Abstract
Considering that the non-classical HLA-G molecule has well-recognized tolerogenic properties, HLA-G expression is expected to be deleterious when present in tumor cells and in cells chronically infected by viruses, whereas HLA-G expression is expected to be advantageous in autoimmune disorders. The expression of HLA-G on tissue or peripheral blood cells, the levels of soluble HLA-G and polymorphic sites along the gene have been studied in several disorders. In this study, we revised the role of the molecule and polymorphic sites along the HLA-G gene in tumors, viral hepatitis, and parasitic disorders. Overall, several lines of evidence clearly show that the induction of HLA-G expression in tumors has been associated with worse disease outcome and disease spread. In addition, the few studies conducted on hepatitis and parasitic disorders indicate that HLA-G may contribute to disease pathogenesis. Few isolated polymorphic sites, primarily located at the coding or 3′ untranslated HLA-G region, have been evaluated in these disorders, and a complete HLA-G typing together with the study of gene regulatory elements may further help on the understanding of the influence of the genetic background on disease susceptibility.
Keywords: HLA-G, tumors, viral hepatitis, parasitic disorders, polymorphism
Introduction
HLA-G is a non-classical class I gene of the human Major Histocompatility Complex (NCBI gene ID: 3135), presenting a restricted tissue expression pattern and encoding molecules with immune modulatory properties. This gene, firstly described by Geraghty and colleagues in 1987 (1), presents a genetic structure that resembles other classical HLA class I genes. However, contrary to that observed for classical class I genes (HLA-A, -B, and -C), the HLA-G gene is quite conserved among different populations and within the same population, presenting only a few non-synonymous mutations and several variation sites characterized as synonymous modifications, intronic variations, or variable sites at the regulatory regions [reviewed at Ref. (2)].
HLA-G does not seem to initiate immune responses as its classical counterparts. Instead, the HLA-G molecule is associated with the induction of inhibitory stimuli for T and B lymphocytes (3, 4), Natural Killer (NK) cells (3), and antigen-presenting cells (APC) (5). The HLA-G molecule may directly interact with multiple inhibitory receptors, including ILT2/CD85j/LILRB1 (ILT2), ILT4/CD85d/LILRB2 (ILT4), and KIR2DL4/CD158d (KIR2DL4).
The HLA-G molecule was firstly detected at the trophoblast in the maternal fetal interface, probably modulating the maternal immune system during pregnancy. Beyond trophoblast expression, HLA-G has been detected in few normal tissues, including cornea (6), thymus (7), and erythroid and endothelial precursors (8), and its upregulation has been detected in several pathological conditions as described in the present review.
Alternative splicing is also an important characteristic of the HLA-G gene. It may produce at least seven protein isoforms generated by alternative splicing of the primary transcript [reviewed at Ref. (2)], in which four isoforms are membrane-bound and three isoforms are soluble due to the lack of a transmembrane domain.
Much effort has been made to evaluate HLA-G worldwide variability. The HLA-G gene seems to present functional polymorphisms mainly in the regulatory regions, probably influencing its expression. Considering data from at least 18 different populations (9–12) the HLA-G locus presents few frequent extended haplotypes. These haplotypes are a combination among a small number of very divergent promoter and 3′ untranslated region (3′UTR) haplotypes (Figures 1 and 2), and a coding allele usually encodes the same HLA-G molecule (Figure 3). The regulatory segments are characterized by the occurrence of several polymorphic sites presenting high heterozygosis. Although there is no consensus regarding where the HLA-G transcription starts (13), the polymorphisms at the 5′ upstream regulatory region (5′URR) have been considered to influence HLA-G expression, mainly because of the fact that polymorphic sites coincides with, or are close to, known transcription factor binding sites (Figure 1) [Reviewed at Ref. (13)]. Likewise, haplotypes at the HLA-G 3′UTR segment have been considered influencing HLA-G expression, mainly because the fact that some polymorphic sites (such as the one at position +3142) may influence the binding of specific microRNAs (14–17) or may influence mRNA stability (such as the one at position +3187) and alternative splicing (such as the 14-bp polymorphism) (Figure 2).
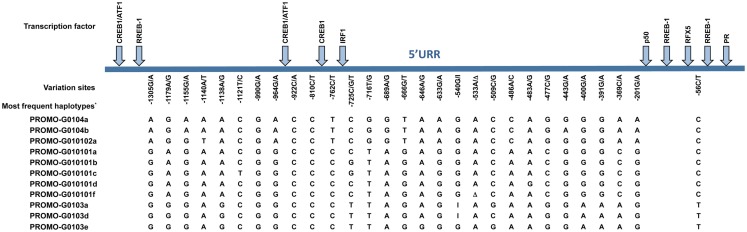
Figure 1.
Variation sites at the 5′ upstream regulatory region (5′URR) of the HLA-G gene (1.4 kb upstream of ATG), as well as the target binding sites of the described transcriptional factors. The position of the variation sites is determined in relation to Adenine of the initiation codon ATG. *Since there is no official nomenclature for 5′URR haplotypes, they were designed as previously reported (10). Transcription factors: CREB1, CAMP responsive element binding protein 1; ATF1, cyclic AMP-dependent transcription factor ATF-1; RREB1, Ras responsive element binding protein 1; IRF1, interferon regulatory factor 1; p50, nuclear factor NF-κ-B p105 subunit; RFX5, DNA-binding protein RFX5 (RFX family); PR, progesterone receptor. I, insertion of a guanine at position −540; Δ, deletion of an adenine at position −533.
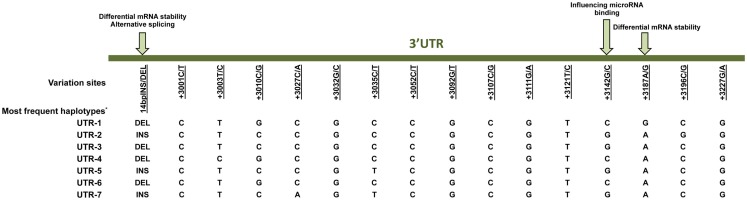
Figure 2.
Variation sites at the 3′ untranslated region (3′UTR) of the HLA-G gene that may influence HLA-G expression. Polymorphic sites associated with diseases presented in this review are underlined. Arrows indicate polymorphic sites that have been functionally studied. *Since there is no official nomenclature for 3′UTR haplotypes, they were designed as previously reported (11). DEL, deletion; INS, insertion.
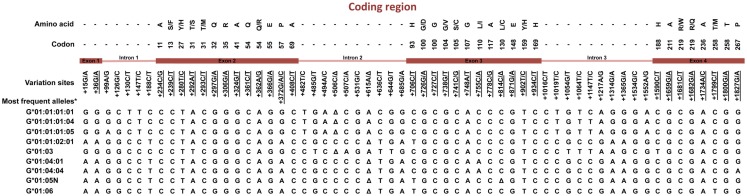
Figure 3.
Variation sites at the coding region of the HLA-G gene from exon 1–4. Polymorphic sites associated with diseases presented in this review are underlined. *Haplotypes presenting a global frequency higher than 1% in worldwide populations. Amino acids: A, alanine; S, serine; F, phenylalanine; Y, tyrosine; T, threonine; M, methionine; Q, glutamine; R, arginine; E, glutamic acid; P, proline; H, histidine; G, glycine; D, aspartic acid; V, valine; C, cysteine; L, leucine; I, isoleucine; W, tryptophan. Δ, deletion.
