–F9. doi: 10.1152/ajprenal.2001.280.1.F1. [DOI] [PubMed] [Google Scholar]
- 18.Flessner MF, Mejia R, Knepper MA. Ammonium and bicarbonate transport in isolated perfused rodent long-loop thin descending limbs. Am. J. Physiol. 1993;264:F388–F396. doi: 10.1152/ajprenal.1993.264.3.F388. [DOI] [PubMed] [Google Scholar]
- 19.Mejia R, Flessner MF, Knepper MA. Model of ammonium and bicarbonate transport along LDL: implications for alkalinization of luminal fluid. Am. J. Physiol. Renal Physiol. 1993;264:F397–F403. doi: 10.1152/ajprenal.1993.264.3.F397. [DOI] [PubMed] [Google Scholar]
- 20.Walter A, Gutknecht J. Permeability of small nonelectrolytes through lipid bilayer membranes. J. Membr. Biol. 1986;90:207–217. doi: 10.1007/BF01870127. [DOI] [PubMed] [Google Scholar]
- 21.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3) Am. J. Physiol. Renal Physiol. 2004;287:F628–F638. doi: 10.1152/ajprenal.00363.2003. [DOI] [PubMed] [Google Scholar]
- 22.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3) Am. J. Physiol. Renal Physiol. 2005;289:F347–F358. doi: 10.1152/ajprenal.00253.2004. [DOI] [PubMed] [Google Scholar]
- 23.DiGiovanni SR, Madsen KM, Luther AD, Knepper MA. Dissociation of ammoniagenic enzyme adaptation in rat S1 proximal tubules and ammonium excretion response. Am. J. Physiol. Renal Physiol. 1994;267:F407–F414. doi: 10.1152/ajprenal.1994.267.3.F407. [DOI] [PubMed] [Google Scholar]
- 24.Nagami GT. Enhanced ammonia secretion by proximal tubules from mice receiving NH4Cl: role of angiotensin II. Am. J. Physiol. Renal Physiol. 2002;282:F472–F477. doi: 10.1152/ajprenal.00249.2001. [DOI] [PubMed] [Google Scholar]
- 25.Nagami GT, Sonu CM, Kurokawa K. Ammonia production by isolated mouse proximal tubules perfused in vitro: effect of metabolic acidosis. J. Clin. Invest. 1986;78:124–129. doi: 10.1172/JCI112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambuhl PM, Amemiya M, Danczkay M, Lotscher M, Kaissling B, et al. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am. J. Physiol. Renal Physiol. 1996;271:F917–F925. doi: 10.1152/ajprenal.1996.271.4.F917. [DOI] [PubMed] [Google Scholar]
- 27.Good DW. Adaptation of HCO3- and NH4+ transport in rat MTAL: effects of chronic metabolic acidosis and Na+ intake. Am. J. Physiol. Renal Physiol. 1990;258:F1345–F1353. doi: 10.1152/ajprenal.1990.258.5.F1345. [DOI] [PubMed] [Google Scholar]
- 28.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J. Clin. Invest. 1988;81:159–164. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsella JL, Aronson PS. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. Am. J. Physiol. Cell Physiol. 1981;241:C220–C226. doi: 10.1152/ajpcell.1981.241.5.C220. [DOI] [PubMed] [Google Scholar]
- 30.Nagami GT. Effect of bath and luminal potassium concentration on ammonia production and secretion by mouse proximal tubules perfused in vitro. J. Clin. Invest. 1990;86:32–39. doi: 10.1172/JCI114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elkjar ML, Kwon TH, Wang W, Nielsen J, Knepper MA, et al. Altered expression of renal NHE3 TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am. J. Physiol. Renal Physiol. 2002;283:F1376–F1388. doi: 10.1152/ajprenal.00186.2002. [DOI] [PubMed] [Google Scholar]
- 32.Nagami GT. Ammonia production and secretion by S3 proximal tubule segments from acidotic mice: role of ANG II. Am. J. Physiol. Renal Physiol. 2004;287:F707–F712. doi: 10.1152/ajprenal.00189.2003. [DOI] [PubMed] [Google Scholar]
