Abstract
Knockout (KO) mice missing the taste signaling protein Trpm5 have greatly attenuated sweetener preferences but develop strong preferences for glucose in 24-h tests, which is attributed to post-oral sugar conditioning. Trpm5 KO mice express mild preferences for galactose but no preferences for fructose in 24-h tests, which suggests that these sugars differ in their post-oral reinforcing effects. Here we investigated sugar-conditioned flavor preferences in Trpm5 KO and C57BL/6J wildtype (B6) mice. The mice were trained to consume a flavored (CS+, e.g. grape) 8% sugar solution and flavored (CS-, e.g., cherry) water on alternating days followed by two-bottle choice tests with CS+ vs. CS- flavors in water and with unflavored sugar vs. water. The KO mice displayed strong preferences (>80%) for the CS+glucose and CS+galactose but not for the CS+fructose flavor. They also preferred glucose and galactose, but not fructose to water. In contrast, the B6 mice preferred all three CS+ flavors to the CS- flavor, and all three sugars to water. In tests with the non-metabolizable sugar α-methyl-D-glucopyranoside (MDG), the KO and B6 mice preferred 8% MDG to water but did not prefer the CS+8%MDG to CS-. However, they preferred a CS+ flavor mixed with 4% MDG over the CS- flavor. Trpm5 KO mice also preferred galactose and MDG to fructose in direct choice tests. The Trpm5 KO data indicate that glucose and, to a lesser extent, galactose and MDG have post-oral reinforcing actions that stimulate intake and preference while fructose has a much weaker effect. The CS+ flavor and sugar preferences of B6 mice may be mediated by the sweet taste and/or post-oral actions of the various sugars. Glucose, galactose, and MDG, but not fructose are ligands for the sodium-glucose transporter 1 (SGLT1) which is implicated in post-oral sugar conditioning in B6 mice.
Keywords: C57BL/6J mice, Glucose, Fructose, Galactose, Flavor-nutrient learning, Flavor-taste learning
1. Introduction
Sugar is a very attractive energy source for humans and other species. In rodents, sugar appetite is initiated by the stimulation of sweet taste receptors in the mouth and is further enhanced by the activation of post-oral sugar sensors in the gut and beyond [20]. The importance of both oral and post-oral sugar sensors to sugar appetite is documented by the behavior of genetically modified mice missing sweet taste signaling elements. These include the T1r2 and T1r3 sweet receptor components and the Trpm5 calcium activated sodium channel. In brief one- and two-bottle lick tests, T1r2, T1r3, and Trpm5 knockout (KO) mice show little or no attraction to sucrose solutions over a range of concentrations, which demonstrates the importance of oral taste signaling in sugar appetite [31,33,34,37]. Yet in 24-h tests, T1r3 KO and Trpm5 KO mice develop strong preferences for concentrated sucrose solutions, which are attributed to a learned association between the non-taste oral properties of the solution and the post-oral nutritive effects of the sugar [8,33,34,37,39]. Trpm5 KO mice can also acquire a conditioned position preference when trained to drink sucrose and water at different bottle positions during 30-min training sessions [9]. Direct evidence for post-oral sugar conditioning in T1r3 KO and Trpm5 KO mice is provided by their acquired preference for a flavored solution (conditioned stimulus, CS+) paired with intragastric (IG) infusions of sucrose or glucose [20,24]. The post-oral intake-stimulating and preference conditioning actions of sugars are referred to as “appetition” to distinguish this process from the satiation process that inhibits sugar intake [18]. The post-oral appetition displayed by T1r3 KO and Trpm5 KO mice indicates that the T1r3 and Trpm5 taste signaling elements located in the gastrointestinal tract are not essential for the post-oral modulation of sugar appetite [20].
Sugar-conditioned flavor preferences can be studied using an oral conditioning protocol. With this method, animals are trained to drink, on alternate days, a sugar solution containing an added flavor (the CS+, e.g., grape) and plain water containing a different flavor (the CS-, e.g. cherry) [17]. Preferences are then assessed in a two-bottle choice test with the CS+ and CS- flavors presented in plain water. Animals with an intact taste system can acquire preferences based on the palatable sweet taste of sugars (flavor-taste conditioning) as well as on the sugar’s post-oral nutrient properties (flavor-nutrient learning) [17]. However, taste ageusic KO mice can acquire only flavor-nutrient based preferences. Using this oral procedure, CS+ flavor preferences have been conditioned by sucrose or monosodium glutamate solutions in taste ageusic P2X2/P2X3, T1r3, Trpm5, and Calhm1 KO mice [3,25,29].
The present study used the oral procedure to investigate preference conditioning in sweet-ageusic Trpm5 KO mice and C57BL/6J (B6) wildtype controls to CS+ flavors added to the monosaccharide sugars glucose, fructose, and galactose. This was of interest because in 24-h sugar vs. water tests Trpm5 KO mice developed strong preferences for glucose, moderate preferences for galactose, and no preference for fructose solutions, which indicated that these sugars differ substantially in their post-oral appetition effects [38]. The glucose and fructose findings are consistent with a recent IG conditioning study in B6 mice showing that IG glucose infusions conditioned a strong CS+ preference whereas IG fructose infusions were ineffective [19]. The galactose preference displayed by the Trpm5 KO mice, however, contrasts with the failure of IG galactose infusions to condition a flavor preference in B6 mice trained 24 h/day [19]. More recently, however, we obtained significant, albeit mild preferences with IG galactose in food-restricted B6 mice trained 1 h/day, indicating that galactose has some post-oral appetition effect [36]; see also [14]. Based on the 24-h sugar preferences displayed by Trpm5 KO mice, we predicted that they would learn preferences for a CS+ flavor added to glucose and galactose solutions but not for a CS+ flavor added to fructose. If so, this would provide further evidence for the differential post-oral appetition actions of these three monosaccharide sugars. In contrast to Trpm5 KO mice, B6 mice might be expected to acquire a preference for the fructose-paired CS+ flavor based on the sweet taste of the sugar (flavor-taste learning). However, this was not certain because in a recent study using a different conditioning protocol, B6 mice failed to prefer a fructose-paired CS+ flavor [16]. Following CS flavor training and testing, we also measured the sugar vs. water preferences of the Trpm5 KO and B6 mice to confirm our prior findings obtained with sugar solutions without added flavors [38]. The sugar solutions were all prepared at a concentration which stimulated maximal or near maximal intakes in 24-h tests [38].
