Abstract
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related mortality worldwide. In the last few decades, there has been a marked increase in therapeutic options for HCC and epidemiological characteristics at HCC diagnosis have also significantly changed. With these changes and advances in medical technology and surveillance program for detecting earlier stage HCC, survival in patients with HCC has significantly improved. Especially, patients with liver cirrhosis are at high risk of HCC development, and regular surveillance could enable early detection of HCC and curative therapy, with potentially improved clinical outcome. However, unfortunately, only 20% of HCC patients are amenable to curative therapy (liver transplantation, surgical resection or ablative therapies). Locoregional therapies such as radiofrequency ablation, percutaneous ethanol injection, microwave coagulation therapy and transcatheter arterial chemoembolization play a key role in the management of unresectable HCC. Currently, molecular-targeted agents such as sorafenib have emerged as a promising therapy for advanced HCC. The choice of the treatment modality depends on the size of the tumor, tumor location, anatomical considerations, number of tumors present and liver function. Furthermore, new promising therapies such as gene therapy and immunotherapy for HCC have emerged. Approaches to the HCC diagnosis and adequate management for patients with HCC are improving survival. Herein, we review changes of epidemiological characteristics, prognosis and therapies for HCC and refer to current knowledge for this malignancy based on our experience of approximately 4000 HCC cases over the last three decades.
Keywords: hepatocellular carcinoma, progress, surveillance, three decades, treatment
Introduction
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related mortality worldwide in terms of incidence with 626 000 new cases per year, accounting for 5.7% of all new cancer cases.1–6 HCC represents more than 90% of primary liver cancer.1–6 Annual incidence rates of HCC are highest in sub-Saharan Africa and East Asia, where approximately 85% of all cases occur.1,6 This malignancy tends to occur in livers damaged through chronic infection with hepatitis B and C or alcohol abuse on a background of cirrhosis.
The therapies of HCC have markedly changed in the last few decades.1–6 Furthermore, in our country, epidemiological characteristics such as age, disease stage at HCC diagnosis and causes of background liver disease for HCC have also significantly changed in the last few decades. With these changes and advances in medical technology such as diagnostic imaging and surveillance programs for detecting earlier stage HCC, survival in patients with HCC has significantly improved. Especially, patients with liver cirrhosis (LC) are at high risk of HCC development. The initiation of surveillance for HCC involves identifying high-risk populations for HCC development that would benefit from cancer screening.7 Regular surveillance for these high-risk populations could enable early detection of HCC and curative therapy, with potentially improved clinical outcome.7 However, unfortunately, only 20% of HCC patients are amenable to curative therapy (liver transplantation [LT], surgical resection [SR] or ablative therapies). HCC often recurs even after curative therapy and survival in HCC patients with advanced stage remains poor.1,2,4,6 Locoregional therapies such as radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), microwave coagulation therapy and transcatheter arterial chemoembolization (TACE) play a key role in the management of unresectable HCC. These non-surgical treatments for HCC have also significantly improved in the last few decades and have shown survival benefits for selected patients with HCC.2,4,8–11 Currently, molecular-targeted agents such as sorafenib have emerged as promising therapies for advanced HCC.5 The choice of the treatment modality depends on the size of the tumor, tumor location, anatomical considerations, number of tumors present and liver function.2,4–11 Furthermore, new promising therapies such as gene therapy and immunotherapy for HCC have emerged.12,13 Approaches to the HCC diagnosis and adequate management for patients with HCC are improving survival.
In this article, we review changes of epidemiological characteristics, prognosis and therapies for HCC and refer to current knowledge for this cancer based on our experience of approximately 4000 HCC cases over the last three decades. Because our experience included vast number of HCC cases, our data will well reflect actual situations of HCC therapy in Japan.
Current Trends in HCC Patients
Baseline characteristics in 4165 patients diagnosed with HCC between 1981 and 2013 in our hospital are shown in Table 1. Current annual trends of age and sex in HCC patients are shown in Figure 1. For over the last three decades, the average age in patients diagnosed with HCC has risen from approximately 60 years to 70 years and the proportion of female HCC patients has been slightly increasing. An aging society means that the number of elderly patients with cancer is predicted to rise in the future.14,15 HCC patients are not an exception. In Japan, 75-year-old men and women have an average expected lifespan of approximately 5 and 10 years, respectively, and Japan has the greatest longevity in the world.16 The increased longevity of the population means that more aged HCC patients are to be expected in the coming years. The proportion of elderly patients with HCC and their average age are increasing in Japan.15,17,18 The age at HCC diagnosis has increased in parallel with the increased proportion of elderly patients infected with HCV.19,20 These trends have thus led to a rising demand in our country for investigations related to clinical characteristics and outcomes of therapy in elderly patients with HCC.
Table 1.