- 33.Choe H, Sackin H, Palmer LG. Permeation properties of inward-rectifier potassium channels and their molecular determinants. J. Gen. Physiol. 2000;115:391–404. doi: 10.1085/jgp.115.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, et al. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc. Natl. Acad. Sci. USA. 2005;102:17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie X, Arrighi I, Kaissling B, Pfaff I, Mann J, et al. Expression and insights on function of potassium channel TWIK-1 in mouse kidney. Pflügers Arch. 2005;451:479–488. doi: 10.1007/s00424-005-1480-9. [DOI] [PubMed] [Google Scholar]
- 36.Merot J, Bidet M, Le MS, Tauc M, Poujeol P. Two types of K+ channels in the apical membrane of rabbit proximal tubule in primary culture. Biochim. Biophys. Acta. 1989;978:134–144. doi: 10.1016/0005-2736(89)90508-7. [DOI] [PubMed] [Google Scholar]
- 37.Yao X, Tian S, Chan HY, Biemesderfer D, Desir GV. Expression of KCNA10, a voltage-gated K channel, in glomerular endothelium and at the apical membrane of the renal proximal tubule. J. Am. Soc. Nephrol. 2002;13:2831–2839. doi: 10.1097/01.asn.0000036866.37886.c5. [DOI] [PubMed] [Google Scholar]
- 38.Cluzeaud F, Reyes R, Escoubet B, Fay M, Lazdunski M, et al. Expression of TWIK-1, a novel weakly inward rectifying potassium channel in rat kidney. Am. J. Physiol. Cell Physiol. 1998;275:C1602–C1609. doi: 10.1152/ajpcell.1998.275.6.C1602. [DOI] [PubMed] [Google Scholar]
- 39.Volkl H, Lang F. Electrophysiology of ammonia transport in renal straight proximal tubules. Kidney Int. 1991;40:1082–1089. doi: 10.1038/ki.1991.318. [DOI] [PubMed] [Google Scholar]
- 40.Good DW. Ammonium transport by the thick ascending limb of Henle’s loop. Annu. Rev. Physiol. 1994;56:623–647. doi: 10.1146/annurev.ph.56.030194.003203. [DOI] [PubMed] [Google Scholar]
- 41.Frank AE, Wingo CS, Weiner ID. Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am. J. Physiol. Renal Physiol. 2000;278:F219–F226. doi: 10.1152/ajprenal.2000.278.2.F219. [DOI] [PubMed] [Google Scholar]
- 42.Haas M, Forbush B. The Na-K-Cl cotransporters. J. Bioenerg. Biomembr. 1998;30:161–172. doi: 10.1023/a:1020521308985. [DOI] [PubMed] [Google Scholar]
- 43.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, et al. Hypotension in NKCC1 null mice: role of the kidneys. Am. J. Physiol. Renal Physiol. 2006;290:F409–F416. doi: 10.1152/ajprenal.00309.2005. [DOI] [PubMed] [Google Scholar]
- 44.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, et al. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1846–H1855. doi: 10.1152/ajpheart.00083.2002. [DOI] [PubMed] [Google Scholar]
- 45.Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, et al. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J. Am. Soc. Nephrol. 1996;7:2533–2542. doi: 10.1681/ASN.V7122533. [DOI] [PubMed] [Google Scholar]
- 46.Wall SM, Fischer MP. Contribution of the Na+-K+-2Cl− cotransporter (NKCC1) to transepithelial transport of H+, NH4+, K+, and Na+ in rat outer medullary collecting duct. J. Am. Soc. Nephrol. 2002;13:827–835. doi: 10.1681/ASN.V134827. [DOI] [PubMed] [Google Scholar]
- 47.Wall SM, Trinh HN, Woodward KE. Heterogeneity of NH4+ transport in mouse inner medullary collecting duct cells. Am. J. Physiol. Renal Physiol. 1995;269:F536–F544. doi: 10.1152/ajprenal.1995.269.4.F536. [DOI] [PubMed] [Google Scholar]
- 48.Glanville M, Kingscote S, Thwaites DT, Simmons NL. Expression and role of sodium, potassium, chloride cotransport (NKCC1) in mouse inner medullary collecting duct (mIMCD-K2) epithelial cells. Pflügers Arch. 2001;443:123–131. doi: 10.1007/s004240100629. [DOI] [PubMed] [Google Scholar]