In a second experiment, we compared the flavor conditioning and preference responses of Trpm5 KO and B6 mice to a non-metabolizable glucose analog, α-methyl-D-glucopyranoside (MDG). This was of interest given our recent findings that IG infusions of MDG conditioned CS+ preferences in B6 mice [36]. MDG, like glucose and galactose, is transported into intestinal cells by sodium glucose transporter 1 (SGLT1) [32]. SGLT1 also functions as a sugar sensor that stimulates intestinal hormone release [30] and is implicated in post-oral sugar conditioning [36]. We recently reported that B6 mice strongly prefer MDG to water in 24-h tests and actually prefer it to glucose in 3-min lick tests [4]. Gustatory nerve recordings and conditioned taste aversion findings indicate that MDG has a sweet taste to gerbils [12]. Based on these findings, we predicted that MDG would condition CS+ preferences in Trpm5 KO and B6 mice, but B6 mice would drink more MDG than KO mice because of their attraction to the sugar’s sweet taste.
In Experiment 3, the relative preference of the Trpm5 KO and B6 mice for galactose vs. fructose and MDG vs. fructose was evaluated in two-bottle tests. Based on the different preference profiles observed in the sugar vs. water tests we predicted that the KO mice would prefer galactose and MDG to fructose, providing additional evidence for the differential post-oral appetition actions of these sugars.
2. Experiment 1. Glucose, fructose and galactose conditioned preferences
2.1. Method
2.1.1. Subjects
C57BL/6J (B6) mice (15 male, 18 female) were derived from individuals purchased from Jackson Laboratories (Bar Harbor, ME). Trpm5 KO mice (14 male, 18 female) were derived from individuals developed on a B6 background [7]. The targeted mutation for the Trpm5 KO is on the isogenic B6 background, having been generated in C57BL6 embryonic stem (Bruce 4) cells. The mice were 10-11 weeks old at the start of training. The Glucose and Fructose groups were run together in one cohort while the Galactose groups were run in a second cohort with the same protocol. Each group had equal or near equal numbers of male and female mice. The mice were singly housed in plastic tub cages with wire tops and provided with ad libitum access to chow (5001, PMI Nutrition International, Brentwood, MO) and deionized water in a room maintained at 22 degrees C with a 12:12 light-dark cycle (lights on 0900 h).
2.1.2. Test Solutions
Solutions were prepared using food grade glucose and fructose (Honeyville Food Products, Rancho Cucamonga, CA), technical grade galactose (G0625, Sigma Chemical Co., St. Louis, MO) and deionized water. The CS+ training solutions contained 8% glucose (Glu), 8% fructose (Fru) or 8% galactose (Gal) and 0.05% cherry or grape Kool-Aid (Kraft Foods, Rye Brook, NY); they are referred to as CS+Glu/Glu, CS+Fru/Fru, and CS+Gal/Gal, respectively. The CS- solution was the other Kool-Aid flavor in water. For half the mice in each group, the CS+ was cherry and the CS- was grape; for the remaining animals the CS flavors were reversed. For the two-bottle tests, the sugar-paired CS+ flavors (referred to as CS+Glu, CS+Fru, and CS+Gal) were presented in water as were the CS- flavors (referred to as CS-, or CS-Glu, CS-Fru, and CS-Gal). Fluid was available through sipper spouts attached to 50-ml plastic tubes that were placed on top of the home cage. The sipper spouts were inserted through holes positioned 3.7 cm apart in a stainless-steel plate positioned to the right of the food bin, and the drinking tubes were fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking bottles on an electronic balance interfaced to a laptop computer. Daily fluid spillage was estimated by recording the change in weight of two bottles that were placed on an empty cage, and intake measures were corrected by this amount.
2.1.3. Procedure
For the first week, the mice were given access to two bottles of water. The Trpm5 KO (n=10) and B6 (n=11) Glucose groups were then given 4 days of one-bottle access to CS- and CS+Glu/Glu on odd and even days, respectively. After 1 day of water only, the mice were given a 2-day choice test (Test 1) with the CS+Glu vs. CS- flavors presented in water; the 2-day test was repeated (Test 2) to determine the persistence of the CS+ preference. After another day of water only, the mice were given a 2-day two-bottle test with unflavored 8% glucose vs. water. The Fructose (KO n=10, B6 n=11) and Galactose (KO n=12, B6 n=11) groups were given the same sequence of tests but with their respective sugars. The left-right positions of the CS+ and CS- bottles were alternated throughout training and testing to minimize the development of side preferences.
Fluid intakes were averaged in 4 or 2-day blocks and evaluated with analysis of variance. Separate tests compared the CS+ and CS- intakes, and sugar and water intakes of the KO and B6 groups given Glucose, Fructose or Galactose tests. Additional tests compared the intakes of the three Glucose, Fructose and Galactose KO groups and three B6 groups. Percent CS+ intakes and sugar intakes (e.g., CS+/Total intake × 100) were calculated for the two-bottle tests and analyzed with analysis of variance or t-tests. A preliminary analysis revealed no sex differences in CS+ or sugar preferences, and therefore the data for male and female mice were combined; this lack of a sex difference is consistent with other findings obtained with B6 mice [11,21,37].
