Abstract
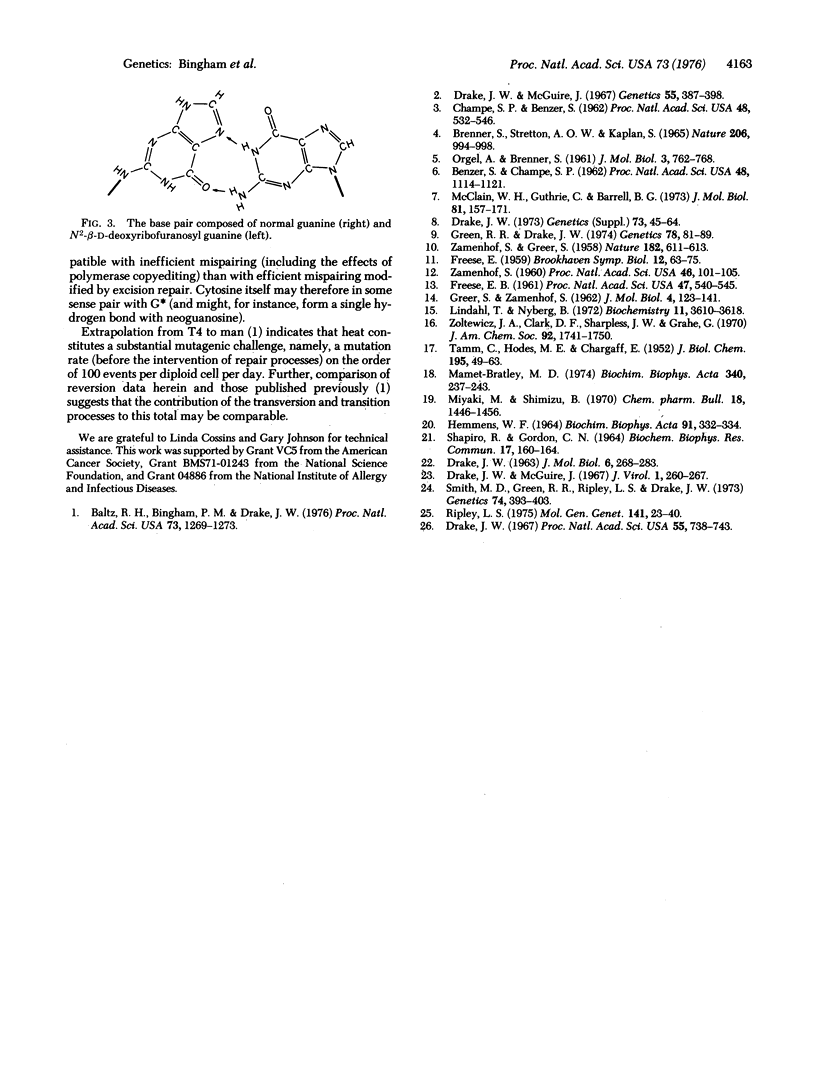
Heat induces transversions (as well as transitions) at G-C base pairs in bacteriophage T4. The target base for transversions is guanine,which is converted to a product which is sometimes replicated and transcribed as a pyrimidine.A model for this process is proposed in which the deoxyguanosine glycosidic bond migrates from N9 to N2: the resulting deoxyneoguanosine may pair with normal guanine to produce G-C leads to C-G transversions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
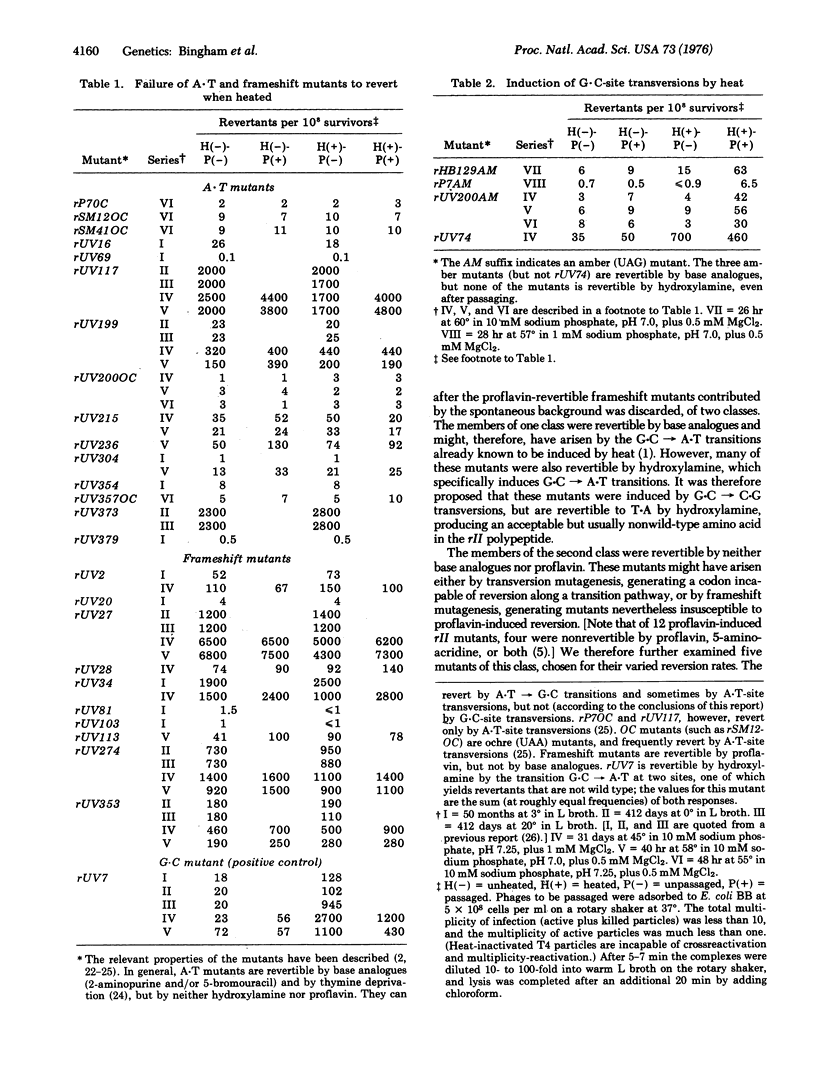
- BENZER S., CHAMPE S. P. A change from nonsense to sense in the genetic code. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1114–1121. doi: 10.1073/pnas.48.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H., Bingham P. M., Drake J. W. Heat mutagenesis in bacteriophage T4: the transition pathway. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1269–1273. doi: 10.1073/pnas.73.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Stretton A. O., Kaplan S. Genetic code: the 'nonsense' triplets for chain termination and their suppression. Nature. 