During the past few decades there has been enormous progress made in understanding and treating cardiovascular diseases. However, heart failure remains a progressive and debilitating condition with generally poor clinical outcomes and high socio-economic burden. The most common cause of heart failure is due to loss of functional cardiomyocytes from myocardial infarction and subsequent fibrosis leading to adverse remodeling, reduced contractile function, and hemodynamic compromise. Given the dire need for better heart failure treatment, investigators have actively explored strategies to improve cardiac function via numerous approaches including cell transplantation, mechanical device support, or whole organ replacement(1). While a detailed comparison of the merit of each of these approaches is beyond the scope of this article, one strategy that has captured tremendous interest in recent years is the use of highly potent transcription factors to reprogram cells into an alternative fate. The remarkable finding of Yamanaka and colleagues to revert a fully differentiated cell back to its most primitive state using a combination of transcript factors brought forth widespread optimism that a similar approach can be used successfully to reprogram any somatic cell into a different cell type(2–5). This subsequently led to a number of follow-on studies to directly reprogram fibroblasts into other cell lineages(6, 7). In 2010 one such study by Ieda, Srivastava, and colleagues described the generation of cardiomyocyte-like cells (iCM) by overexpressing Gata4, Mef2c and Tbx5 (GMT), transcription factors that have been shown to play important roles in cardiac development(8). While this study reported the generation of 5–10% troponin T expressing cells in vitro, two follow up studies by Qian et al.(9) and Song et al.(10) report the ability of GMT or GMT+Hand1 to reprogram resident cardiac fibroblasts within the failing/fibrotic myocardium into functional cardiomyocytes(CMs). Interestingly, both studies found that reprogramming is more efficient in vivo than in vitro where up to 12% of the infected cells within the injured heart express sarcomeric protein markers. A modest yet statistically significant improvement of ejection fraction was found in both studies after injection with reprogramming viruses.
In a parallel effort, Jayawardena et al. had previous reported the generation of iCMs using lentiviruses to overexpress microRNAs in fibroblasts in vitro(11). In this issue of Circulation Research, Jayawardena et al. extend these earlier findings to show that the lentiviral-mediated overexpression of miRNAs 1, 133, 208 and 499 (a.k.a miR combo) in infarcted hearts in vivo results in the formation of fully mature and functional iCM(12). Similar to the study by Song et al, a FSP1-Cre mouse line was bred with a ROSA26tdTOMATO reporter mouse line to generate a double transgenic line that genetically label all fibroblasts including a subset in the heart. When control non-targeting microRNA (negmiR) was given to hearts undergoing permanent LAD ligation, they found 4% of tdTOMATO+ cells express cardiac troponin T (TropT). Following lentiviral transduction of miR combo the frequency of tdTOMATO/TropT double positive cells increased three-fold to 12%. These double positive cells express additional cardiomyocyte genes such as the gap-junction protein Connexin-43 and sarcomeric α-Actinin. Furthermore, these investigators isolated tdTOMATO positive cells at five- to six-weeks post-MI and found cells with a range of phenotypes from rod-shaped fully mature cardiomyocytes to small nonmyocytes. The rod-shaped cardiomyocytes express TropT, α-Actinin, Connexin 43 and N-Cadherin and harbor similar electrophysiological properties as mature ventricular cardiomyocytes. To address whether the virus injection (and by inference, the generation of iCM) had a positive effect on left ventricular (LV) function post-MI, echocardiographic assessment was performed following LAD ligation for up to three months. Interestingly, miR combo treated mice showed statistically significant increase in ejection fraction starting at two months after treatment when compared to negmiR treated animals. The authors also report a significant reduction of fibrosis within the infarct area.