2.2. Results
Glucose groups
Fig. 1 presents the one-bottle training and two-bottle test data. During one-bottle training, both groups consumed more (P < 0.01) CS+Glu/Glu than CS-, although the B6 mice drank more CS+Glu/Glu than the KO mice; CS- intakes did not differ (Group × CS interaction, F(1,19) = 43.4, P < 0.001). Both groups also consumed more (P < 0.01) CS+ than CS- in the two-bottle tests with the flavors presented in water, but in this case, the KO mice consumed more CS+ than did the B6 mice (Group × CS interaction, F(1,19) = 30.8, P < 0.001). CS+ intakes declined from Test 1 to 2 with the drop greater in the KO than B6 mice, although the KO mice still consumed more CS+Glu than B6 mice in Test 2 (Group × Test interaction, F(1,19) = 27.9, P < 0.001). Overall, the percent CS+ preferences of the KO group exceeded those of the B6 group (F(1,19) = 13.3, P < 0.01) and declined in both groups from Test 1 to 2 (F(1,19) = 22.6, P < 0.001). In the two-bottle test with unflavored glucose vs. water, both groups consumed significantly more sugar than water (F(1,19) = 295.5, P < 0.001) but the glucose intake and percent preference were greater (P < 0.01) in the B6 than KO mice.
Fig. 1.
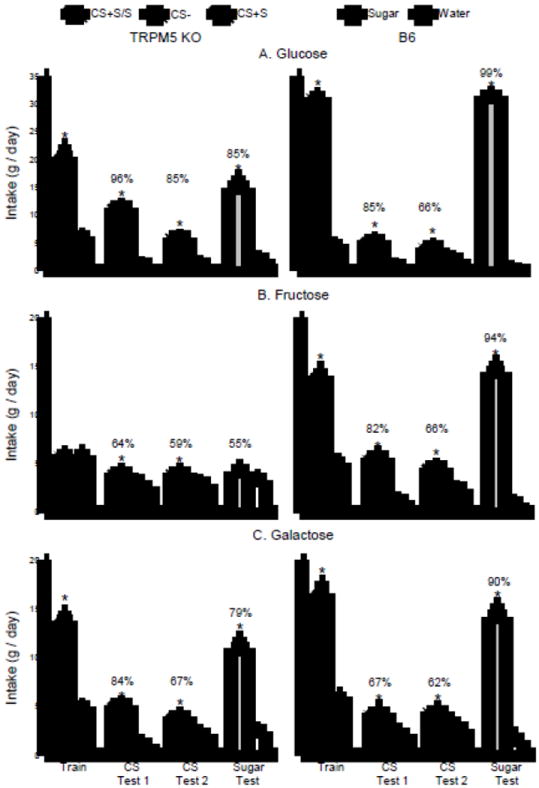
Experiment 1. Mean intakes (+sem) of CS+S/S and CS- during one-bottle training, CS+S and CS- during two-bottle testing, and Sugar and water during two-bottle tests of Trpm5 KO mice (left panels) and B6 mice (right panels). The CS+S/S refers to the CS+ flavored sugar solution used in training, i.e., the CS+Glu/Glu, CS+Fru/Fru and CS+Gal/Gal training solutions used with the Glucose, Fructose and Galactose groups respectively. The CS+S refers to the sugar-paired flavor presented in plain water used in the two-bottle CS choice tests: the CS+Glu, CS+Fru, and CS+Gal flavors. Sugar and water refer to the unflavored sugar (glucose, fructose, or galactose) and unflavored water used in the sugar vs. water tests. The data from the Trpm5 KO and B6 mice (left and right panels) tested with glucose, fructose, and galactose are presented in panels A, B, and C, respectively. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each training period or choice test are indicated by an asterisk (*).
Fructose groups
During one-bottle training, only the B6 mice consumed more (P < 0.01) CS+Fru/Fru than CS- and their CS+Fru/Fru intake exceeded (P < 0.01) that of the KO mice (Group × CS interaction, F(1,19) = 82.1, P < 0.001). The B6 mice also consumed more CS+ than did the KO mice in the CS flavor only test (Group × CS interaction, F(1,19) = 8.3, P < 0.01) and their percent CS+ intakes exceeded those of the KO mice (Group × CS interaction, F(1,19) = 7.7, P < 0.01). Nevertheless, the KO mice, like the B6 mice, consumed more (P < 0.05) CS+ than CS- although their percent preferences were only 59-62% compared to the 66-82% preferences of the B6 mice. The KO mice, however, did not consume more fructose than water in the unflavored sugar test, in contrast to the B6 mice which consumed substantially more sugar than water (Group × Fluid interaction, F(1,19) = 83.3, P < 0.001). The percent sugar intake of the B6 mice also exceeded that of the KO mice (94% vs. 55%, P < 0.01).
Galactose groups
During one-bottle training, the groups did not differ in their CS intakes and overall consumed more (P < 0.01) CS+Gal/Gal than CS- (F(1,20) = 195.8, P < 0.001). Both groups also consumed more (P < 0.01) CS+Gal than CS- in the two-bottle tests (F(1,20) = 28.6, P < 0.001) and they did not differ in their CS intakes. CS+ intakes declined from Test 1 to Test 2 (CS × Test interaction, F(1,20) = 7.9, P < 0.05). The percent CS+Gal preferences were numerically higher in the KO group than the B6 group but these differences were not significant (P < 0.10) and overall percent CS+ intakes declined from Test 1 to 2 (F(1,20) = 7.6, P < 0.05). The KO and B6 groups consumed more galactose than water in the final test (F(1,20) = 85.3, P < 0.001) and their intakes did not differ. The KO and B6 groups also did not significantly differ in their percent galactose intakes (79% vs. 90%, p < 0.07).
Trpm5 KO groups
Analysis of the three Trpm5 KO groups indicated that the mice differed in their CS+sugar but not CS- training intakes as follows: CS+Glu/Glu > CS+Gal/Gal > CS+Fru/Fru (Group × CS interaction, F(2,28) = 35.5, P < 0.001). In the two-bottle flavor test, the KO groups differed in both their CS+ and CS- intakes (Group × CS interaction, F(2,28) = 13.3, P < 0.001). In this case, CS+Glu intake exceeded (P < 0.05) that of CS+Gal and CS+Fru, while CS-Fru exceeded (P < 0.05) that of CS-Gal and CS-Glu. Overall, the percent CS+Glu and CS+Gal intakes exceeded (P < 0.05) that of the CS+Fru (88% ≥ 77% > 61%, F(2,28) = 7.9, P < 0.01). In the sugar test, intake of glucose and galactose exceeded that of fructose (14.8 ≥ 11.1 > 4.2 g, P < 0.01) while water intakes did not differ (Group × Fluid interaction, F(2,28) = 11.5, P < 0.001). Similarly, the percent glucose and galactose intakes exceeded that of the percent fructose intake (85% ≥ 79% > 55%, F(2,28) = 14.4, P < 0.001).