- 49.Wall SM. Ouabain reduces net acid secretion and increases pHi by inhibiting NH4+ uptake on rat tIMCD Na+-K+-ATPase. Am. J. Physiol. Renal Physiol. 1997;273:F857–F868. doi: 10.1152/ajprenal.1997.273.6.F857. [DOI] [PubMed] [Google Scholar]
- 50.Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na+-K+-2Cl− cotransporter, BSC-1. Am. J. Physiol. Renal Physiol. 1996;271:F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 51.Plotkin MD, Kaplan MR, Verlander JW, Lee WS, Brown D, et al. Localization of the thiazide sensitive Na-Cl cotransporter, rTSC1 in the rat kidney. Kidney Int. 1996;50:174–183. doi: 10.1038/ki.1996.300. [DOI] [PubMed] [Google Scholar]
- 52.Kinne R, Kinne-Saffran E, Schutz H, Schölermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl−-cotransporter. J. Membr. Biol. 1986;94:279–284. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- 53.Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M. Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+-K+(NH4+)-2Cl− cotransporter of the rat medullary thick ascending limb. J. Biol. Chem. 1998;273:33681–33691. doi: 10.1074/jbc.273.50.33681. [DOI] [PubMed] [Google Scholar]
- 54.Attmane-Elakeb A, Sibella V, Vernimmen C, Belenfant X, Hebert SC, Bichara M. Regulation by glucocorticoids of expression and activity of rBSC1, the Na+-K+(NH4+)-2Cl− cotransporter of medullary thick ascending limb. J. Biol. Chem. 2000;275:33548–33553. doi: 10.1074/jbc.M006591200. [DOI] [PubMed] [Google Scholar]
- 55.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am. J. Physiol. Renal Physiol. 2006;290:F608–F616. doi: 10.1152/ajprenal.00248.2005. [DOI] [PubMed] [Google Scholar]
- 56.Martin DW. Structure-function relationships in the Na+,K+-pump. Semin. Nephrol. 2005;25:282–291. doi: 10.1016/j.semnephrol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Jorgensen PL. Sodium and potassium ion pump in kidney tubules. Physiol. Rev. 1980;60:864–917. doi: 10.1152/physrev.1980.60.3.864. [DOI] [PubMed] [Google Scholar]
- 58.Kurtz I, Balaban RS. Ammonium as a substrate for Na+-K+-ATPase in rabbit proximal tubules. Am. J. Physiol. Renal Physiol. 1986;250:F497–F502. doi: 10.1152/ajprenal.1986.250.3.F497. [DOI] [PubMed] [Google Scholar]
- 59.Wall SM, Koger LM. NH4+ transport mediated by Na+-K+-ATPase in rat inner medullary collecting duct. Am. J. Physiol. Renal Physiol. 1994;267:F660–F670. doi: 10.1152/ajprenal.1994.267.4.F660. [DOI] [PubMed] [Google Scholar]
- 60.Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am. J. Physiol. Renal Physiol. 1984;247:F729–F738. doi: 10.1152/ajprenal.1984.247.5.F729. [DOI] [PubMed] [Google Scholar]
- 61.Wall SM. NH4+ augments net acid-secretion by a ouabain-sensitive mechanism in isolated-perfused inner medullary collecting ducts. Am. J. Physiol. Renal Physiol. 1996;270:F432–F439. doi: 10.1152/ajprenal.1996.270.3.F432. [DOI] [PubMed] [Google Scholar]
- 62.Wall SM. Mechanisms of NH4+ and NH3 transport during hypokalemia. Acta Physiol. Scand. 2003;179:325–330. doi: 10.1046/j.0001-6772.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 63.Wall SM, Fischer MP, Kim GH, Nguyen BM, Hassell KA. In rat inner medullary collecting duct, NH4+ uptake by the Na,K-ATPase is increased during hypokalemia. Am. J. Physiol. Renal Physiol. 2002;282:F91–F102. doi: 10.1152/ajprenal.0141.2001. [DOI] [PubMed] [Google Scholar]
- 64.Attmane-Elakeb A, Boulanger H, Vernimmen C, Bichara M. Apical location and inhibition by arginine vasopressin of K+/H+ antiport of the medullary thick ascending limb of rat kidney. J. Biol. Chem. 1997;272:25668–25677. doi: 10.1074/jbc.272.41.25668. [DOI] [PubMed] [Google Scholar]
