Abstract
Cognitive decline and dementia are a major cause of disability and mortality among older adults. Cross-sectional evidence from observational studies suggests that greater arterial stiffness is associated with worse cognitive performance. These associations have been observed on measures of global cognition and across multiple domains of cognition. Epidemiologic evidence on the association between arterial stiffness and rate of cognitive decline has been less definitive, and very few studies have investigated the risk of developing dementia. This review summarizes the current research on arterial stiffness and cognition, issues around measurement and the effect that potential intervention might have on the course of cognitive aging. The evidence on pharmacological and non-pharmacological (exercise, nutrition, etc) interventions in older adults with arterial stiffness is promising. Yet there are no studies or trials that directly evaluate how interventions of arterial stiffness reduce or prevent cognitive impairment and risk of developing dementia. More research is needed to elucidate the causal link between arterial stiffness and cognitive decline and dementia, and to identify whether potential interventions to prevent or reduce arterial stiffness may benefit cognitive health of the elderly.
Keywords: Aging, Arterial stiffness, Cognitive decline, Dementia, Epidemiology
Cognitive impairment is very common in elderly persons and is associated with increased disability and mortality [1, 2]. In addition, there is a large societal burden associated with cognitive impairment including, caregiver burden and large health care expenditures [3]. As our population continues to age, understanding the epidemiology of cognitive impairment is becoming very important as it may prevent or delay cognitive decline and dementia pathology.
There is increasing evidence that cardiovascular disease and its risk factors contribute to the development of cognitive impairment and dementia [3-7]. With its rich vascularization and low resistance to flow, the brain is particularly susceptible to cardiovascular dynamics [8-10]. Recently, new vascular markers including, markers of arterial stiffness and arterial pressure [11, 12] have been shown as useful predictors of cardiovascular risk [13-17]. Consequently, there has been increasing interest in evaluating whether these markers are predictors of cognitive impairment and dementia [18-30]. Despite our understanding of the pathophysiologic mechanisms through which arterial stiffness may influence cognitive aging, epidemiologic evidence suggesting its role in cognitive decline and dementia remains unclear and relatively unexplored.
The goal of this review is to summarize the evidence on the relationship between arterial stiffness and cognitive aging. To do so, we will first review evidence from epidemiologic studies on the associations between arterial stiffness and cognitive impairment, cognitive decline, and dementia, then, we will discuss potential underlying mechanisms. Finally, we will summarize current evidence on interventions of arterial stiffness and their influence on the course of cognitive aging.
Cognitive Aging
Cognitive function can be assessed using measures of function for specific cognitive domains or for global cognitive function. We report findings on global cognitive function using measures such as the Mini-Mental State Exam, the Modified Mini Mental State Exam, or some other global composite score. It is suggested that cardiovascular mechanisms, including arterial stiffness, may influence specific cognitive domains [31, 32]. Therefore, we also report findings on specific cognitive domains such as, executive function, memory, and processing speed. There are several ways to characterize cognitive function. While one can examine cognitive performance at a single time point, examining rate of cognitive decline is important for understanding the etiology of cognitive impairment. Therefore, we will provide evidence of associations with both cross-sectional cognitive function and longitudinal/change in cognitive function. Finally, we report studies with findings on dementia. Dementia is defined as a decline in at least two cognitive domains that is severe enough to interfere with functioning [33], and as such dementia provides an important clinical endpoint. Unfortunately, the literature on arterial stiffness and dementia is very scarce and thus does not allow us to examine and report associations with various causes and categories of dementia.
Arterial Aging
As we age, the walls of the aorta undergo increased stiffness that is attributed to changes in the wall structure or function [34-36]. The stiffness is triggered by an increase in collagen and calcium deposition resulting in a thickness of the arterial wall [34-36]. The arterial walls may also undergo hemodynamic-induced elastin fragmentation resulting in loss of function [34-36]. The loss of arterial elastin alters vascular tone and compliance resulting in less extensible walls and thus more stiffened arteries. This has consequences on the ability of the arteries to cushion and accommodate increases in pulse wave propagation.