1965 Jun 5;206(988):994–998. doi: 10.1038/206994a0. [DOI] [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. Reversal of mutant phenotypes by 5-fluorouracil: an approach to nucleotide sequences in messenger-RNA. Proc Natl Acad Sci U S A. 1962 Apr 15;48:532–546. doi: 10.1073/pnas.48.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
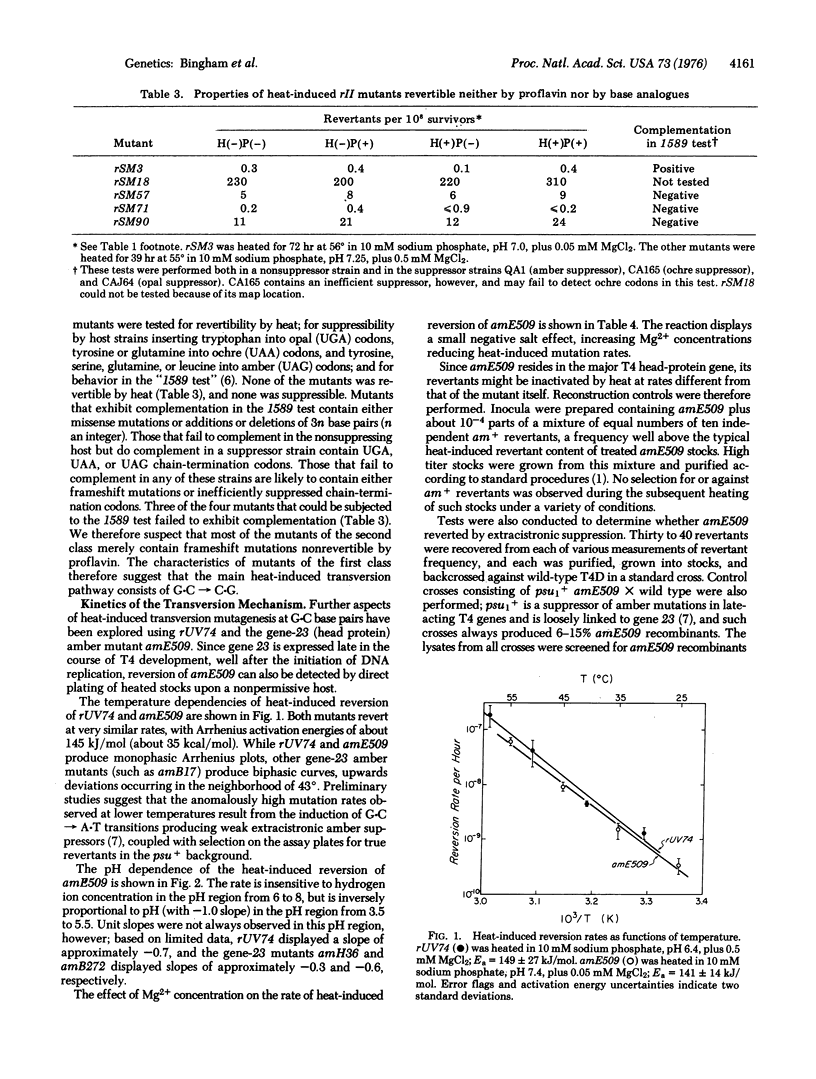
- Drake J. W., McGuire J. Characteristics of mutations appearing spontaneously in extracellular particles of bacteriophage T4. Genetics. 1967 Mar;55(3):387–398. doi: 10.1093/genetics/55.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Properties of r mutants of bacteriophage T4 photodynamically induced in the presence of thiopyronin and psoralen. J Virol. 1967 Apr;1(2):260–267. doi: 10.1128/jvi.1.2.260-267.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Spontaneous mutations accumulating in bacteriophage T4 in the complete absence of DNA replication. Proc Natl Acad Sci U S A. 1966 Apr;55(4):738–743. doi: 10.1073/pnas.55.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. The genetic control of spontaneous and induced mutation rates in bacteriophage T4. Genetics. 1973 Apr;73(Suppl):45–64. [PubMed] [Google Scholar]
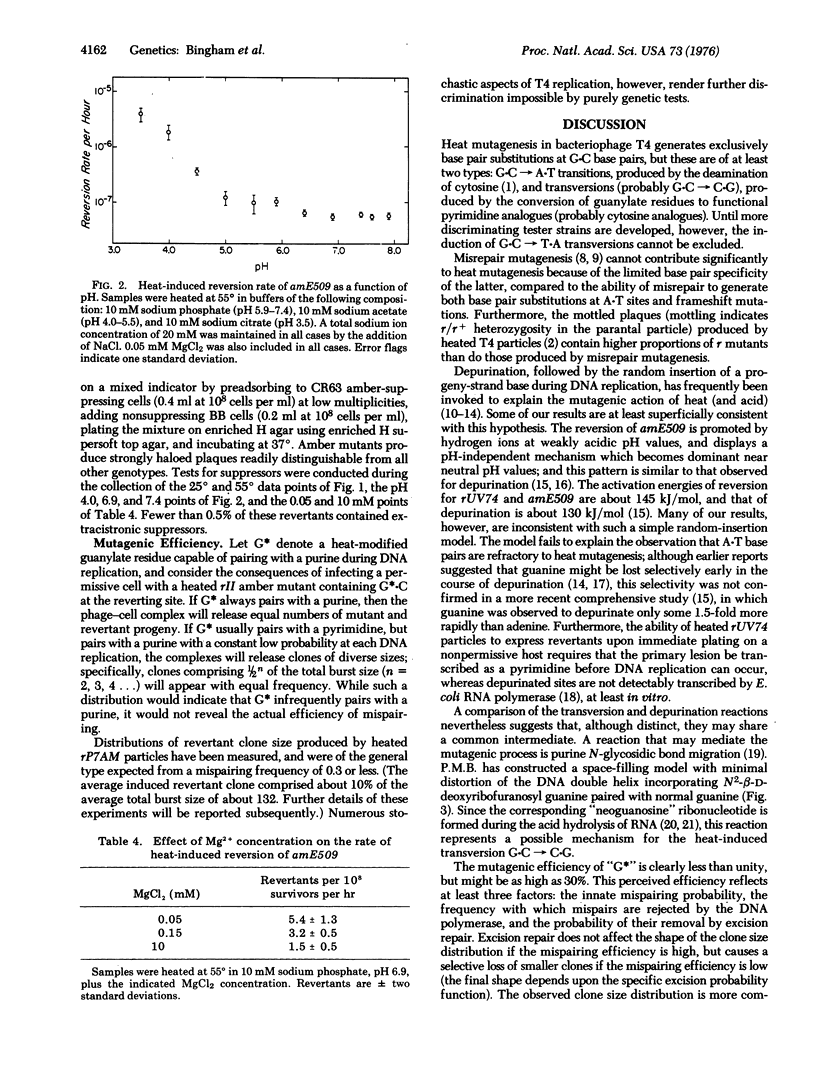