The HLA-G coding region presents mainly synonymous or intronic variation sites. Considering the most frequent HLA-G coding haplotypes found worldwide [reviewed at Ref. (2, 18)], only five different HLA-G full-length molecules are frequently found, in which four are complete molecules encoded by the HLA-G-*01:01, *01:03, *01:04, and *01:06 allele groups, and one is a truncated molecule encoded by the HLA-G*01:05N null allele. Although some different HLA-G molecules were detected worldwide, they are usually quite rare and the same HLA-G coding alleles are usually detected in every population studied so far. Apparently, all these frequently found molecules (exception made to the G*01:05N) present the same modulatory effects described earlier (2). Considering that only a few extended haplotypes are usually found, and considering that most of the HLA-G coding alleles are associated with only one promoter or 3′UTR haplotype, it is possible that most of the associations described so far regarding HLA-G coding polymorphism and pathological conditions are reflecting the presence of specific promoter and 3′UTR sequences and specific HLA-G production capabilities.
In the present review, we report some diseases that have been associated with the modulation of the HLA-G expression, with the presence of specific HLA-G gene variation sites or both, and whenever known, the mechanisms underlying such associations are discussed.
Tumors
The arisen of transformed cells and the spread of cancer cell clones are usually controlled by the immune system cells, particularly by the action of cytototoxic T and NK cells; however, cancer cells have developed several strategies to evade host immune surveillance. Since classical histocompatibility (HLA-A, -B, and -C) molecules present tumor antigens to cytotoxic T cells, tumor cells have developed strategies to escape the cytotoxic effect of T cells by interfering with the expression of these molecules on tumor cell surface. On the other hand, the absence of HLA classical molecules on the surface of tumor cells triggers NK cell activity to eliminate neoplastic cells. If tumor cell expresses HLA-G, the cytotoxic activity of both T and NK cells are inhibited, facilitating tumor cell spread. When the decreased expression of classical HLA molecules is accompanied by an increased expression of immunomodulatory molecules such as HLA-G, the effective cytototoxic immune response against tumor cells is much impoverished [reviewed at Ref. (2)].
Although the study of HLA-G expression in tumor cells has been widely explored [reviewed at Ref. (19–21)], the evaluation of the HLA-G gene polymorphic sites has not been studied at the same extent, and even rarer are the studies evaluating the relationship between HLA-G tumor expression and HLA-G polymorphic sites. Next, we highlight some peculiarities of tumors, for which HLA-G expression (tissue or soluble levels), gene polymorphisms, or both have been evaluated.
HLA-G expression in tumors
Increased HLA-G expression has been observed in different tumor types, including breast cancer (22–29), hepatocellular carcinoma (30–33), papillary thyroid carcinoma (34, 35), follicular thyroid carcinoma (35), follicular adenoma (35), nasopharyngeal carcinoma (36), neuroblastoma (37), bladder transitional cell carcinoma (TCC) (38), melanoma (39–42), colorectal cancer (43–45), gastric cancer (46–48), esophageal carcinoma (49–53), lung cancer (49, 54–57), renal cell carcinoma (58–62), glioblastoma (63–66), acute myeloid leukemia (67, 68), and B-cell chronic lymphocytic leukemia (69–73). Table 1 summarizes the HLA-G expression in many types of tumors described in this review.
Table 1.
Association between HLA-G expression and tumors.
| Tumor | HLA-G molecule |
Reference | |||
|---|---|---|---|---|---|
| n | Expression (%) | Metastasisa | sHLA-G (n) | ||
| Breast cancer | 36 | 36IHC | nd | nd | (22) |
| 46/39 | 26(E)IHC/41(S)IHC | No | nd | (74) | |
| 58 | 70.7IHC | nd | ↑(92)ELISA | (23) | |
| 235 | 66IHC | Yes | ↑(44)ELISA | (24) | |
| 677 | 60IHC | No | nd | (27) | |
| nd | nd | nd | ↑(45)ELISA | (25) | |
| 38 | 58IHC | nd | nd | (28) | |
| nd | nd | nd | ↑(120)ELISA | (75) | |
| 52 | 59.6IHC | No | nd | (29) | |
| 45 | 62IHC | Yes | nd | (26) | |
| Hepatocellular carcinoma | 173 | 57IHC | nd | nd | (30) |
| 219 | 50.2IHC | nd | ↑(19)ELISA | (31) | |
| 36 | 66.7WB | nd | ↑(36)ELISA | (32) | |
| nd | nd | nd | ↑(80)ELISA | (33) | |
| Thyroid cancer | nd | nd | nd | ↑(183)ELISA | (76) |
| 70 | 44.3IHC | Yes | nd | (34) | |
| 72 | 77.5IHC | No | nd | (35) | |
| Nasopharyngeal carcinoma | 552 | 79.2IHC | Yes | nd | (36) |
| Neuroblastoma | 12 | 0IHC | nd | ↑(53)ELISA | (37) |
| Bladder transitional cell carcinoma | 75 | 68IHC | nd | Ø(15)ELISA | (38) |
| Melanoma | nd | nd | nd | ↑(190)ELISA | (39) |
| 79 | 28IHC | nd | nd | (40) | |
| 35 | 34.2IHC | nd | nd | (42) | |
| Colorectal cancer | 39 | 87RT-PCR | nd | nd | (43) |
| 201 | 64.6IHC | Yes | nd | (44) | |
| nd | nd | nd | ↑(144)ELISA | (77) | |
| nd | nd | nd | ↑(37)ELISA | (49) | |
| 251 | 20.3IHC | nd | nd | (45) | |
| Gastric cancer | 160 | 71IHC | Yes | nd | (46) |
| 179 | 49.7IHC | Yes | ↑(179)ELISA | (47) | |
| nd | nd | nd | ↑(28)ELISA | (49) | |
| 52 | 31IHC | Yes | nd | (48) | |
| Esophageal carcinoma | 121 | 90.9IHC | Yes | nd | (52) |
| 79 | 65.8IHC | nd | ↑(41)ELISA | (50) | |
| nd | nd | nd | ↑(58)ELISA | (49) | |
| 60 | 75IHC | No | nd | (53) | |
| 60 | 70IHC | Yes | ↑(60)ELISA | (51) | |
| Lung cancer | 39 | 26IHC | nd | nd | (56) |
| 106 | 75IHC | Yes | nd | (57) | |
| 101 | 41.6IHC | nd | ↑(91)ELISA | (54) | |
| nd | nd | nd | ↑(137)ELISA | (55) | |
| nd | nd | nd | ↑(43)ELISA | (49) | |
| Renal cell carcinoma | 18 | 61IHC | nd | nd | (59) |
| 38 | 76qPCR | nd | nd | (61) | |
| Clear cell renal carcinoma | 12 | 58IHC | nd | nd | (60) |
| 95 | 46.8IHC | nd | ↑(16)ELISA | (62) | |
| Glioblastoma | 5 | 80IHC | nd | nd | (63) |
| 26 | ≥58IHC | nd | nd | (64) | |
| 39 | 64IHC | nd | nd | (65) | |
| 108 | 60.2IHC | nd | nd | (66) | |
| Acute myeloid leukemia | nd | nd | nd | ↑(75)ELISA | (78) |
| 77 | 45FC | nd | nd | (67) | |
| 22 | 68.2FC | nd | nd | (68) | |
| B-cell chronic lymphocytic leukemia | 47 | 1–54FC | nd | nd | (69) |
| 20 | 1–34FC | nd | nd | (72) | |
| 30 | 35.31FC | nd | nd | (73) | |
aAssociation between HLA-G expression and metastasis.
sHLA-G, soluble HLA-G; IHC, imunohistochemistry; nd, not determined; (E), breast carcinoma effusions; (S), breast carcinoma solid lesions; ↑, increased sHLA-G levels in patients; ELISA, enzyme-linked immunosorbent assay; WB, western blotting; Ø, similar sHLA-G levels between patients and controls; RT-PCR, reverse transcriptase-PCR; qPCR, quantitative PCR; FC, flow cytometry.