The aortic pressure waveform is comprised of two pressure waves: a forward pressure wave and a backward reflected pressure wave. In a low-stiffness or non-stiffened aorta, the reflected waves return to the central aorta in early diastole. However in a stiffened aorta, the speed of the arterial wave propagation (i.e. arterial pulse wave velocity (PWV)) increases, and thus the reflected waves that normally return to the central aorta in early diastole return prematurely during systole. The latter will cause an increase in the maximal systolic blood pressure (BP) and aortic central pulse pressure (PP) [8, 9, 37].
Detection of arterial stiffness: markers of arterial aging
The most common measures used to assess arterial stiffness include carotid-femoral pulse wave velocity (cf-PWV), aortic PP, and augmentation index [9, 38-40]. Cf-PWV is regarded as the gold standard measure of arterial stiffness; measurement consists of using applanation tonometry, which measures the velocity of the forward and backward propagation waves between the carotid and femoral arteries. The distance between the carotid and femoral arteries is also measured above the body surface, typically using a measuring tape. Cf-PWV is then calculated as the distance between the carotid and femoral arteries divided by the time differential for the pressure wave to reach both arteries (i.e. PWV=time/distance). As a result of increasing arterial stiffness, the wave propagates faster and the time between the carotid systolic peak and femoral systolic peak decreases, resulting in an increase in PWV. Using ultrasound, PWV may also be measured at the brachial-ankle (ba-PWV) [41] or at the aorta itself (aPWV) [42]. Other measures of arterial stiffness include PP and augmentation index. PP is an indirect measure of arterial stiffness and is calculated as systolic blood pressure minus diastolic blood pressure. Augmentation index is the percentage of aortic pressure augmentation relative to pulse pressure [9]. Measures of PWV, especially the cf-PWV, provide more direct measures of arterial stiffness than other measures such as PP and augmentation index. The increase in central pulse pressure is the consequence of the change in the speed of the arterial wave propagation (described earlier). As such, compared to PP, cfPWV is a more direct measure of arterial stiffness as it measures the speed and distance traveled by the waveform. Furthermore, PP is more dependent on hemodynamic factors, such as arterial diameter and ejection fraction, which makes an increase in PP not necessarily related to arterial stiffness, and thus further making PP not as accurate as cfPWV.
Epidemiologic literature on the cross-sectional association between arterial stiffness and cognitive function
Table 1 summarizes results from cross-sectional studies examining the association between arterial stiffness and cognitive function [18-21, 23, 25, 27-30]. Overall, the evidence suggests that greater arterial stiffness is associated with worse cognitive function. The majority of studies that examined the association between arterial stiffness and global cognitive function, as measured by the MMSE or 3MS, showed consistent results suggesting that greater stiffness is associated with worse global function [18-20, 23, 25, 27, 30]. Similarly, studies reported an association between arterial stiffness and cognitive function in one or more domains, including executive function, processing speed, or verbal memory, but across studies, there is not a consistent association in individual domains. Only one study, the Sidney Memory and Aging Study, failed to find a cross-sectional association between arterial stiffness and at least one domain from various measures of cognitive function [28].
Table 1.