Baseline characteristics of 4165 patients diagnosed with hepatocellular carcinoma (HCC) between 1981 and 2013 in our hospital
| n (%) or mean ± SD | |
|---|---|
| Male/female | 2954 (70.9%)/1211 (29.1%) |
| Age, mean ± SD (range) (years) | 66.2 ± 9.5 (17–95) |
| Child–Pugh classification | |
| Child–Pugh A/B/C | 2571 (65.4%)/1095 (27.9%)/265 (6.7%) |
| Cause of liver disease | |
| B/C/B and C/non-B, non-C | 460 (12.0%)/2734 (71.4%)/83 (2.2%)/551 (14.4%) |
| Background liver | |
| LC/CH/fatty liver/normal liver | 3073 (75.5%)/860 (21.1%)/11 (0.3%)/125 (3.1%) |
| HCC stage | |
| Stage I/II/III/IVA/IVB | 722 (18.1%)/1467 (36.8%)/1175 (29.5%)/501 (12.6%)/121 (3.0%) |
CH, chronic hepatitis; HCC, hepatocellular carcinoma; LC, liver cirrhosis; SD, standard deviation.
Figure 1.
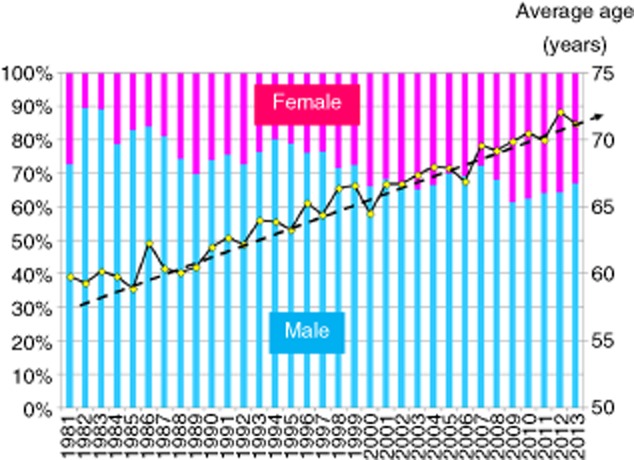
Annual trends in patients with hepatocellular carcinoma (1981–2013, age and sex, Osaka Red Cross Hospital, Japan).
Next, current annual trends of causes of liver disease are shown in Figure 2. In our hospital, the proportion of non-B, non-C (NBNC) HCC patients has been gradually increasing, while the proportion of HCV-related HCC has been gradually decreasing. It is noteworthy that the proportion of NBNC HCC patients was approximately 30% in 2011, 2012 and 2013 in our hospital. Although most HCC is related to viral infection, there is a substantial population of NBNC HCC patients in Japan and the incidence of NBNC HCC has recently tended to increase.3,21–25 The background liver diseases of NBNC HCC vary considerably and they include non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), alcoholic liver disease, autoimmune liver diseases such as autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis, congestive liver diseases such as Budd–Chiari syndrome, congenital metabolic liver diseases such as hereditary hemochromatosis and Wilson's disease, occult B infection and aflatoxins as well as liver diseases of unknown etiology.3 In particular, it is of note that increasing clinical evidences support the fact that NAFLD and NASH can progress to LC and HCC.26,27 NAFLD or NASH may directly promote liver carcinogenesis independent of the presence of LC.3,26 Tokushige et al. conducted a nationwide survey of 14 530 Japanese HCC patients and demonstrated that alcohol-related HCC accounted for 7.2% of all HCC, followed by unknown HCC (5.1%) and NAFLD-related HCC (2.0%) and the characteristics of these three groups were clearly different (median age, 72 years for NAFLD-related HCC, 68 years for alcohol-related HCC and 73 years for unknown HCC, P < 0.01; female sex, 38%, 4% and 37%, respectively, P < 0.01) and obesity and lifestyle-related diseases were significantly more frequent in NAFLD-related HCC than in alcohol-related HCC and unknown HCC.21 On the other hand, one possible reason that the number of HCV-related HCC cases has been recently decreasing is that the rate of HCV eradication has markedly improved due to the progress of treatment for patients with HCV, although antiviral therapies for hepatitis C can prevent but not completely eliminate HCC.28–30 The estimated risk of HCC is reported to be 15–20-times as high among individuals with HCV as it is among those who are not infected with HCV.31 In addition, although the number of patients with HCC-related death has steadily increased over the past 50 years, the incidence of HCC has recently started to decrease in Japan, mainly due to the decrease in rates of HCV-related HCC.19
Figure 2.