In most tumors, the increased HLA-G expression has been associated with advanced disease stages, shorter survival time, presence of metastasis, higher tumor grade, weak host immune response, greater tumor size, tumor recurrence, tumor invasion, poor histological grade, lower classical HLA antigen expression, presence of infiltrating T regulatory cells, cancer progression, increased inflammatory cell lesion infiltration, and tumor differentiation (23, 24, 26, 29–32, 34–36, 40–42, 44, 46–48, 50–54, 56, 57, 66, 69, 72, 73, 79). In other tumors, no association between increased HLA-G expression and clinicopathological features has been observed, including bladder TCC (38) and acute myeloid leukemia (67, 68).
Furthermore, increased sHLA-G levels have been reported for breast cancer (23–25, 75), hepatocellular carcinoma (31–33), papillary thyroid carcinoma (76), neuroblastoma (37), melanoma (39), colorectal cancer (49, 77), gastric cancer (47, 49), esophageal carcinoma (49–51), lung cancer (49, 54, 55), renal cell carcinoma (62), and acute myeloid leukemia (78). Higher sHLA-G levels have been associated with: (i) increased number of CD4+ regulatory T (Treg) cells in breast cancer (23), (ii) more aggressive tumor behavior in papillary thyroid carcinoma (76), (iii) local or disseminated relapse in neuroblastoma (37), (iv) advanced stages of disease and tumor load in melanoma (39), (v) higher IL-10 production in esophageal carcinoma (51), (vi) absence of anterior myelodysplasia along with higher leukocytosis in acute myeloid leukemia (78), and (vii) shorter survival time, high-grade tumors, higher IL-10 production, and loss of HLA classical class I molecules in patients with lung cancer (54–56).
Interestingly, sHLA-G levels were significantly decreased in breast cancer patients at 6 and 12 months after surgery (25). In addition, no association between higher sHLA-G levels and clinicopathological features has been observed in hepatocellular carcinoma (33), colorectal cancer (77), gastric cancer (47), esophageal carcinoma (50, 51), and renal cell carcinoma (62). On the other hand, plasma sHLA-G levels were closely similar when bladder TCC patients and healthy controls were compared (38).
Overall, several laboratory (increased HLA-G tumor expression, increased sHLA-G levels, increased levels of IL-10, and a cytokine that induces HLA-G expression) and clinical (advanced disease stages, worse prognosis, and presence of metastasis) findings do corroborate the malefic role of HLA-G in cancer disorders.
Polymorphic sites at HLA-G gene and tumors
Several isolated segments of the HLA-G gene have been studied in tumors, highlighting the 3′ untranslated and coding regions. Certainly, the 14-bpINS/DEL polymorphism is the most studied. In breast cancer patients, the 14-bpDEL allele and 14-bpDEL/DEL genotype were associated with susceptibility to breast cancer in Southeastern Iranian (80) and Korean patients (81); however, no association has been reported for Brazilians (26). In addition, Korean patients exhibiting the 14-bpINS/INS genotype exhibited no HLA-G expression in breast cancer lesions (81). A meta-analysis evaluating the role of the 14-bpINS/DEL polymorphism in breast cancer reports an overall cancer risk in Asian populations (82).
The 14-bpDEL allele was associated with susceptibility to hepatocellular carcinoma in Brazilian (83) and Chinese (84) patients, but not in Korean patients (84). In addition, Chinese patients exhibiting the 14-bpDEL/DEL genotype presented increased HLA-G expression in hepatocellular carcinoma specimens (84). The 14-bpINS/DEL genotype was associated with decreased risk for childhood neuroblastoma development in Australian and New Zealand patients (85). The HLA-G 3′UTR haplotype known as UTR-3 (86) was associated with susceptibility to acute myeloid leukemia development in Italian patients (68).
Considering the HLA-G coding segment, the +755C/A (non-synonymous Leu/Ile substitution at codon 110, which defines the HLA-G*01:04 protein group) was associated with protection against more severe nasopharyngeal carcinoma tumor stages (87).
Regarding the bladder TCC, the HLA-G*01:04:04 allele, and the HLA-G*01:04 allelic group were associated with susceptibility to bladder TCC in smoking patients and the HLA-G*01:03 allele and the HLA-G*01:04 allelic group was associated with protection against bladder TCC development in non-smoking Brazilian patients. In addition, the HLA-G*01:01 allelic group and HLA-G*01:01/G*01:01 genotype were associated with susceptibility to bladder TCC development in non-smokers. Considering the bladder TCC progression, the following associations were observed: (i) the HLA-G*01:03 allele was associated with high-grade tumors among smokers; (ii) the HLA-G*01:01:01/G*01:01:02 genotype was associated with protection against high-grade tumors in the whole group of patients, whereas the same association was observed with the HLA-G*01:01 group, but only among smokers; and (iii) the HLA-G*01:04 allele group was associated with high-grade tumor development in smoker and in the whole group of patients (88).
No association has been observed for: (i) HLA-G coding region alleles in South Korean and Brazilian breast cancer patients (81, 89); (ii) 14-bpINS/DEL polymorphism in Italian patients presenting thyroid cancer (76); (iii) HLA-G*01:03 allele and HLA-G*01:05N null allele in Tunisian patients with nasopharyngeal carcinoma (87); (iv) HLA-G*01:05N null allele with susceptibility to esophagus carcinoma development in Chinese patients (90); (v) 14-bp INS/DEL polymorphic site in Brazilian bladder TCC patients (88); and (vi) +292A/T, +755C/A, and +1799C/T in Australian and New Zealand childhood neuroblastoma patients (85).
To date, HLA-G polymorphisms have not been investigated in the context of melanoma, glioblastoma, colorectal cancer, gastric cancer, lung cancer, and renal cell carcinoma.
Although some polymorphic sites (14-bpDEL allele) and coding region allele groups (HLA-G*01:04) have been previously associated with increased sHLA-G levels, few convincing associations have been reported, exception made to breast cancer for which an extensive meta-analysis has evidenced the role of this polymorphic site in Asiatic patients. Since several polymorphic sites have been described at the HLA-G regulatory regions, exhibiting putative roles on HLA-G expression, the typing of the complete gene and the study of the regulatory elements (transcription factors and microRNAs) produced in the tumor environment may the helpful to understand the mechanisms of tumor evasion mechanisms.
Viral Hepatitis
Similar to tumor cells, viruses have also developed several strategies to evade the cytotoxic effect of immune effector cells, including downregulation of HLA classical class I molecules and the upregulation of non-classical molecules, or both. As a corollary, the increased HLA-G expression, induced by the virus itself or by the presence of an inflammatory milieu containing transcription and post-transcription factors that positively modulate HLA-G expression, may exacerbate virus morbidity and/or patient mortality. The influence of HLA-G has been studied in several chronic viral infections; some of them associated with neoplastic transformation, including human immunodeficiency virus (HIV), human papillomavirus (HPV), human cytomegalovirus (hCMV), and hepatitis viruses [reviewed at Ref. (2)].
Increased HLA-G hepatocyte expression in HCV-infected liver specimens has been associated with milder stages of fibrosis and hemosiderin deposit (91). Besides hepatocytes, HLA-G expression was observed on mast cells present in areas of liver fibrosis (92). Increased plasma sHLA-G levels were associated with chronic HCV infection and with increased IL-10 and IFN-γ levels (93). Since the treatment of mast cells with IL-10 and class I interferons induces HLA-G expression (92), infiltrating cells may play an important role on the maintenance of chronic infection and induction of chronic complications.
One study has associated increased HLA-G expression in hepatocytes with the HBV viral load (94). Different studies associated the increased serum/plasma sHLA-G levels with hepatitis B virus infection (33, 95, 96), which were associated with increased percentage of CD4+CD25+FoxP3+ T regulatory and HLA-G+CD14+ monocytes cells in patients exhibiting acute or chronic hepatitis (95), active hepatitis B virus infection (33) and HBeAg negative hepatitis, hepatocellular carcinoma, and increased alanine aminotransferase levels (96).