Studies of the cross-sectional association between arterial stiffness and cognitive function
| Publication | Cohort | Age (years) |
Arterial stiffness measure |
Cognitive tests assessed | Result |
|---|---|---|---|---|---|
| Elias et al., 2009 [21] |
Maine-Syracuse Longitudinal study, (N=409), USA |
24-92 | Carotid-femoral PWV |
|
|
| Fukuhara et al., 2006 [18] |
From community, Japan (N=203) |
85 | Brachial-Ankle PWV |
|
|
| Mitchell et al., 2011 [27] |
AGES-Reykjavik Study, (N=668), Iceland |
69-93 | Carotid-femoral PWV, Pulse Pressure (PP) |
|
|
| Poels et al., 2007 [23] |
Rotterdam Study, (N=3714), Netherlands |
55+ | Carotid-femoral PWV |
|
|
| Scuteri et al., 2005 [19] |
From hospital, (N=84), Italy |
78±5 | Carotid-femoral PWV |
|
|
| Singer et al., 2013 [28] |
The community-based Sidney Memory and Aging Study (N=319), Australia |
70-90 | Carotid-femoral PWV |
|
|
| Triantafyllidi et al., 2009 [20] |
From Hospital, (N=110), Greece |
40-80 | Carotid-femoral PWV |
|
|
| Tsao et al., 2013 [29] |
Framingham Offspring cohort study, (N=1559), USA |
61±9 | Carotid-femoral PWV, Pulse pressure |
|
|
| Watson et al., 2011 [25] |
The Cognitive Vitality Substusy (N=552), in the Health, Aging, and Body Composition Study, USA |
73.1±2.7 | Carotid-femoral PWV |
|
|
| Zhong et al., 2014 [30] |
The Epidemiology of Hearing Loss Study, (N=1436), USA |
43-84 | Carotid-femoral PWV |
|
|
Epidemiologic literature on the longitudinal association between arterial stiffness and cognitive function
Examining rate of cognitive decline is important for understanding the etiology of cognitive impairment. Furthermore, longitudinal studies offer a better study design than cross-sectional studies by allowing to establishing temporality. Table 2 summarizes results from longitudinal epidemiologic studies examining the association between arterial stiffness and cognitive decline. As evident in Table 2, only a handful studies have addressed this research question and have reported inconsistent findings [7, 22-26, 43]. Many studies report an association between arterial stiffness and decline in one or more domains, but across studies, there is not a consistent association between arterial stiffness and decline in individual domains.
Table 2.
Studies of the longitudinal association between arterial stiffness and cognitive function
| Publication | Cohort | Age (years) |
Follow-up | Arterial stiffness measure |
Cognitive tests assessed | Result |
|---|---|---|---|---|---|---|
| Benetos et al., 2012 [26] |
PARTAGE study, (N=873), institutionalized patients from France and Italy |
80+ | 1 year | Carotid-femoral PWV |
|
|
| Poels et al., 2007 [23] |
Rotterdam Study, (N=3714), Netherlands |
55+ | Average 5 years |
Carotid-femoral PWV |
|
|
| Scuteri et al., 2007 [24] |
From hospital, N=102, Italy |
79±6 | Median of 12 months (range: 10 to 32) |
Carotid-femoral PWV |
|
|
| Scuteri et al., 2013 [43] |
From hospital, N=105, Italy |
77±5 | Median of 15 months |
Carotid-femoral PWV |
|
|
| Waldstein et al., 2008 [22] |
Baltimore Longitudinal Study of Aging, N=1749 (PP analysis) and N=582 (PWV analysis), USA |
57.1±17.2 (PP) 54.3±17.1 (PWV) |
14 years | Carotid-femoral PWV, Pulse Pressure |
|
|
| Watson et al., 2011 [25] |
The Cognitive Vitality Substusy (N=552), in the Health, Aging, and Body Composition Study, USA |
73.1±2.7 | 6 years | Carotid-femoral PWV |
|
|
| Zeki Al Hazzouri et al., 2013 [7] |
Health, Aging, and Body Composition Study, (N=2,488), USA |
74.2±2.9 | 9 years | Carotid-femoral PWV |
|
|
Findings from the Rotterdam study failed to find an association between arterial stiffness (c-f PWV) and cognitive decline on global cognitive function and domain-specific function. These participants were free of dementia when neuropsychological testing was first performed, and then were followed over an average of five years after which neuropsychological testing was performed again (i.e. two time points), which may not be enough time to observe significant cognitive decline [23]. Furthermore, the Rotterdam study included middle-aged and older-aged participants, which may have diluted any association. In contrast, findings from the Baltimore Longitudinal Study suggest an association between greater arterial stiffness (c-f PWV and PP) and decline in verbal learning memory and non-verbal memory functioning (free recall and visual memory) over 11 years of follow-up of a community-dwelling cohort free of dementia and cerebrovascular diseases [22]. Furthermore, an association was observed between arterial stiffness (c-f PWV) and decline in psychomotor speed in the Cognitive Vitality Substudy of the Health, Aging and Body Composition study [25]. Further findings from the full cohort of the Health, Aging, and Body Composition study, a cohort of well-functioning older adults, showed an association between arterial stiffness (c-f PWV) and decline in global cognitive function measured using the modified Mini Mental Status Exam (3MS) [7]. Similarly, results from the PARTAGE study of patients aged 80+ and without dementia showed an association between greater arterial stiffness and decline in performance on the MMSE over 1 year of follow-up [26]. Similar results were observed among elderly patients followed over a year on average, showing greater c-f PWV associated with decline in MMSE score [24, 43].
