Abstract
The relationship of nontuberculous mycobacterial (NTM) infections and survival among solid organ transplant (SOT) recipients is unknown. We conducted a retrospective cohort study to measure the impact of NTM infection on survival in this patient population, comparing the effect of M. abscessus infection versus infection due to other pathogenic NTM species. We identified 33 patients with NTM infection post-transplantation, 18 with infection that was diagnosed within the first year. Although drug resistance was common among M. abscessus isolates, patients with M. abscessus infection did not have increased mortality compared to patients with other types of NTM infections (p=0.64). In contrast, we observed a significant association overall between early NTM infection and 3-year mortality post-transplantation (HR 8.76, 95% CI 2.69, 28.57). The mortality burden of NTM infection following transplantation may be due to factors other than the virulence of the organisms. Multicenter studies are needed to identify the optimal approach for diagnosing and treating these uncommon but serious infections.
Keywords: Non-tuberculous mycobacteria, Mycobacterium abscessus, Transplantation, Epidemiology, Drug resistance
Introduction
Immunocompromised patients are at increased risk for developing infection with nontuberculous mycobacteria (NTM), environmental pathogens that are responsible for a diverse group of clinical syndromes and are often associated with a delay in diagnosis [1, 2]. Furthermore, treatment of NTM infections in SOT recipients creates challenges because of drug-drug interactions and overlapping drug toxicities [3], with treatment regimens based on observational data from the non-transplant population [4]. Previous studies of the long-term outcomes of NTM infection among SOT recipients have been limited to lung transplant population [5–7], with conflicting findings regarding the relationship between NTM infection and mortality. The relationship between NTM infection and mortality among SOT recipients overall remains undefined.
We recently reported risk factors for NTM infections among SOT recipients at our institution, demonstrating a 11-fold increase in the odds of NTM infection among lung transplant recipients [8]. Among NTM species, M. abscessus presents additional therapeutic challenges, in part due to intrinsic antimicrobial resistance properties [4]. Our current objective was to evaluate the relationship of NTM species and mortality among SOT recipients diagnosed with NTM infection, across organ transplantation types. We hypothesized that NTM infection due to M. abscessus would lead to decreased survival when compared with infections due to all other pathogenic NTM species. As a secondary objective, we sought to measure the relationship between NTM infection and survival among all types of organ transplant recipients, by comparing the survival of SOT recipients with and without NTM infection diagnosed in the post-transplantation period.
Methods
Subjects
We performed a retrospective cohort study of SOT recipients at the Hospital of the University of Pennsylvania. For the primary exposure, we identified all SOT recipients with NTM infection by cross-referencing records from the organ transplant database with the clinical microbiology laboratory records for the period between January 1, 2002 and July 1, 2009. We included all SOT recipients who either 1) met ATS/IDSA criteria for NTM disease during the study period (for pleuropulmonary isolates) or 2) had an NTM organism cultured from a sterile site in association with a clinical syndrome (for all other isolates) [4]. SOT recipients with evidence of NTM disease and/or colonization prior to transplantation were excluded from this study in order to focus on incident NTM disease post-transplantation.
Data collection
Patient information, including demographics and co-morbidities, was collected from the Pennsylvania Integrated Clinical and Administrative Research Database, along with review of consultation records from the Transplant Infectious Disease service. The date of diagnosis of infection was defined as the date of the first positive culture in the context of a clinical syndrome that satisfied our diagnostic criteria.
All mycobacteria were identified by molecular methods, with variations in the methods over the period of the study. Molecular probes (GenProbe, San Diego, CA) were used to identify M. kansasii and M. avium complex (MAC) throughout the study period. For all other mycobacteria, identification was by 500 base-pair 16S rDNA sequence analysis (MicroSeq, Applied Biosystems, Carlsbad, CA). Since 500bp 16S rDNA sequencing can not distinguish between M. chelonae and M. abscessus, biochemical testing and antimicrobial susceptibility phenotype was used to distinguish between these two species for most of the study period. A molecular assay to distinguish between M. chelonae and M. abscessus was used between 2006 to 2009, in addition to phenotyping [9].