This new study by Jayawardena et al raises the exciting prospect that direct overexpression of microRNA can be utilized to effect an alternative cell phenotype in vivo when delivered by lentiviral vector into injured hearts. The findings here, nevertheless, should be considered in the context of prior studies using direct cardiac transcription factor over-expression in vitro as well as in vivo. Earlier study by Protze et al.(13) reported that the three-factor combination of myocardin (Myocd), Mef2c and Tbx5 led to higher expression levels of cardiomyocyte genes when compared with GMT alone but that none of the reprogrammed cells generated were considered functional CMs due to their lack of spontaneous beating and inconsistent sarcomeric protein gene expression. Likewise, Christoforou et al.(14) reported a significant induction of endogenous TropT but almost no α-MHC expression by singular GMT overexpression in murine embryonic fibroblasts. However, these authors showed enhancement of reprogramming efficiency by adding Myocd, SRF, Mesp1 and the chromatin remodeling factor Smarcd3 (BAF60c). Using a fluorescent calcium indicator GCaMP driven by a cardiomyocyte-specific TropT promoter, Addis and colleagues(15) reported a reprograming efficiency of 0.03% by treating murine embryonic fibroblasts with GMT alone. With the addition of Hand2 and Nkx2.5, they were able to increase this efficiency up to 1.6%. The efficiency of cardiomyocyte gene induction in these studies are in line with our previous study using GMT(16) and suggest that significant improvement in the methodology of reprogramming or the reprogramming factors used is needed to raise the in vitro reprogramming efficiency to the ranges of 20% or 30% as seen with neuronal reprogramming in vitro(17).
While the reprogramming work in murine cells have raised significant promise for this approach to generate new patient- or disease-specific cardiomyocytes, the translation of this approach to reprogram human fibroblasts appear to be quite complex. A number of groups have reported that GMT alone or GMT with Hand2 were inefficient to induce cardiomyocyte gene expression in human fibroblasts to generate iCMs. However, the introduction of different modifications to the reprogramming cocktail (e.g. different or more transcription factors, addition of small molecules, co-culturing with murine beating cardiomyocytes) has enabled these investigators to generate significant improvement in cardiomyocyte phenotype in reprogrammed fibroblasts(18–20).
Summary/Perspectives
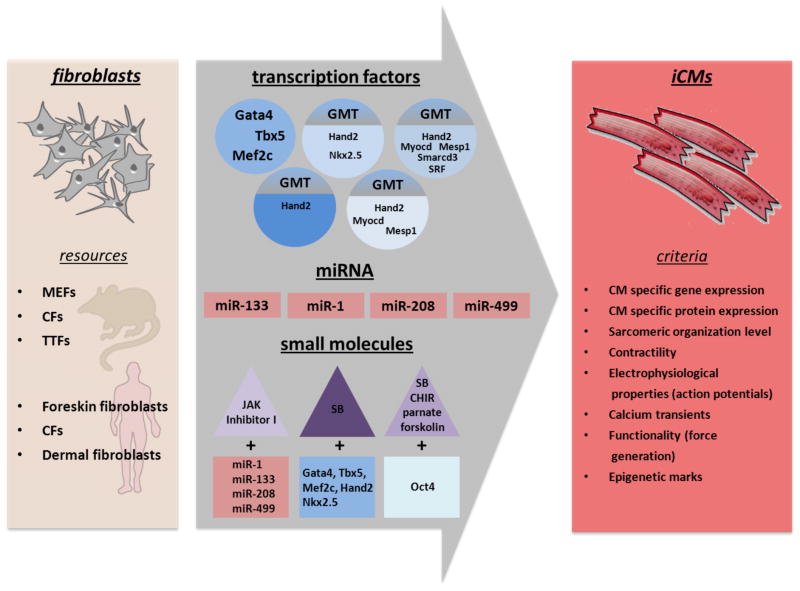
Recent advances in cell lineage reprogramming has open a new era of biology that involves direct cell fate conversion from overexpression of potent transcription factors or in combination with small molecules. (Figure 1) Along this line, Jayawardena and colleagues makes an important contribution here to show the conversion of cardiac fibroblasts into mature cardiomyocytes in vivo using a combination of microRNAs instead of transcription factors. Assuming that the fibroblast marker FSP1-Cre reliably marks only fibroblasts after myocardial injury, the results here suggests that there may be some conversion of fibroblast to CM at baseline since negmiR injection was able to generate a cardiomyocyte phenotype in 4% of the FSP1-Cre labeled fibroblasts. With the addition of miR combo, this efficiency improved three-fold to 12%. These results suggest that direct lineage reprogramming may be remarkably easier in vivo than in vitro and raise the prospect that the identification of the key factor(s) in the heart that helps to improve cardiomyocyte reprogramming should be a major research priority.