Regarding the typing of HLA-G 3′UTR polymorphic sites in HCV- and HBV-infected patients, the +3142C allele and 14-bpDEL/+3142C haplotype were underrepresented in Brazilian HCV-infected patients presenting sickle cells disease compared with HCV-negative group (97). On the other hand, the 14-bpINS/INS genotype was overrepresented in African-Brazilian HIV+ patients co-infected with HCV (HIV+/HCV+) compared with HIV+/HCV− patients. Regarding the HLA-G+3142 C/G and 14-bp INS/DEL variants, no significant association has been reported for HIV+/HCV+- (98) and HBV-infected patients (99), respectively, when compared with their respective controls.
Considering that many viruses have developed evasion strategies that are similar to cancer cells and considering that many chronic viral disorders have been associated with cell transformation and malignancy, the expression of HLA-G in these disorders may predict a worse outcome and greater susceptibility to cell transformation.
Protozoan Parasite Infections
Human malaria infection
Plasmodium spp. is the etiologic agent of the human malaria and little is known about the role of HLA-G during malaria infection, and all studies have been performed to understand the mother to child transmission. One study reported a decreased HLA-G expression in extravillous trophoblast of Plasmodium falciparum-infected placentas compared to uninfected placentas. If by one hand, HLA-G molecule is almost exclusively expressed in extravillous trophoblast of healthy placenta specimens, on the other hand, HLA-G is detected in intervillous space macrophages of Plasmodium-infected placentas. In addition, NK cells are increased in infected compared to uninfected placentas (100). Furthermore, increased cord plasma levels of sHLA-G have been associated with low birth weight and increased risk of P. falciparum infection in infancy (101).
A family based association study performed on individuals from Niakhar, Senegal, reported that the +3187G allele was associated with higher transmission to children and lower level of parasite density during asymptomatic P. falciparum infection. The HLA-G 3′UTR haplotype known as UTR-1 was associated with a decreased level of parasite density during asymptomatic infection under a dominant model, whereas the HLA-G UTR-3 haplotype was associated with an increased level of parasite density during the follow-up and increased intensity of asymptomatic infection under a recessive model (102).
A second family based association study also conducted on Senegalese population has tested the association of HLA-G 3′UTR variants with acquired anti-malarial humoral immunity. The +3010G and +3142C alleles were overtransmitted to children with increased total IgG and IgG1 antibodies levels against glutamate-rich protein (GLURP) of P. falciparum, and the +3196G allele had a preferential transmission to children with a lower IgG3 response against merozoite surface protein 2 (MSP2). The HLA-G 3′UTR-2 haplotype was associated with a decreased IgG3 response against MSP2, suggesting a role of HLA-G on the regulation of immune humoral response during P. falciparum infection (103).
Human African trypanosomiasis
Human African trypanosomiasis, also known as sleeping sickness, is caused by protozoan parasites of the Trypanosoma brucei species. Although no studies are available regarding HLA-G expression, genetic studies report associations of HLA-G gene single nucleotide variation sites with the disease. A family based association study reported that the HLA-G 3′UTR-14-bpINS and +3196G alleles had a preferential transmission from heterozygote parents to children and were associated with susceptibility to human African trypanosomiasis (HAT) development. In contrast, the HLA-G 3′UTR +3003C, +3010G, and +3187G alleles showed lower transmission from parents to children and were associated with decreased risk of developing the disease. Regarding HLA-G 3′UTR haplotypes, UTR-2 and UTR-5 haplotypes were associated with higher susceptibility to HAT development, whereas the HLA-G UTR-4 haplotype was associated with decreased risk for HAT development (104).
American trypanosomiasis
The parasite Trypanosoma cruzi is the etiologic agent of American trypanosomiasis, also known as Chagas disease (105). In the chronic phase, four major clinical forms are observed: (i) cardiac that presents progressive congestive heart failure, various cardiac arrhythmias, thromboembolic events, and sudden death; (ii) digestive that is characterized by clinical signs of megaesophagus, megacolon, or both; (iii) cardiodigestive that comprises clinical and pathological signs of cardiac and digestive involvement; and (iv) indeterminate that develops without evident clinical and pathological signs (106). Recently, our group reported a decreased HLA-G expression on cardiac muscle and colonic cells in patients presenting cardiac or digestive clinical variants, respectively. On the other hand, no significant differences were observed regarding HLA-G expression in the esophagus of patients with digestive form when compared to non-chagasic patients.
Furthermore, we evaluated the polymorphic sites at the HLA-G 3′UTR region in Brazilian chagasic patients. The +3003T allele and +3003TT and +3187GG genotypes were overrepresented, whereas the +3003C allele and +3003CT, +3010GC, and +3042GC genotypes were underrepresented in symptomatic patients. In addition, the +3027CC and +3035CC genotypes, and the +3027C and +3035C alleles were associated with the digestive form of Chagas disease. Regarding HLA-G 3′UTR haplotypes, decreased UTR-4 and UTR-7 frequencies were associated with symptomatic patients and with the digestive form, respectively. On the other hand, UTR-13 was associated with the indeterminate variant and UTR-14 with the cardiac form (107).
Overall, studies on the association between HLA-G and parasitic disorders are still scarce and only the HLA-G 3′UTR has been evaluated.
Conclusion
Considering the tolerogenic properties of HLA-G and considering the aphorism that the induced expression of HLA-G may be detrimental in tumors and chronic viral infection, the overall findings reported is this revision corroborates this idea. Noteworthy, is the induced expression of HLA-G on the surface of tumor cells, which has been associated with greater tumor morbidity, tumor progression, and spreading. In addition, in chronic viral infections associated with pre-neoplastic and neoplastic transformation. On the other hand, the repression of HLA-G expression is less well studied; i.e., the decreased expression of HLA-G in organs or conditions in which a constitutive expression of the molecule is expected. For instance, the decreased expression of HLA-G (placentas of P. falciparum-infected mothers or heart and colonic specimens of Chagas disease) has been associated with morbidity of the chronic parasitic infection.
Studies on the association of the HLA-G gene with diseases of diverse etiology have underestimated the myriad of polymorphic sites present at the various gene segments and have primarily focused on the evaluation of one or few polymorphic sites, particularly at the 3′UTR. Considering that many polymorphic sites along the HLA-G gene can be readily performed and analyzed, and considering the relevant role of isolated polymorphic sites or HLA-G haplotypes on HLA-G expression, HLA-G typing on diseases should add an additional tool on the understanding of the role of HLA-G on disease associations.
Theoretically, polymorphic sites observed along the coding region may modify the encoded protein and consequently the interaction with HLA-G receptors and the formation of HLA-G dimers that may more efficiently bind to HLA-G receptors. Thus, a particular allele and a particular molecule could provide susceptibility or protection against a disease development; however, such associations have not been strong enough to be considered a disease marker, as has been observed for the classical association between HLA-B27 and ankylosing spondylitis. On the other hand, polymorphic sites observed along the HLA-G promoter and 3′UTR gene segments may modify gene expression, accounting for disease morbidity. Unfortunately, few polymorphic sites along regulatory regions have extensively been evaluated regarding their function, and probably a combination of regulatory transcriptional and posttranscriptional elements may account for the final HLA-G production. Therefore, a complete gene evaluation together with the availability of transcription and protein profiles may provide light to the understanding of the mechanisms of HLA-G induction or repression in a specific disorder.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The Review Editor, Joel LeMaoult, declares that despite having co-authored a manuscript and being affiliated with the same institution as author Philippe Moreau, there has been no conflict of interest during the review and handling of this manuscript.
Acknowledgments
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [grant number CAPES/COFECUB 653/09], Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq Science Without Borders Program, grant number 236754/2012-2; Special Visiting Researcher, grant number 406594/2013-9; Young Talents, grant number 401641/2013-9; CNPq edital 71/2013, grant number 406594/2013-9; CNPq Universal, grant number 466036/2013-5; CNPq/MS/SCTIE/DECIT No. 31/2014 – Pesquisas sobre Doença de Chagas, grant number 467157/2014-6], and Núcleo de Apoio a Pesquisa em Doenças Inflamatórias (NAP-DIN).