Analysis
For the primary analysis, we constructed Kaplan-Meier survival curves to compare the survival of patients with NTM infection caused by M. abscessus, and patients with NTM infection caused by all other organisms. We chose to right-censor after 3 years of follow-up time because of the typical time period required to complete a treatment course for NTM infection. For all infections, we characterized the number of effective drugs that were used at the time of therapy initiation, based on susceptibility results that were later obtained.
As a secondary objective, we determined the overall relationship between NTM infection that was diagnosed in the first year post-transplantation and 3-year survival. For a comparison group, we selected SOT recipients without NTM infection from the transplant registry, matched 3:1 by date of transplantation for each patient with early NTM infection. We chose this approach in order to account for changes in clinical practices in the management of solid organ transplant recipients that occurred during the study period. We constructed Kaplan-Meier survival curves to describe the relationship between early NTM infection and 3-year mortality post-transplantation, and performed Cox regression modeling to determine the association between early NTM infection and survival, adjusting for type of transplantation (lung versus non-lung). We selected additional variables for the adjusted analysis based on their confounding effect on the relationship of early NTM infection and survival, which we defined as a change of 15% or greater in the hazard ratio when included in the model [10].
Results
Survival of SOT recipients with M. abscessus infection versus infection due to other NTM species
We identified 33 SOT recipients with NTM infection that met ATS/IDSA criteria post-transplantation, including M. abscessus (14), M. avium (12), M. fortuitum (2), M. kansasii (2), M. marinum (2), and M. chelonae (1). Sites of infection included pleuropulmonary (21), disseminated (5), abdominal (3), cutaneous (2), deep wound (1) and bone (1). The median time between transplantation and diagnosis of NTM infection was 9.2 months (range 4 days to 12.8 years).
We observed a bimodal distribution in the time between transplantation and the development of NTM infection. Eighteen of 33 patients were diagnosed with NTM infection within the first year post-transplantation, after a median of 2.2 months (IQR 1.5 to 4.8 months). In contrast, the median time to diagnosis of NTM infection among 15 patients that developed infection after the first year post-transplantation was 7.5 years (IQR 4.7 to 9.4 years).
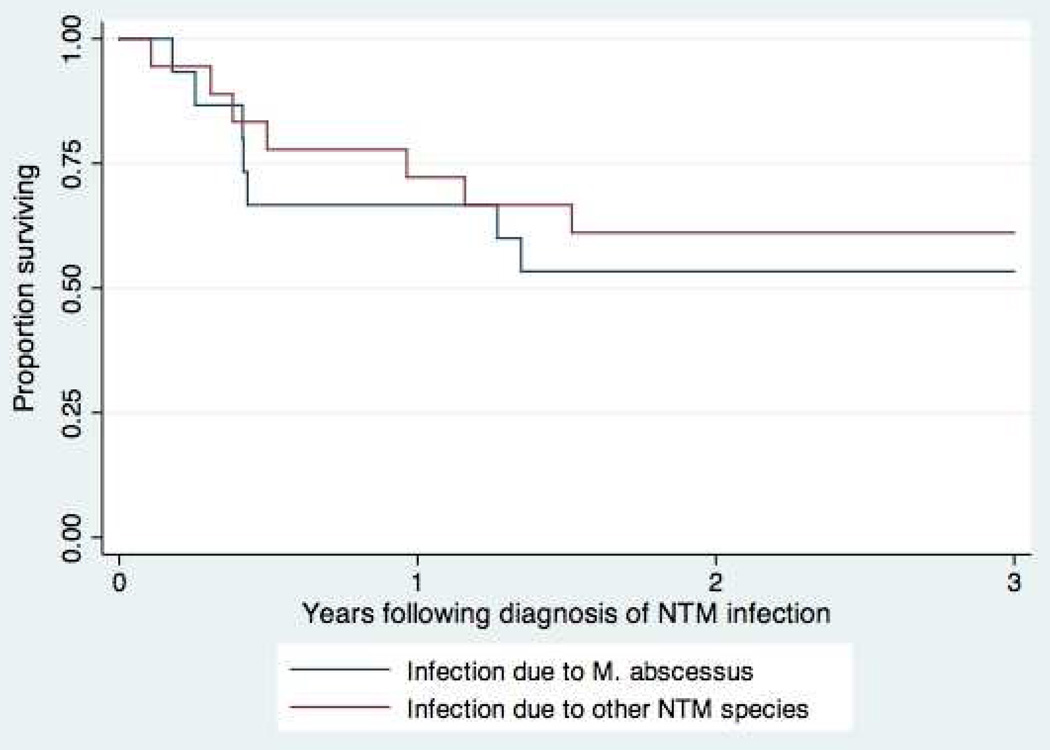
There was no significant difference in mortality during the 3-year period following the diagnosis of infection among SOT receipients with M. abscessus infection versus all other types of NTM infections, as shown in Figure 1 (p = 0.64 by log-rank test).