B6 groups
During one-bottle training, the B6 groups consumed more CS+Glu/Glu than CS+Gal/Gal and CS+Fru/Fru; CS- intakes did not differ (Group × CS interaction, F(2,30) = 232.6, P < 0.001). In the two-bottle CS flavor tests, overall the B6 groups consumed more CS+ than CS- (F(1,30) = 64.2, P < 0.001) and there were no other differences. The groups also did not differ in their percent CS+ intakes although percent CS+Glu and CS+Fru intakes were somewhat higher than CS+Gal intakes (75% = 74% ≥ 64%). The B6 groups differed in the sugar test, however, in that glucose intake was twice as much as that of fructose and galactose (Group × Fluid interaction, F(2,30) = 67.7, P < 0.001) although percent sugar intakes did not differ (99%, vs. 94% vs. 90%).
3. Experiment 2. MDG conditioned flavor preferences
The conditioning response of Trpm5 KO and B6 mice to a CS+ flavor mixed into an MDG solution and their preference for MDG over water were investigated using the protocol of Experiment 1. However, separate groups were tested with 8% MDG and 4% MDG. These concentrations were tested because in our prior study IG infusion of 8% MDG, which was diluted to 4% in the stomach by the ingested CS+ solution, conditioned a stronger preference than did IG infusion of 16% MDG, which was diluted to 8% in the stomach [36].
3.1. Method
8% MDG
Naïve Trpm5 KO (5 male, 5 female) and B6 (5 male, 5 female) were housed as in Experiment 1. The CS+MDG/MDG training solution contained 8% MDG (α-methyl-D-glucopyranoside, Sigma) flavored with 0.05% grape or cherry Kool-Aid. The CS- solution contained the alternate Kool-Aid flavor in water. The mice were trained and tested with the flavored solutions followed by a two-bottle test with unflavored MDG vs. water as in Experiment 1.
4% MDG
Naïve Trpm5 KO (5 male, 5 female) and B6 (5 male, 5 female) were treated as above except that they were trained and tested with 4% MDG solutions.
3.2. Results
8% MDG
Fig. 2A presents the one-bottle training and two-bottle test data. During one-bottle training, both groups consumed more (P < 0.001) CS+MDG/MDG than CS-, although the B6 mice drank more CS+MDG/MDG than the KO mice (P < 0.001); CS- intakes did not differ (Group × CS interaction, F(1,17) = 13.9, P < 0.01). In the two-bottle CS flavor tests, neither group preferred the CS+MDG to the CS- and their intakes and preferences did not differ. Yet, in the 8% MDG vs. water test, both groups drank significantly more MDG than water (P < 0.001), although the B6 mice drank more than twice as much 8% MDG as did the KO mice (Group × CS interaction, F(1,18) = 27.9, P < 0.001) and displayed a stronger MDG preference (91% vs. 68%, P < 0.01).
Fig. 2.
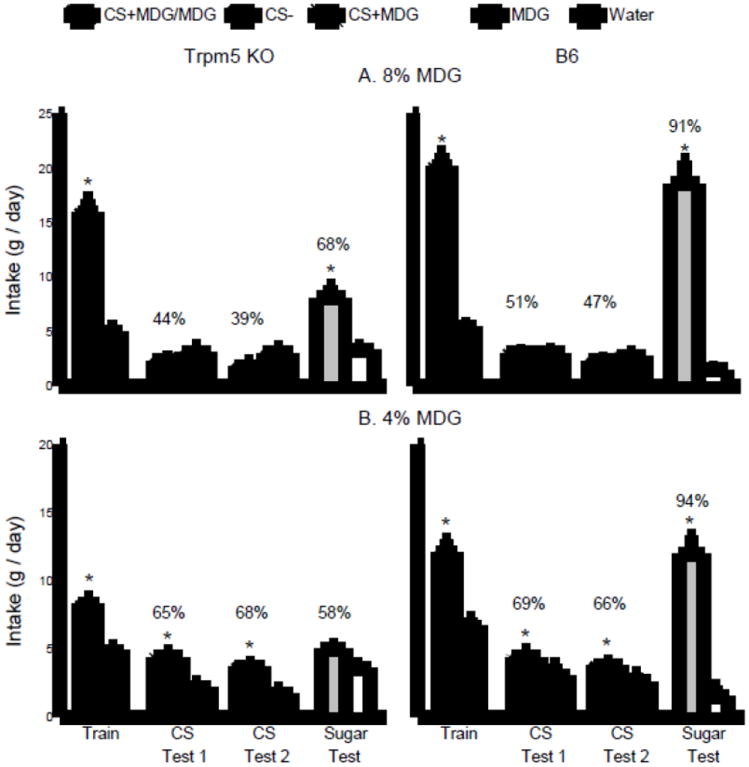
Experiment 2. Mean intakes (+sem) of CS+MDG/MDG and CS- during one-bottle training, CS+MDG and CS- during two-bottle testing, and unflavored MDG and water during two-bottle tests of Trpm5 KO mice (left panels) and B6 mice (right panels). The data from the mice tested with 8% MDG and 4% MDG are presented in panels A and B, respectively. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each training period or choice test are indicated by an asterisk (*).