- FREESE E. B. Transitions and transversions induced by depurinating agents. Proc Natl Acad Sci U S A. 1961 Apr 15;47:540–545. doi: 10.1073/pnas.47.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E. On the molecular explanation of spontaneous and induced mutations. Brookhaven Symp Biol. 1959 Nov;NO:63–75. [PubMed] [Google Scholar]
- GREER S., ZAMENHOF S. Studies on depurination of DNA by heat. J Mol Biol. 1962 Mar;4:123–141. doi: 10.1016/s0022-2836(62)80046-1. [DOI] [PubMed] [Google Scholar]
- Green R. R., Drake J. W. Misrepair mutagenesis in bacteriophage T4. Genetics. 1974 Sep;78(1):81–89. doi: 10.1093/genetics/78.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMMENS W. F. NEO-GUANYLIC ACID PRODUCED BY THE ACTION OF ACID ON RIBONUCLEIC ACID. Biochim Biophys Acta. 1964 Oct 16;91:332–334. doi: 10.1016/0926-6550(64)90260-9. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Mamet-Bratley M. D. Transcription in vitro from a DNA template containing apurinic sites. Biochim Biophys Acta. 1974 Mar 27;340(3):237–243. doi: 10.1016/0005-2787(74)90269-x. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Guthrie C., Barrell B. G. The psu1+ amber suppressor gene of bacteriophage T4: identification of its amino acid and transfer RNA. J Mol Biol. 1973 Dec 5;81(2):157–171. doi: 10.1016/0022-2836(73)90186-1. [DOI] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- Ripley L. S. Transversion mutagenesis in bacteriophage T4. Mol Gen Genet. 