Figure 1.
Survival following diagnosis of NTM infection, M. abscessus vs other NTM species
Multidrug resistance was commonly observed among 13 M. abscessus isolates with susceptibility results available (Table 1). Although none of the isolates were characterized as susceptible to either imipenem or cefoxitin, 5 of 10 isolates demonstrated intermediate susceptibility to imipenem, and all 13 isolates demonstrated intermediate suscepibility to cefoxitin. Only clarithromycin and amikacin displayed consistent in vitro activity against M. abscessus isolates from SOT recipients.
Table 1.
In vitro susceptibilities of initial M. abscessus isolates from SOT recipients (n=13)
| Drug (# tested) | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Clarithromycin (13) | 12 | 0 | 1 |
| Ciprofloxacin (13) | 0 | 0 | 13 |
| Amikacin (13) | 13 | 0 | 0 |
| Cefoxitin (13) | 0 | 13 | 0 |
| Imipenem (10) | 0 | 5 | 5 |
| Linezolid (13) | 2 | 6 | 5 |
There were 16 of 33 patients with 2 active drugs initiated at diagnosis, and 15 of 33 patients with 3 or more active drugs initiated at diagnosis. One patient with M. marinum infection was treated with minocycline monotherapy, and a second patient with M. fortuitum infection was treated with azithromycin and ethambutol, but had macrolide resistant infection demonstrated on susceptibility testing.
Secondary analysis of survival of SOT recipients with and without NTM infection
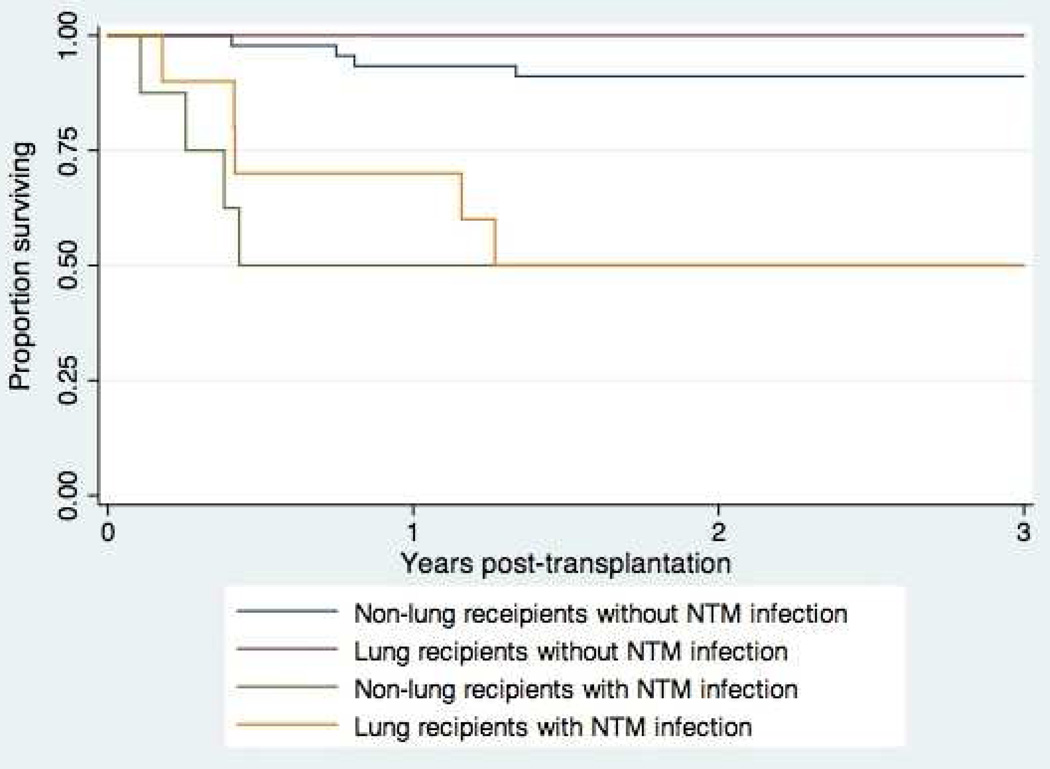
In order to characterize the overall effect of early NTM infection on survival post-transplantation, we performed a secondary survival analysis limited to 18 SOT recipients with infection during the first year post-transplantation, and selected a comparison group of SOT recipients without NTM infection by matching in a 3:1 ratio by date of transplantation (selected from the organ transplant recipient database). Kaplan-Meier survival curves for the 3-year period post-transplantation are shown in Figure 2. At 3 years post transplantation, 9 of 18 (50%) of SOT recipients with early NTM infection had died, compared with 7 of 54 (13%) SOT recipients without NTM infection. Among 8 of 9 patients with death certificates available for review, NTM infection was listed as a contributing factor in 3 patients, infections due to other organisms were listed in 4 patients, and rejection was listed in 2 patients.
Figure 2.
Survival post-transplantation of 18 SOT recipients with early NTM infection, compared with 54 SOT recipients without NTM infection (matched 3:1 by date of transplantation)
In a Cox regression model, after adjusting for type of transplantation (lung versus non-lung), early NTM infection remained significantly associated with decreased survival during the 3-year period post-transplantation (Table 4). Surprisingly, lung transplantation was not associated with 3-year mortality in either unadjusted or adjusted analyses.
Discussion