4% MDG
During one-bottle training (Fig. 2B), both groups consumed more (P < 0.001) CS+MDG/MDG than CS-, and the B6 mice drank more CS+MDG/MDG than the KO mice (P < 0.001); CS- intakes did not differ (Group × CS interaction, F(1,17) = 6.1, P < 0.05). In the two-bottle CS flavor tests (Fig. 2B), both groups consumed more CS+MDG than CS- (F(1,17) = 24.2, P < 0.001) and did not differ in their CS intakes or CS+MDG preferences. However, in the 4% MDG vs. water test, the B6 mice drank substantially more MDG than water (P < 0.01) whereas the KO mice consumed only slightly and not significantly more MDG than water (P < 0.10) (Group × Fluid interaction, F(1,17) = 78.7, P < 0.001); the percent MDG preference of the B6 mice also exceeded that of the KO mice (94% vs. 58%, P < 0.01).
4. Experiment 3. Galactose and MDG Preferences vs. Fructose
The results of Experiments 1 and 2 revealed that KO and B6 mice were similar in that they consumed more glucose, galactose and MDG than water. They differed, however, in that only the B6 mice consumed more fructose than water, which confirms prior findings [38]. The present experiment further investigated the differential response of KO and B6 mice to the various sugars by giving them direct two-bottle tests with galactose vs. fructose and MDG vs. fructose. Novel flavors were added to the sugars to make them more distinctive, particularly for the Trpm5 KO mice because of their insensitivity to sweet taste.
4.1. Method
Galactose vs. Fructose
Naïve Trpm5 KO (5 male, 7 female) and B6 (6 male, 6 female) mice were housed as in Experiment 1 and tested with flavored 8% galactose and 8% fructose. The sugar solutions contained 0.05% grape or cherry Kool-Aid. Half of the animals had grape added to fructose and cherry added to galactose; the flavors were reversed for the remaining animals. The mice were given an initial two-bottle choice test (Test 1) with flavored galactose vs. fructose for 2 days. They were then given alternating one-bottle access to the flavored galactose and fructose solutions for 4 days to allow them to associate the flavor of each sugar with its post-oral consequence. A second two-bottle choice test (Test 2) was then conducted with the two flavored sugars.
MDG vs. Fructose
Naïve Trpm5 KO (6 male, 5 female) and B6 (5 male, 5 female) mice were given a series of two- and one-bottle tests with flavored 8% MDG and 8% fructose as described above.
4.2. Results
Galactose vs. Fructose
Fig. 3A shows the data for galactose vs. fructose choice tests and one-bottle training days. In the initial sugar choice, the KO and B6 mice consumed comparable amounts overall but they significantly differed in their sugar preferences: the KO mice consumed more flavored galactose than fructose while the B6 mice displayed the opposite intake pattern (Group × Sugar interaction, F(1,22) = 79.1, P < 0.001). The percent galactose intakes were 77% and 9% (P <0.01), respectively, for the KO and B6 mice. On the one-bottle training days the KO mice consumed more (P < 0.01) galactose but less fructose than the B6 mice (Group × Sugar interaction, F(1,22) = 49.0, P < 0.001). In addition, the KO mice drank more galactose than fructose while the B6 consumed comparable amounts of the two flavored sugars. In Test 2 with the two flavored sugars, the groups again showed differential preferences and the KO mice consumed more galactose than fructose while the B6 mice showed the opposite pattern (Group × Sugar interaction, F(1,22) = 248.6, P < 0.001). They also differed substantially in their percent galactose intakes (93% vs. 6%, P < 0.001). The KO mice increased their galactose preference from Test 1 to 2 (77 vs. 93%) while percent galactose intakes did not differ for the B6 mice (9% vs. 6%).
Fig. 3.
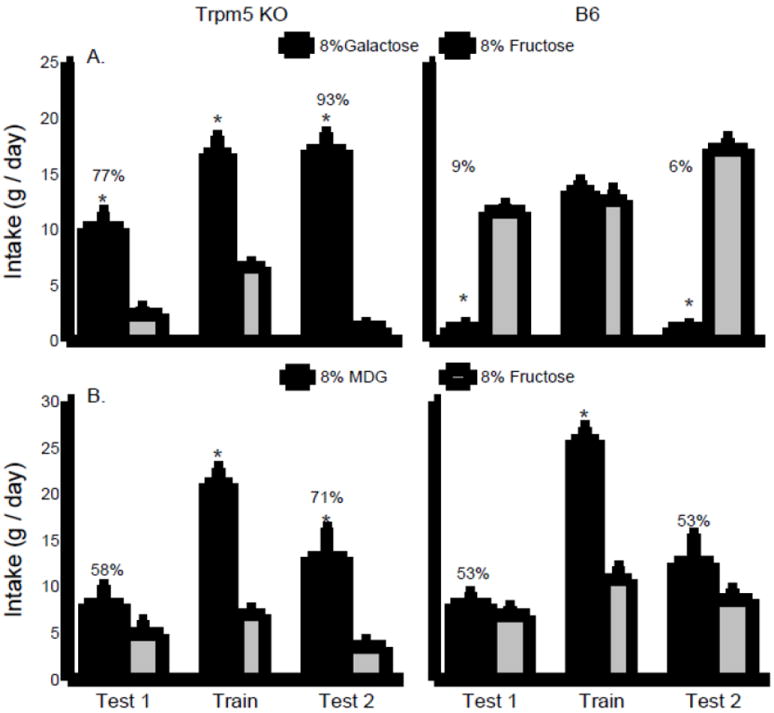
Experiment 3. A. Mean intakes (+sem) of flavored 8% galactose and 8% fructose during two-bottle choice tests 1 and 2 and one-bottle training sessions. The data from the Trpm5 KO and B6 mice are presented in the left and right panels, respectively. B. Mean intakes (+sem) of flavored 8% MDG and 8% fructose during two-bottle choice tests 1 and 2 and one-bottle training sessions. The data from the Trpm5 KO and B6 mice are presented in the left and right panels, respectively. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) differences within each training period or choice test are indicated by an asterisk (*).