According to a recent meta-analysis by Pase et al. 2012 [44], there was an overall association between arterial stiffness and cognitive decline measured on the MMSE. However, the association was of small magnitude which, as the authors note, could be due to ceiling effects and the inability of the neuropsychological test, in this case the MMSE, to detect cognitive changes in well-functioning and healthy adults. As for the association between arterial stiffness and decline in specific cognitive domains, the findings are inconsistent across studies but with many studies reporting an association between arterial stiffness and decline in one or more domains [22, 25].
The inconsistency of results across studies may reflect a weak relationship between arterial stiffness and cognitive decline, or may be due to differences in the cognitive domains assessed, differences in aspects of the study design, including the specific neuropsychological tests used, how rate of cognitive decline was modeled, length of follow-up, inclusion and exclusion criteria, or differences in participants’ characteristics. The sensitivity of some of the neuropsychological tests such as the MMSE may be compromised in well-functioning and healthier cohorts, which may explain some of the inconsistencies between studies. Furthermore, shared determinants of arterial stiffness and cognitive decline may confound the association and result in between-studies inconsistency. Possible confounders include age, socioeconomic factors, behavioral factors, such as diet, smoking, and physical activity, and co-morbidities, such as obesity, hypertension, diabetes, and depressive symptoms. While many studies adjust for some or all of these factors, residual confounding may still mask the true association. Future studies, especially those with a wide age range, should examine whether the association between arterial stiffness and cognitive function is age-dependent. Finally, potential publication bias may have also contributed to an underrepresentation of null findings from longitudinal studies.
Epidemiologic literature on the association between arterial stiffness and dementia
The association between arterial stiffness and dementia remains relatively unexplored. Cross-sectional evidence from one study of elderly with memory complaints attending a geriatric clinic suggests that subjects with mild cognitive impairment, vascular dementia, and Alzheimer’s disease show greater arterial stiffness (c-f PWV) compared to those with normal cognitive function [45]. Other cross-sectional results have reported that subjects with vascular dementia show greater arterial stiffness than subjects with AD or without dementia [46]. There is only one prospective study of dementia that has been conducted, the Rotterdam study, and which failed to find an association between arterial stiffness and risk of dementia [23].
Taken together, current epidemiologic evidence suggests that arterial stiffness is associated with cognitive impairment and less definitely with cognitive decline. There is less evidence to make any conclusions about the association of arterial stiffness and risk of dementia. More research is needed to better understand the pathological effects of arterial stiffness on the brain and to evaluate any causal link.
Pathophysiologic mechanisms between arterial stiffness and cognitive function
As described earlier, age-related changes in arterial stiffness cause an increase in arterial pulse pressure which ultimately results in hemodynamic stress in the heart and in high-flow end-organs such as the brain. The brain has low resistance to flow. When subjected to high pulsatile stress, the brain arteries become compromised, especially with age and in the presence of certain cardiovascular diseases such as hypertension [8-10, 40].
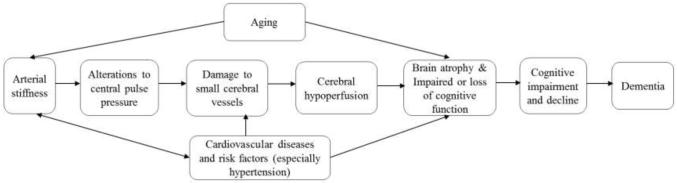
A low stiffened aorta has the ability to cushion pulsatile flow so that it is delivered to the brain in a non-pulsatile form. However in the presence of stiffness, the aorta loses its cushioning capacity and thus pulsatile pressure is transmitted to the brain. Pulsatile pressure predisposes the brain to damages of its small cerebral vessels and results in small arterial diseases [9, 40]. Indeed, high levels of central PP in the brain result in structural changes and dysfunction of its microcirculation [8, 47], resulting in microvascular damage and impaired microvascular function. This in turn may lead to brain atrophy and impaired or loss of cognitive function [8]. In particular, small-vessel disease of the brain has been shown to be associated with changes in the frontal-subcortical regions of the brain that control executive and motor functions; thus suggesting that arterial stiffness may be associated with these specific cognitive domains[31, 32].