- 65.Cougnon M, Bouyer P, Jaisser F, Edelman A, Planelles G. Ammonium transport by the colonic H+-K+-ATPase expressed in Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1999;277:C280–C287. doi: 10.1152/ajpcell.1999.277.2.C280. [DOI] [PubMed] [Google Scholar]
- 66.Codina J, Pressley TA, DuBose TD., Jr The colonic H+,K+-ATPase functions as a Na+-dependent K+(NH4+)-ATPase in apical membranes from rat distal colon. J. Biol. Chem. 1999;274:19693–19698. doi: 10.1074/jbc.274.28.19693. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura S, Amlal H, Galla JH, Soleimani M. NH4+ secretion in inner medullary collecting duct in potassium deprivation: role of colonic H+-K+-ATPase. Kidney Int. 1999;56:2160–2167. doi: 10.1046/j.1523-1755.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 68.Swarts HGP, Koenderink JB, Willems PHGM, De Pont JJ. The non-gastric H,K-ATPase is oligomycin-sensitive and can function as an H+, NH4+-ATPase. J. Biol. Chem. 2005;280:33115–33122. doi: 10.1074/jbc.M504535200. [DOI] [PubMed] [Google Scholar]
- 69.Frank AE, Wingo CS, Andrews PM, Ageloff S, Knepper MA, Weiner ID. Mechanisms through which ammonia regulates cortical collecting duct net proton secretion. Am. J. Physiol. Renal Physiol. 2002;282:F1120–F1128. doi: 10.1152/ajprenal.00266.2001. [DOI] [PubMed] [Google Scholar]
- 70.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 71.Knepper MA. The aquaporin family of molecular water channels. Proc. Natl. Acad. Sci. USA. 1994;91:6255–6258. doi: 10.1073/pnas.91.14.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH in oocytes expressing aquaporin-1. Am. J. Physiol. Renal Physiol. 2001;281:F255–F263. doi: 10.1152/ajprenal.2001.281.2.F255. [DOI] [PubMed] [Google Scholar]
- 73.Holm LM, Jahn TP, Moller AL, Schjoerring JK, Ferri D, et al. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pfügers Arch. 2005;450:415–428. doi: 10.1007/s00424-005-1399-1. [DOI] [PubMed] [Google Scholar]
- 74.Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/ arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl. Acad. Sci. USA. 2006;103:269–274. doi: 10.1073/pnas.0507225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elkjar ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, et al. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am. J. Physiol. Renal Physiol. 2001;281:F1047–F1057. doi: 10.1152/ajprenal.0158.2001. [DOI] [PubMed] [Google Scholar]
- 77.Yang B, Song Y, Zhao D, Verkman AS. Phenotype analysis of aquaporin-8 null mice. Am. J. Physiol Cell Physiol. 2005;288:C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- 78.Yang B, Zhao D, Solenov E, Verkman AS. Evidence from knockout mice against physiologically significant aquaporin 8-facilitated ammonia transport. Am. J. Physiol. Cell Physiol. 2006;291:C417–C423. doi: 10.1152/ajpcell.00057.2006. [DOI] [PubMed] [Google Scholar]
- 79.de Groot BL, Grubmuller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. doi: 10.1126/science.1066115. [DOI] [PubMed] [Google Scholar]
- 80.von Wiren N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000;3:254–261. [PubMed] [Google Scholar]
- 81.Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, vonWiren N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell. 1999;11:937–948. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J. Biol. Chem. 2001;276:1424–1433. doi: 10.1074/jbc.M007528200. [DOI] [PubMed] [Google Scholar]