References
- 1.Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A (1987) 84(24):9145–9. 10.1073/pnas.84.24.9145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donadi EA, Castelli EC, Arnaiz-Villena A, Roger M, Rey D, Moreau P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell Mol Life Sci (2011) 68(3):369–95. 10.1007/s00018-010-0580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A (1997) 94(21):11520–5. 10.1073/pnas.94.21.11520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naji A, Menier C, Morandi F, Agaugue S, Maki G, Ferretti E, et al. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J Immunol (2014) 192(4):1536–46. 10.4049/jimmunol.1300438 [DOI] [PubMed] [Google Scholar]
- 5.Horuzsko A, Lenfant F, Munn DH, Mellor AL. Maturation of antigen-presenting cells is compromised in HLA-G transgenic mice. Int Immunol (2001) 13(3):385–94. 10.1093/intimm/13.3.385 [DOI] [PubMed] [Google Scholar]
- 6.Le Discorde M, Moreau P, Sabatier P, Legeais JM, Carosella ED. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum Immunol (2003) 64(11):1039–44. 10.1016/j.humimm.2003.08.346 [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre S, Adrian F, Moreau P, Gourand L, Dausset J, Berrih-Aknin S, et al. Modulation of HLA-G expression in human thymic and amniotic epithelial cells. Hum Immunol (2000) 61(11):1095–101. 10.1016/S0198-8859(00)00192-0 [DOI] [PubMed] [Google Scholar]
- 8.Menier C, Rabreau M, Challier JC, Le Discorde M, Carosella ED, Rouas-Freiss N. Erythroblasts secrete the nonclassical HLA-G molecule from primitive to definitive hematopoiesis. Blood (2004) 104(10):3153–60. 10.1182/blood-2004-03-0809 [DOI] [PubMed] [Google Scholar]
- 9.Tan Z, Shon AM, Ober C. Evidence of balancing selection at the HLA-G promoter region. Hum Mol Genet (2005) 14(23):3619–28. 10.1093/hmg/ddi389 [DOI] [PubMed] [Google Scholar]
- 10.Castelli EC, Mendes-Junior CT, Veiga-Castelli LC, Roger M, Moreau P, Donadi EA. A comprehensive study of polymorphic sites along the HLA-G gene: implication for gene regulation and evolution. Mol Biol Evol (2011) 28(11):3069–86. 10.1093/molbev/msr138 [DOI] [PubMed] [Google Scholar]
- 11.Sabbagh A, Luisi P, Castelli EC, Gineau L, Courtin D, Milet J, et al. Worldwide genetic variation at the 3’ untranslated region of the HLA-G gene: balancing selection influencing genetic diversity. Genes Immun (2014) 15(2):95–106. 10.1038/gene.2013.67 [DOI] [PubMed] [Google Scholar]
- 12.Santos KE, Lima TH, Felicio LP, Massaro JD, Palomino GM, Silva AC, et al. Insights on the HLA-G evolutionary history provided by a nearby Alu insertion. Mol Biol Evol (2013) 30(11):2423–34. 10.1093/molbev/mst142 [DOI] [PubMed] [Google Scholar]
- 13.Castelli EC, Veiga-Castelli LC, Yaghi L, Moreau P, Donadi EA. Transcriptional and posttranscriptional regulations of the HLA-G gene. J Immunol Res (2014) 2014:734068. 10.1155/2014/734068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan Z, Randall G, Fan J, Camoretti-Mercado B, Brockman-Schneider R, Pan L, et al. Allele-specific targeting of microRNAs to HLA-G and risk of asthma. Am J Hum Genet (2007) 81(4):829–34. 10.1086/521200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manaster I, Goldman-Wohl D, Greenfield C, Nachmani D, Tsukerman P, Hamani Y, et al. MiRNA-mediated control of HLA-G expression and function. PLoS One (2012) 7(3):e33395. 10.1371/journal.pone.0033395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelli EC, Moreau P, Oya e Chiromatzo A, Mendes-Junior CT, Veiga-Castelli LC, Yaghi L, et al. In silico analysis of microRNAS targeting the HLA-G 3’ untranslated region alleles and haplotypes. Hum Immunol (2009) 70(12):1020–5. 10.1016/j.humimm.2009.07.028 [DOI] [PubMed] [Google Scholar]
- 17.Martelli-Palomino G, Pancotto JA, Muniz YC, Mendes-Junior CT, Castelli EC, Massaro JD, et al. Polymorphic sites at the 3’ untranslated region of the HLA-G gene are associated with differential HLA-G soluble levels in the Brazilian and French population. PLoS One (2013) 8(10):e71742. 10.1371/journal.pone.0071742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castelli EC, Ramalho J, Porto IO, Lima TH, Felicio LP, Sabbagh A, et al. Insights into HLA-G genetics provided by worldwide haplotype diversity. Front Immunol (2014) 5:476. 10.3389/fimmu.2014.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res (2005) 65(22):10139–44. 10.1158/0008-5472.CAN-05-0097 [DOI] [PubMed] [Google Scholar]
- 20.Carosella ED, Moreau P, Lemaoult J, Rouas-Freiss N. HLA-G: from biology to clinical benefits. Trends Immunol (2008) 29(3):125–32. 10.1016/j.it.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Bukur J, Jasinski S, Seliger B. The role of classical and non-classical HLA class I antigens in human tumors. Semin Cancer Biol (2012) 22(4):350–8. 10.1016/j.semcancer.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre S, Antoine M, Uzan S, McMaster M, Dausset J, Carosella ED, et al. Specific activation of the non-classical class I histocompatibility HLA-G antigen and expression of the ILT2 inhibitory receptor in human breast cancer. J Pathol (2002) 196(3):266–74. 10.1002/path.1039 [DOI] [PubMed] [Google Scholar]
- 23.Chen HX, Lin A, Shen CJ, Zhen R, Chen BG, Zhang X, et al. Upregulation of human leukocyte antigen-G expression and its clinical significance in ductal breast cancer. Hum Immunol (2010) 71(9):892–8. 10.1016/j.humimm.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 24.He X, Dong DD, Yie SM, Yang H, Cao M, Ye SR, et al. HLA-G expression in human breast cancer: implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann Surg Oncol (2010) 17(5):1459–69. 10.1245/s10434-009-0891-9 [DOI] [PubMed] [Google Scholar]
- 25.Sayed D, Badr G, Maximous D, Mikhail NN, Abu-Tarboush F, Alhazza IM. HLA-G and its relation to proliferation index in detection and monitoring breast cancer patients. Tissue Antigens (2010) 75(1):40–7. 10.1111/j.1399-0039.2009.01393.x [DOI] [PubMed] [Google Scholar]
- 26.Ramos CS, Goncalves AS, Marinho LC, Gomes Avelino MA, Saddi VA, Lopes AC, et al. Analysis of HLA-G gene polymorphism and protein expression in invasive breast ductal carcinoma. Hum Immunol (2014) 75(7):667–72. 10.1016/j.humimm.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 27.de Kruijf EM, Sajet A, van Nes JG, Natanov R, Putter H, Smit VT, et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol (2010) 185(12):7452–9. 10.4049/jimmunol.1002629 [DOI] [PubMed] [Google Scholar]
- 28.Elliott RL, Jiang XP, Phillips JT, Barnett BG, Head JF. Human leukocyte antigen G expression in breast cancer: role in immunosuppression. Cancer Biother Radiopharm (2011) 26(2):153–7. 10.1089/cbr.2010.0924 [DOI] [PubMed] [Google Scholar]