Recent brain imaging studies have found arterial stiffness to be associated with cerebral microvascular disease and structural brain changes, including stroke [48], white matter hyperintensities, lacunar infarctions, and cortical brain atrophy [47, 49, 50]. Furthermore, high central PP may result in structural changes to cerebral blood vessels which may in turn interfere with the transport of important nutrients to the brain as well as interfere with the clearance of toxic byproducts out of the brain [51]. Arterial stiffness has also been demonstrated as an independent predictor of cardiovascular events and cardiovascular risk factors [21, 52-54] which are in turn important predictors of cognitive decline. Figure 1 illustrates the potential causal pathways linking arterial stiffness and cognitive decline and dementia.
Figure 1.
Markers of arterial stiffness: potential for intervention
In prior sections, we discussed how arterial stiffness can be detected using various measures that assess pulse wave propagation. To be used as prognostic factors, markers of arterial stiffness have to be easy to implement and of clinical utility. Most measures of arterial stiffness are relatively simple and performed non-invasively. The carotid-femoral PWV, which is the gold standard measure of arterial stiffness, is robust and reproducible and has well-documented clinical reference values [9, 55]. One limitation of measures of PWV is that they are more time consuming compared to other simpler measures of arterial stiffness (e.g. brachial BP), and may require a trained technician [9].
The public health significance of markers of arterial stiffness depends on whether arterial stiffness is modifiable meaning whether it can be reduced, and consequently reduce risk of cognitive aging. The literature on potential pharmacologic treatments of arterial stiffness has largely focused on anti-hypertensive drugs that reduce blood pressure [9, 10, 37, 40, 56, 57] due to the role and potential causal link between hypertension, vascular stiffness, and cognitive impairment. Anti-hypertensive therapy and drugs such as ACEIs (angiotensin-converting-enzyme inhibitors), ARBs (angiotensin receptor blockers), CCBs (calcium channel blockers), and nitrates have been shown to reduce arterial stiffness by reducing the backward-traveling wave reflections which consequently reduces central augmentation and PP [58-60]. While reduction in arterial stiffness may benefit the microvascular function [61], it remains unknown whether modification of arterial stiffness will be causally related to cognitive decline and dementia incidence. This is an important area of future examination.
Non-pharmacological interventions that reduce arterial stiffness have been promising. There is a growing literature suggesting that lifestyle and dietary changes may reduce blood pressure and arterial stiffness [62, 63]. Indeed, aerobic exercise through improved endothelial function [64, 65], smoking cessation [62], and dietary factors such as omega-3 and flavonoids have been shown to reduce blood pressure and arterial stiffness. While it remains unknown whether reduction in arterial stiffness will be causally related to a reduced risk of cognitive decline and dementia, many of these behavioral and lifestyle factors have been shown to benefit cognitive health.
CONCLUSION
There is evidence that arterial stiffness in the elderly is cross-sectionally associated with cognitive impairment. The evidence of an association with cognitive decline is less definitive, and conclusions about its association with dementia incidence cannot yet be made. Future research should carefully implement cognitive testing that is sensitive to subtle cognitive changes, especially in younger and healthier populations. Future studies should also seek to understand whether arterial stiffness has a causal effect on cognitive decline and dementia and if so, through what mechanisms. Finally, the evidence that exists on interventions in older adults with arterial stiffness, especially non-pharmacological, is promising. Thus, the beneficial effects of these interventions on reducing or preventing cognitive decline are yet to be determined.
Acknowledgments
Sources of funding Dr. Zeki Al Hazzouri was supported by a grant from the NIH, National Institute on Aging (K01AG047273) and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2TR000143.