- 83.Liu Z, Chen Y, Mo R, Hui C, Cheng JF, et al. Characterization of human RhCG and mouse Rhcg as novel nonerythroid Rh glycoprotein homologues predominantly expressed in kidney and testis. J. Biol. Chem. 2000;275:25641–25651. doi: 10.1074/jbc.M003353200. [DOI] [PubMed] [Google Scholar]
- 84.Marini AM, Urrestarazu A, Beauwens R, Andre B. The Rh (rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem. Sci. 1997;22:460–461. doi: 10.1016/s0968-0004(97)01132-8. [DOI] [PubMed] [Google Scholar]
- 85.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat. Genet. 2000;26:341–344. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 86.Westhoff CM, Ferreri-Jacobia M, Mak DD, Foskett JK. Identification of the erythrocyte Rh-blood group glycoprotein as a mammalian ammonium transporter. J. Biol. Chem. 2002;277:12499–12502. doi: 10.1074/jbc.C200060200. [DOI] [PubMed] [Google Scholar]
- 87.Westhoff CM, Siegel DL, Burd CG, Foskett JK. Mechanism of genetic complementation of ammonium transport in yeast by human erythrocyte Rh-associated glycoprotein (RhAG) J. Biol. Chem. 2004;279:17443–17448. doi: 10.1074/jbc.M311853200. [DOI] [PubMed] [Google Scholar]
- 88.Ripoche P, Bertrand O, Gane P, Birkenmeier C, Colin Y, Cartron JP. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc. Natl. Acad. Sci. USA. 2004;101:17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benjelloun F, Bakouh N, Fritsch J, Hulin P, Lipecka J, et al. Expression of the human erythroid Rh glycoprotein (RhAG) enhances both NH3 and NH4+ transport in HeLa cells. Pflügers Arch. 2005;450:155–167. doi: 10.1007/s00424-005-1381-y. [DOI] [PubMed] [Google Scholar]
- 90.Cartron JP. RH blood group system and molecular basis of Rh-deficiency. Baillieres Best Pract. Res. Clin. Haematol. 1999;12:655–689. doi: 10.1053/beha.1999.0047. [DOI] [PubMed] [Google Scholar]
- 91.Liu Z, Huang CH. The mouse Rhl1 and Rhag genes: sequence, organization, expression, and chromosomal mapping. Biochem. Genet. 1999;37:119–138. doi: 10.1023/a:1018726303397. [DOI] [PubMed] [Google Scholar]
- 92.Conroy MJ, Bullough PA, Merrick M, Avent ND. Modelling the human rhesus proteins: implications for structure and function. Br. J. Haematol. 2005;131:543–551. doi: 10.1111/j.1365-2141.2005.05786.x. [DOI] [PubMed] [Google Scholar]
- 93.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, et al. Expression of the ammonia transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the intestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1036–G1047. doi: 10.1152/ajpgi.00418.2004. [DOI] [PubMed] [Google Scholar]
- 94.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, et al. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J. Am. Soc. Nephrol. 2003;14:545–554. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- 95.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C glycoprotein, in the mouse kidney. Am. J. Physiol. Renal Physiol. 2003;284:F323–F337. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 96.Lopez C, Metral S, Eladari D, Drevensek S, Gane P, et al. The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J. Biol. Chem. 2004;280:8221–8228. doi: 10.1074/jbc.M413351200. [DOI] [PubMed] [Google Scholar]
- 97.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology. 2003;124:1432–1440. doi: 10.1016/s0016-5085(03)00277-4. [DOI] [PubMed] [Google Scholar]
- 98.Kaiser S, Gerok W, Haussinger D. Ammonia and glutamine metabolism in human liver slices: new aspects on the pathogenesis of hyperammonaemia in chronic liver disease. Eur. J. Clin. Invest. 1988;18:535–542. doi: 10.1111/j.1365-2362.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 99.Czarnowski D, Gorski J. Sweat ammonia excretion during submaximal cycling exercise. J. Appl. Physiol. 1991;70:371–374. doi: 10.1152/jappl.1991.70.1.371. [DOI] [PubMed] [Google Scholar]