- 29.da Silva GB, Silva TG, Duarte RA, Neto NL, Carrara HH, Donadi EA, et al. Expression of the classical and nonclassical HLA molecules in breast cancer. Int J Breast Cancer (2013) 2013:250435. 10.1155/2013/250435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai MY, Xu YF, Qiu SJ, Ju MJ, Gao Q, Li YW, et al. Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection. Clin Cancer Res (2009) 15(14):4686–93. 10.1158/1078-0432.CCR-09-0463 [DOI] [PubMed] [Google Scholar]
- 31.Lin A, Chen HX, Zhu CC, Zhang X, Xu HH, Zhang JG, et al. Aberrant human leukocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med (2010) 14(8):2162–71. 10.1111/j.1582-4934.2009.00917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Ye Z, Meng XQ, Zheng SS. Expression of HLA-G in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int (2011) 10(2):158–63 10.1016/S1499-3872(11)60025-8 [DOI] [PubMed] [Google Scholar]
- 33.Park Y, Park Y, Lim HS, Kim YS, Hong DJ, Kim HS. Soluble human leukocyte antigen-G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue Antigens (2012) 79(2):97–103. 10.1111/j.1399-0039.2011.01814.x [DOI] [PubMed] [Google Scholar]
- 34.Nunes LM, Ayres FM, Francescantonio IC, Saddi VA, Avelino MA, Alencar Rde C, et al. Association between the HLA-G molecule and lymph node metastasis in papillary thyroid cancer. Hum Immunol (2013) 74(4):447–51. 10.1016/j.humimm.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 35.de Figueiredo Feitosa NL, Crispim JC, Zanetti BR, Magalhaes PK, Soares CP, Soares EG, et al. HLA-G is differentially expressed in thyroid tissues. Thyroid (2014) 24(3):585–92. 10.1089/thy.2013.0246 [DOI] [PubMed] [Google Scholar]
- 36.Cai MB, Han HQ, Bei JX, Liu CC, Lei JJ, Cui Q, et al. Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma. Int J Biol Sci (2012) 8(6):891–900. 10.7150/ijbs.4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morandi F, Levreri I, Bocca P, Galleni B, Raffaghello L, Ferrone S, et al. Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res (2007) 67(13):6433–41. 10.1158/0008-5472.CAN-06-4588 [DOI] [PubMed] [Google Scholar]
- 38.Gan LH, Huang LF, Zhang X, Lin A, Xu DP, Wang Q, et al. Tumor-specific upregulation of human leukocyte antigen-G expression in bladder transitional cell carcinoma. Hum Immunol (2010) 71(9):899–904. 10.1016/j.humimm.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen – G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer (2001) 92(2):369–76. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim EC, Aractingi S, Allory Y, Borrini F, Dupuy A, Duvillard P, et al. Analysis of HLA antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-A1 genotype. Int J Cancer (2004) 108(2):243–50. 10.1002/ijc.11456 [DOI] [PubMed] [Google Scholar]
- 41.Bezuhly M, Howlett A, Colp P, Conrad DM, Walsh N, Rowden G, et al. Quantitative HLA-G expression in metastasising and non-metastasising primary thin cutaneous melanomas. Dermatology (2008) 217(3):281–3 10.1159/000150602 [DOI] [PubMed] [Google Scholar]
- 42.Fang X, Zhang X, Li J. Up-regulation of human leukocyte antigen G expression in primary cutaneous malignant melanoma associated with host-vs-tumor immune response. J Huazhong Univ Sci Technolog Med Sci (2008) 28(2):219–21. 10.1007/s11596-008-0227-1 [DOI] [PubMed] [Google Scholar]
- 43.Fukushima Y, Oshika Y, Nakamura M, Tokunaga T, Hatanaka H, Abe Y, et al. Increased expression of human histocompatibility leukocyte antigen-G in colorectal cancer cells. Int J Mol Med (1998) 2(3):349–51. [DOI] [PubMed] [Google Scholar]
- 44.Ye SR, Yang H, Li K, Dong DD, Lin XM, Yie SM. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol (2007) 20(3):375–83. 10.1038/modpathol.3800751 [DOI] [PubMed] [Google Scholar]
- 45.Zeestraten EC, Reimers MS, Saadatmand S, Dekker JW, Liefers GJ, van den Elsen PJ, et al. Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer (2014) 110(2):459–68 10.1038/bjc.2013.696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leukocyte antigen G (HLA-G) correlates with poor prognosis in gastric carcinoma. Ann Surg Oncol (2007) 14(10):2721–9. 10.1245/s10434-007-9464-y [DOI] [PubMed] [Google Scholar]
- 47.Du L, Xiao X, Wang C, Zhang X, Zheng N, Wang L, et al. Human leukocyte antigen-G is closely associated with tumor immune escape in gastric cancer by increasing local regulatory T cells. Cancer Sci (2011) 102(7):1272–80. 10.1111/j.1349-7006.2011.01951.x [DOI] [PubMed] [Google Scholar]
- 48.Tuncel T, Karagoz B, Haholu A, Ozgun A, Emirzeoglu L, Bilgi O, et al. Immunoregulatory function of HLA-G in gastric cancer. Asian Pac J Cancer Prev (2013) 14(12):7681–4. 10.7314/APJCP.2013.14.12.7681 [DOI] [PubMed] [Google Scholar]
- 49.Cao M, Yie SM, Liu J, Ye SR, Xia D, Gao E. Plasma soluble HLA-G is a potential biomarker for diagnosis of colorectal, gastric, esophageal and lung cancer. Tissue Antigens (2011) 78(2):120–8. 10.1111/j.1399-0039.2011.01716.x [DOI] [PubMed] [Google Scholar]
- 50.Lin A, Zhang X, Zhou WJ, Ruan YY, Xu DP, Wang Q, et al. Human leukocyte antigen-G expression is associated with a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Cancer (2011) 129(6):1382–90. 10.1002/ijc.25807 [DOI] [PubMed] [Google Scholar]
- 51.Zheng J, Xu C, Chu D, Zhang X, Li J, Ji G, et al. Human leukocyte antigen G is associated with esophageal squamous cell carcinoma progression and poor prognosis. Immunol Lett (2014) 161(1):13–9. 10.1016/j.imlet.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 52.Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of HLA-G is associated with prognosis in esophageal squamous cell carcinoma. Am J Clin Pathol (2007) 128(6):1002–9. 10.1309/JNCW1QLDFB6AM9WE [DOI] [PubMed] [Google Scholar]
- 53.Hu J, Li L, Liu Y, Chen Y, Liu C, Liang W, et al. Overexpression of HLA-G Is positively associated with Kazakh esophageal squamous cell carcinoma in Xinjiang, China. Viral Immunol (2013) 26(3):180–4. 10.1089/vim.2012.0085 [DOI] [PubMed] [Google Scholar]
- 54.Lin A, Zhu CC, Chen HX, Chen BF, Zhang X, Zhang JG, et al. Clinical relevance and functional implications for human leukocyte antigen-g expression in non-small-cell lung cancer. J Cell Mol Med (2010) 14(9):2318–29. 10.1111/j.1582-4934.2009.00858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schutt P, Schutt B, Switala M, Bauer S, Stamatis G, Opalka B, et al. Prognostic relevance of soluble human leukocyte antigen-G and total human leukocyte antigen class I molecules in lung cancer patients. Hum Immunol (2010) 71(5):489–95. 10.1016/j.humimm.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 56.Urosevic M, Kurrer MO, Kamarashev J, Mueller B, Weder W, Burg G, et al. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol (2001) 159(3):817–24. 10.1016/S0002-9440(10)61756-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leukocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer (2007) 58(2):267–74. 10.1016/j.lungcan.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 58.Hanak L, Slaby O, Lauerova L, Kren L, Nenutil R, Michalek J. Expression pattern of HLA class I antigens in renal cell carcinoma and primary cell line cultures: methodological implications for immunotherapy. Med Sci Monit (2009) 15(12):CR638–43. [PubMed] [Google Scholar]