Contrary to our hypothesis, we did not observe an increased mortality among SOT recipients with M. abscessus infection, compared to SOT recipients with other types of NTM infection. This finding was surprising given the high level of antimicrobial resistance that was observed among M. abscessus isolates, with only 2 of 13 isolates displaying in vitro susceptibility to at least 3 drug classes. The similar survival that we observed between these groups may be related to the proportion of non-M. abscessus infections that presented with deep or disseminated infection in this cohort (8), or a reflection of the competing risks for mortality that may occur among SOT recipients following the initial diagnosis of NTM infection [11].
We observed a clear bimodal distribution in the incidence of NTM infection post-transplantation. Development of NTM infection during the first year post-transplantation was strongly associated with decreased survival, which was independent of organ type. Similar to CMV infection, the mechanism for the impact of early NTM infection on survival may be multifactorial, including indirect effects exerted through immune modulation [12], transplant rejection [13], or development of secondary nosocomial infections [14], as suggested by our review of death certificates where available.
Our study had several limitations. As a single center investigation, generalization of the observed relationships to other transplant centers with differences in practices may be limited. In addition, the ATS/IDSA criteria for pleuropulmonary infections have not been validated in the transplant population, and it is possible in some instances the isolation of NTM organisms may have represented colonization rather than infection. In addition, changes in diagnostic approaches evolved over time, as described in Methods, above. Death was ascertained from the clinical records, which may be subject to information bias if the patients that developed NTM infection were more likely to be followed within the health system, as compared with patients that had not developed NTM infection. By limiting the survival analysis to a 3-year time horizon, we attempted to capture the period when patients are likely to receive their follow-up care at the institution where transplantation was performed. Strengths of the study included the use of microbiology records to identify patients with NTM infection and the broad range of transplant procedures performed at our institution.
In summary, we did not observe a difference in survival between M. abscessus and non-M. abscessus infections among SOT recipients. However, NTM infection in the first year post-transplantation negatively influenced 3-year survival as compared to SOT recipients without NTM infection. Prospective, multicenter studies are needed to provide an evidence base in support of the optimal approach for identifying and treating NTM infections in this complex patient population.