MDG vs. Fructose
Fig. 3B shows the data for flavored MDG vs. fructose choice tests and the one-bottle training days. In the initial sugar choice, the KO and B6 mice consumed comparable amounts overall and drank slightly but not significantly more MDG than fructose. During the one-bottle days, the B6 mice consumed more sugar than the KO mice (F(1,19) = 6.6, P < 0.05) and both groups drank significantly more MDG than fructose (F(1,19) = 257.3, P < 0.001). Overall, the two groups consumed more flavored MDG than fructose in choice Test 2 (F(1,19) = 7.7, P < 0.05). The Group × Sugar interaction was not significant, but only the KO mice consumed significantly more MDG than fructose in Test 2. The percent MDG intake of the KO mice exceeded that of the B6 mice in Test 2 (71% vs. 53%) but this difference was not significant.
5. Discussion
The present study demonstrates that sweet ageusic Trpm5 KO, like normal B6 mice, learned to prefer flavors added to glucose, galactose and MDG solutions but the strains differed in their conditioning response to fructose. The Trpm5 KO and B6 mice also substantially differed in their preference for galactose vs. fructose solutions. As discussed below, the findings can be explained by the different oral and post-oral actions of the sugars in the KO and B6 mice.
Glucose and Fructose conditioning
Based on our recent report that Trpm5 KO mice express a significant preference for 8% glucose but not for 8% fructose in 24-h sugar vs. water tests [38], we predicted that the KO mice would also differ in learning to prefer flavors paired with these two sugars. This was confirmed in Experiment 1, in which the KO mice displayed a very strong (96%) preference for the CS+Glu flavor but only a weak (64%) preference for the CS+Fru flavor. Their significant CS+Fru preference was unexpected, given that the KO mice did not consume more CS+Fru/Fru than CS- during training and did not prefer unflavored 8% fructose to water. Thus, at best, 8% fructose has only a weak post-oral conditioning effect in Trpm5 KO mice. Training KO mice with higher fructose concentrations would not be expected to enhance flavor conditioning, because Trpm5 KO mice actually consumed less 16% or 32% fructose than water in 24-h two-bottle tests [38].
In contrast to the KO mice, the B6 mice expressed similar preferences for the CS+Glu and CS+Fru flavors in the CS flavor tests as well as for unflavored glucose and fructose in the sugar vs. water tests. Yet, in our prior study, B6 mice trained with CS+ flavors paired with IG infusions of 16% glucose or fructose (which were diluted to 8% sugar in the stomach by the ingested CS+ solution), expressed a strong preference for the CS+Glu (86%) but not for the CS+Fru (53%) [19]. The IG findings indicate that 8% fructose has little or no post-oral appetition effect in B6 mice, which is supported by other data obtained with this strain [35]. The significant preference displayed by the B6 mice for the CS+Fru flavor added to the 8% fructose solution in Experiment 1 is therefore attributed to a learned association between the CS+ flavor and the sweet taste of fructose (flavor-taste learning) rather than to a flavor post-ingestive association. Since Trpm5 KO mice display a greatly attenuated gustatory nerve response to sugars [7] they would not be expected to learn flavor-taste preferences.
The significant CS+Fru flavor preference displayed by B6 mice in Experiment 1 contrasts with the failure of B6 mice to express a CS+Fru preference using a different training protocol. Pinhas et al. [16] reported that food-restricted B6 mice trained to drink CS+ flavored fructose (8% or 16%) and CS- flavored saccharin (0.05% or 0.2%) solutions 1 h/day did not prefer the CS+Fru flavor over the CS-Sac flavor when both flavors were presented in saccharin solutions, unlike other mouse strains (e.g., SWR, BALB/c). The present protocol differed in several respects from that used by Pinhas (deprivation level, session length, saccharin in CS- solution) and it is not certain why different outcomes were obtained in the two studies. Nevertheless, the fact the B6 mice in the Pinhas study were trained with a saccharin-sweetened CS-, in order to stimulate them to drink the solution in the 1-h session, whereas the B6 mice in Experiment 1 were trained with a non-sweetened CS-, would appear to be a critical factor.
The B6 mice were sensitive to the oral sweetness and post-oral conditioning actions of glucose while the KO mice could detect only the latter. This suggests that B6 mice, which also consumed more CS+Glu/Glu in training, should have displayed stronger CS+/Glu preferences, but instead, the KO mice expressed stronger preferences. Perhaps the post-oral actions of glucose are more reinforcing to Trpm5 KO mice than B6 mice, but this is not supported by our finding of comparable CS+ flavor preferences conditioned by IG glucose infusions in the KO and B6 mice [20]. Rather, the sweet taste of glucose may have competed with the CS+ flavor as an associative cue in the B6 mice [10]. In addition, only the B6 mice would have experienced a change in taste from CS+Glu/Glu (sweet, training) to CS+Glu (nonsweet, testing), which may have reduced their flavor preference due to generalization decrement.
The present and prior findings obtained with Trpm5 KO mice and their B6 controls are consistent in demonstrating that fructose, unlike glucose, has weak if any post-oral appetite stimulating effects [19,36,38]. However, this is not the case with other inbred mouse strains. In particular, we recently reported that IG fructose infusions conditioned significant CS+ preferences in FVB/NJ inbred mice tested 1- or 24-h/day, although the preferences were weaker than those induced by IG glucose infusions [27]. Studies of Sprague-Dawley (SD) rats have produced intermediate results: IG fructose infusions conditioned significant flavor preferences with long training sessions but not with short sessions [2,5,22,23]. The reason for the variable post-oral appetition effects of fructose in rodent species/strains remains to be determined.
Galactose preference conditioning
In 24-h sugar vs. water tests, B6 mice displayed stronger preferences for glucose and fructose than for galactose over a range of concentrations [38]. This is consistent with gustatory nerve data indicating that galactose is less sweet than the other sugars to mammals [13,15]. Sweet ageusic Trpm5 KO and T1r3 KO mice, on the other hand, displayed stronger preferences for glucose than galactose and no preference for fructose [38]. Since sugar preferences in KO mice are driven primarily by post-oral effects, these data indicated that the relative appetition potency of the three sugars is glucose > galactose ≫ fructose in KO mice. Experiment 1 revealed a similar CS+ preference profile in the Trpm5 KO: their preference for the CS+Glu exceeded that for the CS+Gal, which in turn was stronger than that for CS+Fru. The B6 mice also displayed a stronger preference for CS+Glu than for CS+Gal, which may have been due to both the taste and post-oral differences between the two sugars.