- 59.Ibrahim EC, Guerra N, Lacombe MJ, Angevin E, Chouaib S, Carosella ED, et al. Tumor-specific up-regulation of the nonclassical class I HLA-G antigen expression in renal carcinoma. Cancer Res (2001) 61(18):6838–45. [PubMed] [Google Scholar]
- 60.Ibrahim EC, Allory Y, Commo F, Gattegno B, Callard P, Paul P. Altered pattern of major histocompatibility complex expression in renal carcinoma: tumor-specific expression of the nonclassical human leukocyte antigen-G molecule is restricted to clear cell carcinoma while up-regulation of other major histocompatibility complex antigens is primarily distributed in all subtypes of renal carcinoma. Am J Pathol (2003) 162(2):501–8. 10.1016/S0002-9440(10)63844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kren L, Valkovsky I, Dolezel J, Capak I, Pacik D, Poprach A, et al. HLA-G and HLA-E specific mRNAs connote opposite prognostic significance in renal cell carcinoma. Diagn Pathol (2012) 7:58. 10.1186/1746-1596-7-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li BL, Lin A, Zhang XJ, Zhang X, Zhang JG, Wang Q, et al. Characterization of HLA-G expression in renal cell carcinoma. Tissue Antigens (2009) 74(3):213–21. 10.1111/j.1399-0039.2009.01302.x [DOI] [PubMed] [Google Scholar]
- 63.Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol (2002) 168(9):4772–80. 10.4049/jimmunol.168.9.4772 [DOI] [PubMed] [Google Scholar]
- 64.Kren L, Muckova K, Lzicarova E, Sova M, Vybihal V, Svoboda T, et al. Production of immune-modulatory nonclassical molecules HLA-G and HLA-E by tumor infiltrating ameboid microglia/macrophages in glioblastomas: a role in innate immunity? J Neuroimmunol (2010) 220(1–2):131–5. 10.1016/j.jneuroim.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 65.Kren L, Slaby O, Muckova K, Lzicarova E, Sova M, Vybihal V, et al. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: an unexpected prognostic significance? Neuropathology (2011) 31(2):129–34. 10.1111/j.1440-1789.2010.01149.x [DOI] [PubMed] [Google Scholar]
- 66.Wastowski IJ, Simoes RT, Yaghi L, Donadi EA, Pancoto JT, Poras I, et al. Human leukocyte antigen-G is frequently expressed in glioblastoma and may be induced in vitro by combined 5-aza-2’-deoxycytidine and interferon-gamma treatments: results from a multicentric study. Am J Pathol (2013) 182(2):540–52. 10.1016/j.ajpath.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo QY, Chen BG, Ruan YY, Lin A, Yan WH. HLA-G expression is irrelevant to prognosis in patients with acute myeloid leukemia. Leuk Res (2011) 35(10):1350–4. 10.1016/j.leukres.2011.05.036 [DOI] [PubMed] [Google Scholar]
- 68.Locafaro G, Amodio G, Tomasoni D, Tresoldi C, Ciceri F, Gregori S. HLA-G expression on blasts and tolerogenic cells in patients affected by acute myeloid leukemia. J Immunol Res (2014) 2014:636292. 10.1155/2014/636292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nuckel H, Rebmann V, Durig J, Duhrsen U, Grosse-Wilde H. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood (2005) 105(4):1694–8. 10.1182/blood-2004-08-3335 [DOI] [PubMed] [Google Scholar]
- 70.Rebmann V, Nuckel H, Duhrsen U, Grosse-Wilde H. HLA-G in B-chronic lymphocytic leukaemia: clinical relevance and functional implications. Semin Cancer Biol (2007) 17(6):430–5. 10.1016/j.semcancer.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 71.Giannopoulos K, Dmoszynska A, Bojarska-Junak A, Schmitt M, Rolinski J. Expression of HLA-G in patients with B-cell chronic lymphocytic leukemia (B-CLL). Folia Histochem Cytobiol (2008) 46(4):457–60. 10.2478/v10042-008-0072-x [DOI] [PubMed] [Google Scholar]
- 72.Erikci AA, Karagoz B, Ozyurt M, Ozturk A, Kilic S, Bilgi O. HLA-G expression in B chronic lymphocytic leukemia: a new prognostic marker? Hematology (2009) 14(2):101–5. 10.1179/102453309X385197 [DOI] [PubMed] [Google Scholar]
- 73.Attia MA, Nosair NA, Gawally A, Elnagar G, Elshafey EM. HLA-G expression as a prognostic indicator in B-cell chronic lymphocytic leukemia. Acta Haematol (2014) 132(1):53–8. 10.1159/000353757 [DOI] [PubMed] [Google Scholar]
- 74.Kleinberg L, Florenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, et al. Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch (2006) 449(1):31–9. 10.1007/s00428-005-0144-7 [DOI] [PubMed] [Google Scholar]
- 75.Provatopoulou X, Kalogera E, Sagkriotis A, Zagouri F, Nonni A, Zografos GC, et al. Soluble human leukocyte antigen-G expression in patients with ductal and lobular breast malignancy. Anticancer Res (2012) 32(3):1021–6. [PubMed] [Google Scholar]
- 76.Dardano A, Rizzo R, Polini A, Stignani M, Tognini S, Pasqualetti G, et al. Soluble human leukocyte antigen-g and its insertion/deletion polymorphism in papillary thyroid carcinoma: novel potential biomarkers of disease? J Clin Endocrinol Metab (2012) 97(11):4080–6. 10.1210/jc.2012-2231 [DOI] [PubMed] [Google Scholar]
- 77.Zhu CB, Wang CX, Zhang X, Zhang J, Li W. Serum sHLA-G levels: a useful indicator in distinguishing colorectal cancer from benign colorectal diseases. Int J Cancer (2011) 128(3):617–22. 10.1002/ijc.25372 [DOI] [PubMed] [Google Scholar]
- 78.Gros F, Sebti Y, de Guibert S, Branger B, Bernard M, Fauchet R, et al. Soluble HLA-G molecules increase during acute leukemia, especially in subtypes affecting monocytic and lymphoid lineages. Neoplasia (2006) 8(3):223–30. 10.1593/neo.05703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morandi F, Scaruffi P, Gallo F, Stigliani S, Moretti S, Bonassi S, et al. Bone marrow-infiltrating human neuroblastoma cells express high levels of calprotectin and HLA-G proteins. PLoS One (2012) 7(1):e29922. 10.1371/journal.pone.0029922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eskandari-Nasab E, Hashemi M, Hasani SS, Omrani M, Taheri M, Mashhadi MA. Association between HLA-G 3’UTR 14-bp ins/del polymorphism and susceptibility to breast cancer. Cancer Biomark (2013) 13(4):253–9. 10.3233/CBM-130364 [DOI] [PubMed] [Google Scholar]
- 81.Jeong S, Park S, Park BW, Park Y, Kwon OJ, Kim HS. Human leukocyte antigen-G (HLA-G) polymorphism and expression in breast cancer patients. PLoS One (2014) 9(5):e98284. 10.1371/journal.pone.0098284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge YZ, Ge Q, Li MH, Shi GM, Xu X, Xu LW, et al. Association between human leukocyte antigen-G 14-bp insertion/deletion polymorphism and cancer risk: a meta-analysis and systematic review. Hum Immunol (2014) 75(8):827–32. 10.1016/j.humimm.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 83.Teixeira AC, Mendes-Junior CT, Souza FF, Marano LA, Deghaide NH, Ferreira SC, et al. The 14bp-deletion allele in the HLA-G gene confers susceptibility to the development of hepatocellular carcinoma in the Brazilian population. Tissue Antigens (2013) 81(6):408–13. 10.1111/tan.12097 [DOI] [PubMed] [Google Scholar]