References
- [1].Gill TM, Richardson ED, Tinetti ME. Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. J Gerontol A Biol Sci Med Sci. 1995;50:M235–241. doi: 10.1093/gerona/50a.5.m235. [DOI] [PubMed] [Google Scholar]
- [2].Liu IY, LaCroix AZ, White LR, Kittner SJ, Wolf PA. Cognitive impairment and mortality: a study of possible confounders. Am J Epidemiol. 1990;132:136–143. doi: 10.1093/oxfordjournals.aje.a115625. [DOI] [PubMed] [Google Scholar]
- [3].Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- [5].Zeki Al Hazzouri A, Haan MN, Yingzi D, Neuhaus JM, Yaffe K. Reduced heart rate variability is associated with worse cognitive performance in elderly Mexican Americans. Hypertension. 2014;63:181–187. doi: 10.1161/HYPERTENSIONAHA.113.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zeki Al Hazzouri A, Haan MN, Neuhaus JM, Pletcher M, Peralta CA, Lopez L, Perez Stable EJ. Cardiovascular risk score, cognitive decline, and dementia in older mexican americans: the role of sex and education. Journal of the American Heart Association. 2013;2:e004978. doi: 10.1161/JAHA.113.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N, Satterfield S, Harris T, Yaffe K. Pulse Wave Velocity and Cognitive Decline in Elders: The Health, Aging, and Body Composition Study. Stroke; a journal of cerebral circulation. 2013;44:388–393. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pase MP. Modifiable vascular markers for cognitive decline and dementia: the importance of arterial aging and hemodynamic factors. Journal of Alzheimer’s disease : JAD. 2012;32:653–663. doi: 10.3233/JAD-2012-120565. [DOI] [PubMed] [Google Scholar]
- [10].O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- [11].Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- [12].Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- [13].La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- [14].Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- [15].Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- [16].Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA : the journal of the American Medical Association. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- [17].Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- [18].Fukuhara M, Matsumura K, Ansai T, Takata Y, Sonoki K, Akifusa S, Wakisaka M, Hamasaki T, Fujisawa K, Yoshida A, Fujii K, Iida M, Takehara T. Prediction of cognitive function by arterial stiffness in the very elderly. Circulation journal : official journal of the Japanese Circulation Society. 2006;70:756–761. doi: 10.1253/circj.70.756. [DOI] [PubMed] [Google Scholar]
- [19].Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23:1211–1216. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- [20].Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, Trivilou P, Zerva L, Stamboulis E, Kremastinos DT. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens. 2009;22:525–530. doi: 10.1038/ajh.2009.35. [DOI] [PubMed] [Google Scholar]
- [21].Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–673. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- [23].Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke; a journal of cerebral circulation. 2007;38:888–892. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- [24].Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–1040. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- [25].Watson NL, Sutton-Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM, Najjar SS, Launer LJ, Yaffe K, Atkinson HH, Satterfield S, Newman AB. Arterial Stiffness and Cognitive Decline in Well-Functioning Older Adults. J Gerontol A Biol Sci Med Sci. 2011;66:1336–1342. doi: 10.1093/gerona/glr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, Manckoundia P, Agnoletti D, Labat C, Gautier S. Pulse Wave Velocity is Associated With 1-Year Cognitive Decline in the Elderly Older than 80 Years: The PARTAGE Study. Journal of the American Medical Directors Association. 2012;13:239–243. doi: 10.1016/j.jamda.2010.08.014. [DOI] [PubMed] [Google Scholar]
- [27].Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain : a journal of neurology. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Singer J, Trollor JN, Crawford J, O’Rourke MF, Baune BT, Brodaty H, Samaras K, Kochan NA, Campbell L, Sachdev PS, Smith E. The association between pulse wave velocity and cognitive function: the Sydney Memory and Ageing Study. PloS one. 2013;8:e61855. doi: 10.1371/journal.pone.0061855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhong W, Cruickshanks KJ, Schubert CR, Carlsson CM, Chappell RJ, Klein BE, Klein R, Acher CW. Pulse wave velocity and cognitive function in older adults. Alzheimer Dis Assoc Disord. 2014;28:44–49. doi: 10.1097/WAD.0b013e3182949f06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [32].Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- [33].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- [35].Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- [36].Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055. doi: 10.1161/01.HYP.0000164580.39991.3d. [DOI] [PubMed] [Google Scholar]