Although the CS+Gal preference of the Trpm5 KO mice indicates that the sugar has a significant post-oral conditioning effect, we previously observed that B6 mice did not learn a preference for a flavored saccharin solution (CS+) paired with IG infusions of 8 or 16% galactose solutions [19]. It may be that galactose has differential post-oral reward actions in Trpm5 KO and B6 mice. Alternatively, the IG infusion in our prior study may have failed to condition a CS+ preference because it induced relatively high galactose intakes due to the palatability of the 0.2% saccharin used to sweeten the CS+ solution. Mice have a limited ability to metabolize galactose [28] and high galactose intakes may limit the sugar’s post-oral appetition effects. Consistent with this interpretation, B6 mice trained with a minimally sweet CS solution (0.025% saccharin) acquired a significant preference for a CS+ paired with IG galactose infusions [36]. Another factor that may have enhanced galactose conditioning in the present study is that the mice were trained with a sweet CS+ galactose solution vs. a non-sweet CS- solution whereas in the IG study they were trained with equally sweet saccharin CS+ and CS- solutions [19].
The stronger preferences of Trpm5 KO mice for CS+Gal and 8% galactose, than for CS+Fru and 8% fructose (vs. CS- and water, respectively) suggested that they would also prefer galactose to fructose in a direct choice test. This was confirmed in Experiment 3: the Trpm5 KO mice drank substantially more flavored galactose than flavored fructose in the two-bottle choice tests. In marked contrast, the B6 mice displayed a very strong preference for fructose over galactose. The fructose preference of the B6 mice was presumably driven by the sweeter taste of this sugar. The KO mice, in contrast, were insensitive to the sweet taste of the two sugars and their galactose preference reflects the differential post-oral conditioning actions of the galactose and fructose [36].
MDG preference conditioning
The present findings along with prior data [4] indicate that B6 mice are very attracted to the non-metabolizable sugar MDG. In the sugar vs. water test the B6 mice strongly preferred 8% MDG to water. They also consumed more 8% MDG than 8% fructose or 8% galactose (18.6 vs. 14.4 and 10.9 g/day) in the sugar vs. water tests although less than 8% glucose (31.3 g/day). Yet, unlike 8% glucose and galactose, 8% MDG failed to condition a preference for the CS+ over the CS- in the B6 mice. The elevated intakes and strong preference for 8% MDG over water displayed by the B6 mice can be attributed to the sweet taste and post-oral appetition actions of this sugar analog. The Trpm5 KO mice were insensitive to the sweet taste of MDG and their elevated intake of the CS+/8% MDG during training and preference for the 8% MDG in the two-bottle test is attributed primarily to the post-oral appetition actions of the sugar [36]. This can also account for why the KO mice preferred flavored 8% MDG to flavored 8% fructose. Given the attractive taste (for B6 mice) and post-oral stimulatory actions (for KO and B6 mice) of 8% MDG it is surprising that the mice did not acquire a preference for the CS+ flavor over the CS- flavor.
The 4% MDG solution, on the other hand, conditioned significant albeit mild CS+MDG preferences in the Trpm5 KO and B6 mice, although it stimulated less CS+MDG/MDG overconsumption during one-bottle training and in the MDG vs. water choice test compared to 8% MDG. The CS+ preference results are consistent with our prior findings obtained with B6 mice trained 1 h/day with IG infusions of 8% and 16% MDG, which were diluted in the stomach to 4% and 8% concentrations, respectively, by the ingested CS+ solutions. That is, the 8% MDG infusions conditioned a stronger preference than did the 16% MDG infusions (70% vs. 62%) [36]. Like glucose and galactose, MDG is transported from the lumen into intestinal cells by SGLT1, but unlike these other sugars, MDG is not actively transported across the basal membrane of intestinal cells by the sugar transporter GLUT2 [32]. It is possible, therefore, that cellular accumulation of the non-metabolizable MDG in intestinal and/or other cells generates inhibitory signals that limit the flavor conditioning actions of concentrated MDG solutions [36]. Yet, the Trpm5 KO and B6 mice consumed more 8% MDG than 4% MDG solution during one-bottle training and in the sugar vs. water tests. Thus, there is a discrepancy between the ability of 4% and 8% MDG to stimulate intake vs. condition a flavor preference that remains to be elucidated.
In contrast to the MDG conditioned flavor preference observed in B6 mice with oral (Experiment 2) and IG procedures [36], IG infusions of 8% MDG conditioned a CS+ flavor avoidance in SD rats [4]. SD rats also avoid rather than prefer CS+Gal flavors in experiments using oral and IG conditioning procedures [23,26]. This species difference in response to MDG and galactose may reflect greater sensitivities of rats to the negative effects related to the accumulation of poorly metabolized sugars.
Conclusions
The present findings confirm and extend our recent findings that Trpm5 KO mice express significant preferences for 8% glucose and galactose, but not for 8% fructose in 24-h sugar vs. water tests [38]. The KO mice also learned to strongly prefer a CS+ flavor mixed into 8% glucose or 8% galactose but showed only a weak preference for a CS+fructose paired flavor. Furthermore, in a direct choice test, KO mice significantly preferred 8% galactose to 8% fructose. These findings are consistent with IG infusion data indicating that glucose and to a lesser degree galactose have post-oral effects in B6 mice that support flavor conditioning while fructose has little or no post-oral conditioning action [19,36]. The fructose-conditioned flavor preference displayed by B6 mice may be mediated by a flavor-sweet taste association rather than a flavor-nutrient association. In addition, the B6 mice presumably preferred 8% fructose to galactose because of its sweeter taste. Experiment 3 revealed for the first time that normal B6 and sweet ageusic Trpm5 KO mice not only prefer glucose to water but they also prefer the non-metabolizable glucose analog MDG to water. Furthermore, the Trpm5 KO mice preferred MDG to fructose. This supports our recent IG conditioning findings indicating that sugar metabolism is not essential for glucose-based preferences [36]. There was a dissociation between the intake stimulating and flavor conditioning actions of MDG at 8% and 4% concentrations and further research is needed to understand these components of post-oral appetition.