- 84.Jiang Y, Chen S, Jia S, Zhu Z, Gao X, Dong D, et al. Association of HLA-G 3’ UTR 14-bp insertion/deletion polymorphism with hepatocellular carcinoma susceptibility in a Chinese population. DNA Cell Biol (2011) 30(12):1027–32. 10.1089/dna.2011.1238 [DOI] [PubMed] [Google Scholar]
- 85.Lau DT, Norris MD, Marshall GM, Haber M, Ashton LJ. HLA-G polymorphisms, genetic susceptibility, and clinical outcome in childhood neuroblastoma. Tissue Antigens (2011) 78(6):421–7. 10.1111/j.1399-0039.2011.01781.x [DOI] [PubMed] [Google Scholar]
- 86.Castelli EC, Mendes-Junior CT, Deghaide NH, de Albuquerque RS, Muniz YC, Simoes RT, et al. The genetic structure of 3’untranslated region of the HLA-G gene: polymorphisms and haplotypes. Genes Immun (2010) 11(2):134–41. 10.1038/gene.2009.74 [DOI] [PubMed] [Google Scholar]
- 87.Ghandri N, Gabbouj S, Farhat K, Bouaouina N, Abdelaziz H, Nouri A, et al. Association of HLA-G polymorphisms with nasopharyngeal carcinoma risk and clinical outcome. Hum Immunol (2011) 72(2):150–8. 10.1016/j.humimm.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 88.Castelli EC, Mendes-Junior CT, Viana de Camargo JL, Donadi EA. HLA-G polymorphism and transitional cell carcinoma of the bladder in a Brazilian population. Tissue Antigens (2008) 72(2):149–57. 10.1111/j.1399-0039.2008.01091.x [DOI] [PubMed] [Google Scholar]
- 89.Rolfsen GB, Castelli EC, Donadi EA, Duarte RA, Soares CP. HLA-G polymorphism and breast cancer. Int J Immunogenet (2014) 41(2):143–8 10.1111/iji.12092 [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, Gao XJ, Deng YC, Zhang HX. Relationship between HLA-G gene polymorphism and the susceptibility of esophageal cancer in Kazakh and Han nationality in Xinjiang. Biomarkers (2012) 17(1):9–15. 10.3109/1354750X.2011.633242 [DOI] [PubMed] [Google Scholar]
- 91.de Oliveira Crispim JC, Silva TG, Souto FJ, Souza FF, Bassi CL, Soares CP, et al. Upregulation of soluble and membrane-bound human leukocyte antigen G expression is primarily observed in the milder histopathological stages of chronic hepatitis C virus infection. Hum Immunol (2012) 73(3):258–62. 10.1016/j.humimm.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 92.Amiot L, Vu N, Rauch M, L’Helgoualc’h A, Chalmel F, Gascan H, et al. Expression of HLA-G by mast cells is associated with hepatitis C virus-induced liver fibrosis. J Hepatol (2014) 60(2):245–52. 10.1016/j.jhep.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 93.Weng PJ, Fu YM, Ding SX, Xu DP, Lin A, Yan WH. Elevation of plasma soluble human leukocyte antigen-G in patients with chronic hepatitis C virus infection. Hum Immunol (2011) 72(5):406–11. 10.1016/j.humimm.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 94.Souto FJ, Crispim JC, Ferreira SC, da Silva AS, Bassi CL, Soares CP, et al. Liver HLA-G expression is associated with multiple clinical and histopathological forms of chronic hepatitis B virus infection. J Viral Hepat (2011) 18(2):102–5. 10.1111/j.1365-2893.2010.01286.x [DOI] [PubMed] [Google Scholar]
- 95.Shi WW, Lin A, Xu DP, Bao WG, Zhang JG, Chen SY, et al. Plasma soluble human leukocyte antigen-G expression is a potential clinical biomarker in patients with hepatitis B virus infection. Hum Immunol (2011) 72(11):1068–73. 10.1016/j.humimm.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 96.Han Q, Li N, Zhu Q, Li Z, Zhang G, Chen J, et al. Association of serum soluble human leukocyte antigen-G levels with chronic hepatitis B virus infection. Clin Exp Med (2014) 14(1):35–43. 10.1007/s10238-012-0214-5 [DOI] [PubMed] [Google Scholar]
- 97.Cordero EA, Veit TD, da Silva MA, Jacques SM, Silla LM, Chies JA. HLA-G polymorphism influences the susceptibility to HCV infection in sickle cell disease patients. Tissue Antigens (2009) 74(4):308–13. 10.1111/j.1399-0039.2009.01331.x [DOI] [PubMed] [Google Scholar]
- 98.da Silva GK, Vianna P, Veit TD, Crovella S, Catamo E, Cordero EA, et al. Influence of HLA-G polymorphisms in human immunodeficiency virus infection and hepatitis C virus co-infection in Brazilian and Italian individuals. Infect Genet Evol (2014) 21:418–23. 10.1016/j.meegid.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 99.Kim SK, Chung JH, Jeon JW, Park JJ, Cha JM, Joo KR, et al. Association between HLA-G 14-bp insertion/deletion polymorphism and hepatocellular carcinoma in Korean patients with chronic hepatitis B viral infection. Hepatogastroenterology (2013) 60(124):796–8. 10.5754/hge11180 [DOI] [PubMed] [Google Scholar]
- 100.Sartelet H, Schleiermacher D, Le-Hesran JY, Graesslin O, Gaillard D, Fe M, et al. Less HLA-G expression in Plasmodium falciparum-infected third trimester placentas is associated with more natural killer cells. Placenta (2005) 26(6):505–11. 10.1016/j.placenta.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 101.Sadissou I, d’Almeida T, Cottrell G, Luty A, Krawice-Radanne I, Massougbodji A, et al. High plasma levels of HLA-G are associated with low birth weight and with an increased risk of malaria in infancy. Malar J (2014) 13(1):312. 10.1186/1475-2875-13-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia A, Milet J, Courtin D, Sabbagh A, Massaro JD, Castelli EC, et al. Association of HLA-G 3’UTR polymorphisms with response to malaria infection: a first insight. Infect Genet Evol (2013) 16:263–9. 10.1016/j.meegid.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 103.Sabbagh A, Courtin D, Milet J, Massaro JD, Castelli EC, Migot-Nabias F, et al. Association of HLA-G 3’ untranslated region polymorphisms with antibody response against Plasmodium falciparum antigens: preliminary results. Tissue Antigens (2013) 82(1):53–8. 10.1111/tan.12140 [DOI] [PubMed] [Google Scholar]
- 104.Courtin D, Milet J, Sabbagh A, Massaro JD, Castelli EC, Jamonneau V, et al. HLA-G 3’ UTR-2 haplotype is associated with human African trypanosomiasis susceptibility. Infect Genet Evol (2013) 17:1–7. 10.1016/j.meegid.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 105.Ayo CM, Dalalio MM, Visentainer JE, Reis PG, Sippert EA, Jarduli LR, et al. Genetic susceptibility to Chagas disease: an overview about the infection and about the association between disease and the immune response genes. Biomed Res Int (2013) 2013:284729. 10.1155/2013/284729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marin-Neto JA, Rassi A., Jr Update on Chagas heart disease on the first centennial of its discovery. Rev Esp Cardiol (2009) 62(11):1211–6 10.1016/S1885-5857(09)73346-8 [DOI] [PubMed] [Google Scholar]
- 107.Dias FC, Mendes-Junior CT, da Silva MC, Tristão FSM, Dellalibera-Joviliano R, Moreau P, et al. Human leukocyte antigen-G (HLA-G) and its murine functional homolog Qa2 in the Trypanosoma cruzi infection. Mediators Inflamm (2014) 2014:595289. 10.1155/2014/595829 [DOI] [PMC free article] [PubMed] [Google Scholar]