1975 Nov 3;141(1):23–40. doi: 10.1007/BF00332376. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Green R. R., Ripley L. S., Drake J. W. Thymineless mutagenesis in bacteriophage T4. Genetics. 1973 Jul;74(3):393–403. doi: 10.1093/genetics/74.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM C., HODES M. E., CHARGAFF E. The formation apurinic acid from the desoxyribonucleic acid of calf thymus. J Biol Chem. 1952 Mar;195(1):49–63. [PubMed] [Google Scholar]
- ZAMENHOF S., GREER S. Heat as an agent producing high frequency of mutations and unstable genes in Escherichia coli. Nature. 1958 Aug 30;182(4635):611–613. doi: 10.1038/182611a0. [DOI] [PubMed] [Google Scholar]
- Zamenhof S. EFFECTS OF HEATING DRY BACTERIA AND SPORES ON THEIR PHENOTYPE AND GENOTYPE. Proc Natl Acad Sci U S A. 1960 Jan;46(1):101–105. doi: 10.1073/pnas.46.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltewicz J. A., Clark D. F., Sharpless T. W., Grahe G. Kinetics and mechanism of the acid-catalyzed hydrolysis of some purine nucleosides. J Am Chem Soc. 1970 Mar 25;92(6):1741–1749. doi: 10.1021/ja00709a055. [DOI] [PubMed] [Google Scholar]