- 100.Brusilow SW, Gordes EH. Ammonia secretion in sweat. Am. J. Physiol. 1968;214:513–517. doi: 10.1152/ajplegacy.1968.214.3.513. [DOI] [PubMed] [Google Scholar]
- 101.Mak DD, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am. J. Physiol. Renal Physiol. 2006;290:F297–F305. doi: 10.1152/ajprenal.00147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ludewig U. Electroneutral ammonium transport by basolateral Rhesus B glycoprotein. J. Physiol. 2004;559:751–759. doi: 10.1113/jphysiol.2004.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zidi-Yahiaoui N, Mouro-Chanteloup I, D’Ambrosio AM, Lopez C, Gane P, et al. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem. J. 2005;391:33–40. doi: 10.1042/BJ20050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am. J. Physiol. Renal Physiol. 2004;288:F170–F181. doi: 10.1152/ajprenal.00419.2003. [DOI] [PubMed] [Google Scholar]
- 105.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, et al. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am. J. Physiol. Renal Physiol. 2006;290:F397–F408. doi: 10.1152/ajprenal.00162.2005. [DOI] [PubMed] [Google Scholar]
- 106.Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin WK, et al. Intercalated cell H+/OH− transporter expression is reduced in Slc26a4 null mice. Am. J. Physiol. Renal Physiol. 2005;289:F1262–F1272. doi: 10.1152/ajprenal.00206.2005. [DOI] [PubMed] [Google Scholar]
- 107.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, et al. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am. J. Physiol. Renal Physiol. 2005;289:F1281–F1290. doi: 10.1152/ajprenal.00172.2005. [DOI] [PubMed] [Google Scholar]
- 108.Weiner ID. Expression of the non-erythroid Rh glycoproteins in mammalian tissues. Transfus. Clin. Biol. 2006;13:159–163. doi: 10.1016/j.tracli.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, et al. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J. Am. Soc. Nephrol. 2002;13:1999–2008. doi: 10.1097/01.asn.0000025280.02386.9d. [DOI] [PubMed] [Google Scholar]
- 110.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, et al. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am. J. Physiol. Renal Physiol. 2006;290:F1443–F1452. doi: 10.1152/ajprenal.00459.2005. [DOI] [PubMed] [Google Scholar]
- 111.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, et al. Expression of the ammonia transporter, Rh C glycoprotein, in normal and neoplastic human kidney. J. Am. Soc. Nephrol. 2006 doi: 10.1681/ASN.2006020160. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, et al. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J. Biol. Chem. 2004;279:15975–15983. doi: 10.1074/jbc.M308528200. [DOI] [PubMed] [Google Scholar]
- 113.Nakhoul NL, Palmer SA, Abdulnour-Nakhoul S, Hering-Smith K, Hamm LL. Ammonium transport in oocytes expressing Rheg. FASEB J. 2002;16(4):A53. (Abstr.) [Google Scholar]
- 114.Weiner ID. The Rh gene family and renal ammonium transport. Curr. Opin. Nephrol. Hypertens. 2004;13:533–540. doi: 10.1097/00041552-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 115.Conroy MJ, Jamieson SJ, Blakey D, Kaufmann T, Engel A, et al. Electron and atomic force microscopy of the trimeric ammonium transporter AmtB. EMBO Rep. 2004;5:1153–1158. doi: 10.1038/sj.embor.7400296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khademi S, O’Connell J, III, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 117.Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc. Natl. Acad. Sci. USA. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Javelle A, Severi E, Thornton J, Merrick M. Ammonium sensing in Escherichia coli: role of the ammonium transporter AmtB and AmtB-GlnK complex formation. J. Biol. Chem. 2004;279:8530–8538. doi: 10.1074/jbc.M312399200. [DOI] [PubMed] [Google Scholar]