Table 2.
Association of early NTM infection with 3-year mortality post- transplantation
| Characteristic | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
|---|---|---|---|---|
| Early NTM infection | 8.76 (2.69, 28.57) | <0.001 | 11.25 (2.74, 51.17) | 0.002 |
| Lung transplantation | 2.21 (0.72, 6.77) | 0.16 | 0.46 (0.11, 1.85) | 0.28 |
| Male sex | 0.36 (0.12, 1.08) | 0.07 | ||
| Age categories | ||||
| <45 | Reference | |||
| 45–55 | 1.48 (0.25, 8.85) | 0.67 | ||
| 55–65 | 1.65 (0.34, 7.94) | 0.53 | ||
| >65 | 0.85 (0.08, 9.35) | 0.89 | ||
| Malnutrition | 6.43 (2.08, 19.86) | 0.001 | 2.46 (0.71, 8.59) | 0.16 |
| Diabetes mellitus | 1.37 (0.46, 4.07) | 0.58 | ||
| CMV Disease | 4.74 (1.45, 15.53) | 0.01 | 7.41 (1.77, 30.96) | 0.006 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in abstract form at the 2013 Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC)
References
- 1.De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006;42:1756–1763. doi: 10.1086/504381. [DOI] [PubMed] [Google Scholar]
- 2.Appelgrean P, Farnebo F, Dotevall L, et al. Late-onset posttraumatic skin and soft-tissue infections caused by rapid-growing mycobacteria in tsunami survivors. Clin Infect Dis. 2008;47:e11–e16. doi: 10.1086/589300. [DOI] [PubMed] [Google Scholar]
- 3.Philley JV, Griffith DE. Management of nontuberculous mycobacterial (NTM) lung disease. Semin Respir Crit Care Med. 2013;34:135–142. doi: 10.1055/s-0033-1333575. [DOI] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Knoll BM, Kappagoda S, Gill RR, et al. Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study. Transpl Infect Dis. 2012;14:452–460. doi: 10.1111/j.1399-3062.2012.00753.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang HC, Weigt SS, Derhovanessian A, et al. Non-tuberculous mycobacterium infection after lung transplantation is associated with increased mortality. J Heart Lung Transplant. 2011;30:790–798. doi: 10.1016/j.healun.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalermskulrat W, Sood N, Neuringer IP, et al. Non-tuberculous mycobacteria in end stage cystic fibrosis: implications for transplantation. Thorax. 2006;61:507–513. doi: 10.1136/thx.2005.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longworth SA, Vinnard C, Lee I, et al. Risk factors for nontuberculous mycobacterial Infections in solid organ transplant recipients: a case-control study. Transpl Infect Dis. doi: 10.1111/tid.12170. In press. [DOI] [PubMed] [Google Scholar]
- 9.Cloud JL, Hoggan K, Belousov E, et al. Use of the MGB Eclipse system and SmartCycler PCR for differentiation of Mycobacterium chelonae and M. abscessus. J Clin Microbiol. 2005;43(8):4205–4207. doi: 10.1128/JCM.43.8.4205-4207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 11.Baddley JW, Andes DR, Marr KA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis. 2010;15:1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah AM, Savage ND, van Zon M, et al. The ESX-5 secretion system of Mycobacterium marinum modulates the macrophage response. J Immunol. 2008;181:7166–7175. doi: 10.4049/jimmunol.181.10.7166. [DOI] [PubMed] [Google Scholar]
- 13.Marques ID, Azevedo LS, Pierrotti LC, et al. Clinical features and outcomes of tuberculosis in kidney transplant recipients in Brazil: a report of the last decade. Clin Transplant. 2013;27:E169–E176. doi: 10.1111/ctr.12077. [DOI] [PubMed] [Google Scholar]
- 14.Linares L, Cervera C, Hoyo I, et al. Klebsiella pneumoniae infection in solid organ transplant recipients: epidemiology and antibiotic resistance. Transplant Proc. 2010;42:2941–2943. doi: 10.1016/j.transproceed.2010.07.080. [DOI] [PubMed] [Google Scholar]