The results obtained here and in other mouse and rat studies clearly indicate that monosaccharide sugars differ in their post-oral appetite-stimulating effects. This also extends to disaccharide and polysaccharide carbohydrates. That is, glucose-only carbohydrates (maltose, maltodextrins) promote stronger preferences than does sucrose, which is a glucose + fructose disaccharide [1,6]. These findings suggest that altering the carbohydrate components (glucose, fructose, galactose) in mixed macronutrient foods would alter their post-oral appetite stimulating effects, but this remains to be demonstrated.
Highlights.
Trpm5 knockout (KO) mice have a greatly impaired sweet taste.
KO mice learn to prefer sugar-paired flavors based on post-oral nutritive effects.
They show strong preferences for glucose and galactose, but not fructose flavors.
They also prefer a flavor paired with a non-metabolizable glucose analog
Acknowledgments
This research was supported by grant DK-31135 from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Damien Glass, Kevin Lemaire, and Mohammed Riad for their technical assistance and Robert F. Margolskee for providing us with the Trpm5 KO mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Sucrose to Polycose preference shifts in rats: The role of taste, osmolality and the fructose moiety. Physiol Behav. 1991;49:1047–1060. doi: 10.1016/0031-9384(91)90330-q. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Ackroff K, Sclafani A. Flavor preference conditioned by oral monosodium glutamate in mice. Chem Senses. 2013;38:745–758. doi: 10.1093/chemse/bjt049. [DOI] [PubMed] [Google Scholar]
- 4.Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning in rats by glucose but not a non-metabolizable glucose analog. Physiol Behav. 2014;133:92–98. doi: 10.1016/j.physbeh.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 2001;72:691–703. doi: 10.1016/s0031-9384(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 6.Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: Maltose is more reinforcing than sucrose. Physiol Behav. 1998;64:535–541. doi: 10.1016/s0031-9384(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 7.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 8.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 9.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Dwyer DM, Haselgrove M, Jones PM. Cue interactions in flavor preference learning: A configural analysis. J Exp Psychol :Anim Behav Process. 2011;37:41–57. doi: 10.1037/a0021033. [DOI] [PubMed] [Google Scholar]
- 11.Glendinning JI, Beltran F, Benton L, Cheng S, Gieseke J, Gillman J, Spain HN. Taste does not determine daily intake of dilute sugar solutions in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1333–R1341. doi: 10.1152/ajpregu.00331.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakinovich W., Jr Taste aversion to sugars by the gerbil. Physiol Behav. 1982;28:1065–1071. doi: 10.1016/0031-9384(82)90176-7. [DOI] [PubMed] [Google Scholar]
- 13.Jakinovich W, Jr, Goldstein IJ. Stimulation of the gerbil’s gustatory receptors by monosaccharides. Brain Res. 1976;110:491–504. doi: 10.1016/0006-8993(76)90860-x. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura S, Yoneda T, Aki S, Eguchi A, Manabe Y, Tsuzuki S, Inoue K, Fushiki T. Intragastric infusion of glucose enhances the rewarding effect of sorbitol fatty acid ester ingestion as measured by conditioned place preference in mice. Physiol Behav. 2010;99:509–514. doi: 10.1016/j.physbeh.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Noma A, Goto J, Sato M. The relative taste effectiveness of various sugars and sugar alcohols for the rat. Kumamoto Med J. 1971;24:1–9. [PubMed] [Google Scholar]
- 16.Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, Kakuriev L, Guskova L, Fuzailov I, Touzani K, Sclafani A, Bodnar RJ. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 2012;105:451–459. doi: 10.1016/j.physbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sclafani A. How food preferences are learned - laboratory animal models. Proc Nutr Soc. 1995;54:419–427. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- 18.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71:454–458. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sclafani A, Ackroff K. Advantame sweetener preference in C57BL/6J mice and Sprague-Dawley rats. Chem Senses. 2014 doi: 10.1093/chemse/bju070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 23.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 24.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sclafani A, Marambaud P, Ackroff K. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol Behav. 2014;126:25–29. doi: 10.1016/j.physbeh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sclafani A, Williams DL. Galactose consumption induces conditioned flavor avoidance in rats. J Nutr. 1999;129:1737–1741. doi: 10.1093/jn/129.9.1737. [DOI] [PubMed] [Google Scholar]
- 27.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: Strain differences in post-oral sugar appetition. Am J Physiol Regul Integr Comp Physiol. 2014 doi: 10.1152/ajpregu.00312.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solberg DH, Diamond JM. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 1987;252:G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- 29.Stratford JM, Finger TE. Central representation of postingestive chemosensory cues in mice that lack the ability to taste. J Neurosci. 2011;31:9101–9110. doi: 10.1523/JNEUROSCI.0404-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. In: Joost H-G, editor. Appetite Control, Handbook of Experimental Pharmacology. Vol. 209. Berlin Heidelberg: Springer; 2012. pp. 309–335. [DOI] [PubMed] [Google Scholar]
- 31.Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: Implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright EM, Sala-Rabanal M, Loo DDF, Hirayama BA. Sugar absorption. In: Johnson L, editor. Physiology of the Gastrointestinal Tract. Boston: Academic Press; 2012. pp. 1583–1593. [Google Scholar]
- 33.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJP. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 35.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1635–R1647. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. Impact of deleting T1r3 or Trpm5 on carbohydrate preference and acceptance in C57BL/6 mice. Chem Senses. 2013;38:421–437. doi: 10.1093/chemse/bjt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 2009;34:685–694. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]