- [37].O’Rourke MF, Adji A, Namasivayam M, Mok J. Arterial aging: a review of the pathophysiology and potential for pharmacological intervention. Drugs Aging. 2011;28:779–795. doi: 10.2165/11592730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [38].Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- [39].Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- [40].Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. Journal of Alzheimer’s disease : JAD. 2012;32:541–549. doi: 10.3233/JAD-2012-120757. [DOI] [PubMed] [Google Scholar]
- [41].Sugawara N, Yasui-Furukori N, Umeda T, Kaneda A, Sato Y, Takahashi I, Matsuzaka M, Danjo K, Nakaji S, Kaneko S. Comparison of ankle-brachial pressure index and pulse wave velocity as markers of cognitive function in a community-dwelling population. BMC psychiatry. 2010;10:46. doi: 10.1186/1471-244X-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vappou J, Luo J, Okajima K, Di Tullio M, Konofagou E. Aortic Pulse Wave Velocity Measured by Pulse Wave Imaging (Pwi): A Comparison with Applanation Tonometry. Artery research. 2011;5:65–71. doi: 10.1016/j.artres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Scuteri A, Tesauro M, Guglini L, Lauro D, Fini M, Di Daniele N. Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol. 2013;169:371–377. doi: 10.1016/j.ijcard.2013.09.009. [DOI] [PubMed] [Google Scholar]
- [44].Pase MP, Herbert A, Grima NA, Pipingas A, O’Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Internal medicine journal. 2012;42:808–815. doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- [45].Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke; a journal of cerebral circulation. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- [46].Mizushima Y, Oobasawa H, Yoshida S, Irie H, Urata T, Shimoda H. Pulse wave velocity in persons with vascular dementia. J Am Geriatr Soc. 2003;51:1329–1330. doi: 10.1046/j.1532-5415.2003.514208.x. [DOI] [PubMed] [Google Scholar]
- [47].Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- [48].Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke; a journal of cerebral circulation. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- [49].Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke; a journal of cerebral circulation. 2009;40:1229–1236. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- [50].Ohmine T, Miwa Y, Yao H, Yuzuriha T, Takashima Y, Uchino A, Takahashi-Yanaga F, Morimoto S, Maehara Y, Sasaguri T. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:75–81. doi: 10.1291/hypres.31.75. [DOI] [PubMed] [Google Scholar]
- [51].Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke; a journal of cerebral circulation. 2009;40:S40–44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- [53].Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- [54].Marioni RE, Strachan MW, Reynolds RM, Lowe GD, Mitchell RJ, Fowkes FG, Frier BM, Lee AJ, Butcher I, Rumley A, Murray GD, Deary IJ, Price JF. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:710–713. doi: 10.2337/db09-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409–413. doi: 10.1161/HYPERTENSIONAHA.109.133801. [DOI] [PubMed] [Google Scholar]
- [57].Matsui Y, Eguchi K, O’Rourke MF, Ishikawa J, Miyashita H, Shimada K, Kario K. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension. 2009;54:716–723. doi: 10.1161/HYPERTENSIONAHA.109.131466. [DOI] [PubMed] [Google Scholar]
- [58].Hirata K, Vlachopoulos C, Adji A, O’Rourke MF. Benefits from angiotensin-converting enzyme inhibitor ‘beyond blood pressure lowering’: beyond blood pressure or beyond the brachial artery? J Hypertens. 2005;23:551–556. doi: 10.1097/01.hjh.0000160211.56103.48. [DOI] [PubMed] [Google Scholar]
- [59].Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension. 2003;41:297–301. doi: 10.1161/01.hyp.0000049622.07021.4f. [DOI] [PubMed] [Google Scholar]
- [60].Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17:118–123. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- [61].Safar ME. Peripheral pulse pressure, large arteries, and microvessels. Hypertension. 2004;44:121–122. doi: 10.1161/01.HYP.0000135448.73199.75. [DOI] [PubMed] [Google Scholar]
- [62].Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens. 2005;18:137–144. doi: 10.1016/j.amjhyper.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [63].Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- [64].Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32:1531–1535. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Collier SR, Kanaley JA, Carhart R, Jr., Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–686. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]