Figure 1.
Direct reprogramming strategies for the induction of cardiomyocyte-like cells (iCMs) from different sources of mouse and human fibroblasts. MEFs - murine embryonic fibroblasts; CFs - cardiac fibroblasts; TTFs - tail tip fibroblasts; SB - SB431542: CHIR - CHIR99021.
Beyond the issue of reprogramming efficiency, a number of translational challenges need to be overcome before direct CM reprogramming can be applied clinically. First, the use of lentiviruses as delivery vehicles to target cardiac fibroblasts is problematic from a regulatory standpoint given the known oncogenic potential of these viruses when integrated into the genome. Second, lentiviruses are not specific for fibroblasts and it is unclear whether the generation of induced CMs from coronary endothelial or smooth muscle cells or other cardiac cells would lead adverse consequences. Third, it remains unclear whether the presence of iCMs with heterogeneous phenotypes after direct reprogramming can lead to arrhythmia. Recent finding that the transplantation of human embryonic stem cell-derived CMs into primate heart can generate transient ventricular fibrillation/tachycardia raises the possibility that the presence of immature and heterogeneous but electrically-coupled CMs in the diseased heart may be problematic(21). Finally, we need demonstration of successful direct CM reprogramming and improved in vivo cardiac function in a relevant large animal model. The lack of an identical (or highly overlapping) set of reprogramming factors that works for both mouse and human fibroblasts raises concerns that new factors may need to be discovered de novo for reprogramming of fibroblasts in large animals.
Nevertheless, the prospect for cellular reprogramming to revolutionize cardiac regenerative therapy is exceptionally promising. By acquiring a greater understanding of the epigenetic landscape that regulates cardiomyocyte gene expression and function and the key factors that induces and maintains this landscape, we may one day be able to devise the most optimal strategy to treat damaged hearts in patients with heart failure.
Acknowledgments
We thank the members of the Krane and Wu labs for manuscript critique. MAD is supported by Dr. Rusche Forschungsprojekt (2014) DSHF and DGTHG; MK is supported by Deutsche Stiftung für Herzforschung (F/37/11), Deutsches Zentrum für Herz Kreislauf Forschung (DZHK B 13-050A; DZHK B 14-013SE) and Deutsche Forschungsgemeinschaft (KR3770/7-1; KR3770/9-1); SMW is supported by NIH U01 HL099776, NIH Director’s Pioneer Award (DP1 LM012179-01), American Heart Association Grant-in-Aid (14GRNT18630016), and an Endowed Faculty Scholar Award from the Lucile Packard Foundation for Children and Child Health Research Institute at Stanford.
References
- 1.Doppler SA, Deutsch MA, Lange R, Krane M. Cardiac regeneration: current therapies-future concepts. J Thorac Dis. 2013;5(5):683–97. doi: 10.3978/j.issn.2072-1439.2013.08.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 6.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–9. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 7.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110(11):1465–73. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayawardena TM, Finch EA, Zhang L, Zhang H, Hodgkinson C, Pratt RE, Rosenberg PB, Mirotsou M, Dzau VJ. MicroRNA Induced Cardiac Reprogramming In Vivo: Evidence for Mature Cardiac Myocytes and Improved Cardiac Function. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.116.304510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012;53(3):323–32. doi: 10.1016/j.yjmcc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Christoforou N, Chellappan M, Adler AF, Kirkton RD, Wu T, Addis RC, Bursac N, Leong KW. Transcription factors MYOCD, SRF, Mesp1 and SMARCD3 enhance the cardio-inducing effect of GATA4, TBX5, and MEF2C during direct cellular reprogramming. PLoS One. 2013;8(5):e63577. doi: 10.1371/journal.pone.0063577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, Gearhart JD. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J Mol Cell Cardiol. 2013;60:97–106. doi: 10.1016/j.yjmcc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111(1):50–5. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Sudhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9(4):374–82. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu JD, Stone NR, Liu L, Spencer CI, Qian L, Hayashi Y, Delgado-Olguin P, Ding S, Bruneau BG, Srivastava D. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 2013;1(3):235–47. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013;110(14):5588–93. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A. 2013;110(31):12667–72. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]