- 119.Zidi-Yahiaoui N, Ripoche P, Le Van KC, Gane P, D’Ambrosio AM, et al. Ammonium transport properties of HEK293 cells expressing RhCG mutants: preliminary analysis of structure/function by site-directed mutagenesis. Transfus. Clin. Biol. 2006;13:128–131. doi: 10.1016/j.tracli.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 120.Kim KS, Feild E, King N, Yaoi T, Kustu S, Inwood W. Spontaneous mutations in the ammonium transport gene AMT4 of Chlamydomonas reinhardtii. Genetics. 2005;170:631–644. doi: 10.1534/genetics.105.041574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soupene E, Inwood W, Kustu S. Lack of the Rhesus protein Rh1 impairs growth of the green alga Chlamydomonas reinhardtii at high CO2. Proc. Natl. Acad. Sci. USA. 2004;101:7787–7792. doi: 10.1073/pnas.0401809101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soupene E, King N, Feild E, Liu P, Niyogi KK, et al. Rhesus expression in a green alga is regulated by CO2. Proc. Natl. Acad. Sci. USA. 2002;99:7769–7773. doi: 10.1073/pnas.112225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang CH, Peng J. Evolutionary conservation and diversification of Rh family genes and proteins. Proc. Natl. Acad. Sci. USA. 2005;102:15512–15517. doi: 10.1073/pnas.0507886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Endeward V, Cartron JP, Ripoche P, Gros G. Red cell membraneCO2 permeability in normal human blood and in blood deficient in various blood groups, and effect of DIDS. Transfus. Clin. Biol. 2006;13:123–127. doi: 10.1016/j.tracli.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 125.Ripoche P, Goossens D, Devuyst O, Gane P, Colin Y, et al. Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: a stopped-flow analysis. Transfus. Clin. Biol. 2006;13:117–122. doi: 10.1016/j.tracli.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Mudry B, Guy RH, Gado-Charro MB. Transport numbers in transdermal iontophoresis. Biophys. J. 2006;90:2822–2830. doi: 10.1529/biophysj.105.074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Atkins PW. Molecules in motion: ion transport and molecular diffusion. In: Atkins PW, editor. Physical Chemistry. Oxford, UK: Oxford Univ. Press; 1978. pp. 819–848. [Google Scholar]
- 128.Falk KG. Transference numbers of electrolytes in aqueous solutions. In: Washburn EW VI, editor. International Critical Tables of Numerical Data, Physics, Chemistry and Technology. New York: McGraw-Hill; 1929. pp. 809–811. [Google Scholar]
- 129.LeMasurier M, Heginbotham L, Miller C. KcsA: It’s a potassium channel. J. Gen. Physiol. 2001;118:303–314. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heginbotham L, MacKinnon R. Conduction properties of the cloned Shaker K+ channel. Biophys. J. 1993;65:2089–2096. doi: 10.1016/S0006-3495(93)81244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eisenman G, Latorre R, Miller C. Multi-ion conduction and selectivity in the high-conductance Ca++-activated K+ channel from skeletal muscle. Biophys. J. 1986;50:1025–1034. doi: 10.1016/S0006-3495(86)83546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]