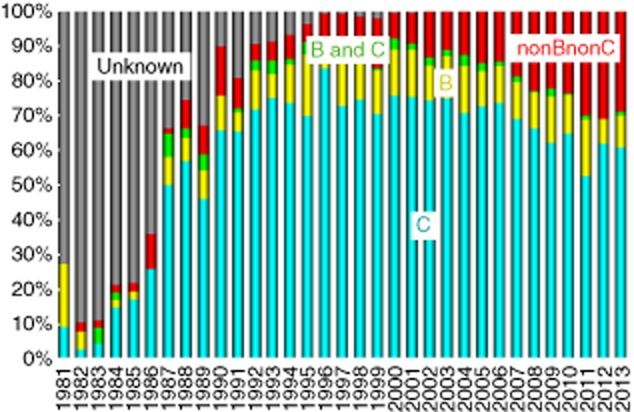
Annual trends in patients with hepatocellular carcinoma (1981–2013, causes of liver disease [virus], Osaka Red Cross Hospital, Japan).
Next, current annual trends of proportions of patients with HCC stage I, II, III, IVA and IVB at initial HCC diagnosis in our hospital are shown in Figure 3. The proportion of early stage HCC patients (HCC stage I or II) in the 1980s was 34.1%. However, that in the 1990s increased to 50.6% and that after 2000 increased to over 60%. These improvements may be due to adequate selection of patients at high risk of HCC occurrence and progress in diagnostic imaging technique of HCC such as introductions of contrast-enhanced ultrasonography, multidetector computed tomography and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) magnetic resonance imaging (MRI).32 In particular, Gd-EOB-DTPA MRI including the hepatobiliary phase had the highest accuracy with sensitivities for detecting early stage HCC.32 Success of a surveillance program depends on both target population and surveillance modality.7
Figure 3.
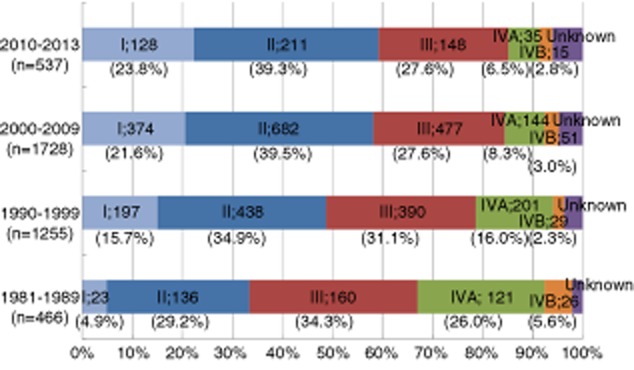
Hepatocellular carcinoma stage at diagnosis (1981–2013, Osaka Red Cross Hospital, Japan).
Cumulative Overall Survival for All HCC Cases for the Last Three Decades and Cumulative Overall Survival According to Child-Pugh Stage, HCC Stage and Causes of Liver Disease
The cumulative overall survival (OS) curve for all HCC cases from 1981 to 2013 (n = 4165) in our hospital is demonstrated in Figure 4. The 1-, 3-, 5-, 8-, 10- and 15-year survival rates were 79.8%, 55.4%, 37.5%, 21.8%, 14.8% and 6.1%, respectively. One report from the USA showed that the 5-year survival rate in HCC patients in the United States has remained below 12%.6 As compared with their results of survival, our results are reasonably good, although the reasons for these discrepancies are unclear.
Figure 4.
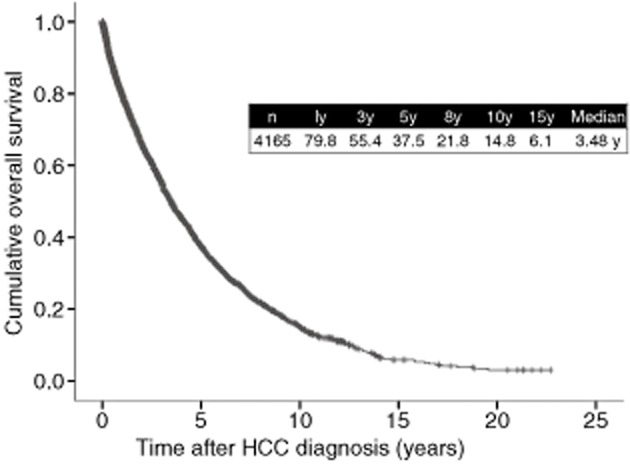
Cumulative overall survival for hepatocellular carcinoma (HCC) patients (4165 cases, Osaka Red Cross Hospital, Japan). y, years.
The main predictors affecting survival in HCC patients are liver function and tumor burden.1–6 Herein, we present the cumulative OS rates according to Child–Pugh stage and HCC stage in Figures 5 and 6. As for Child–Pugh stage, 1-, 3- and 5-year survival rates are 86.3%, 64.6% and 44.8%, respectively, in patients with Child–Pugh A (n = 2571), 71.7%, 40.5% and 22.8%, respectively, in patients with Child–Pugh B (n = 1095), and 44.0%, 17.1% and 8.3%, respectively, in patients with Child–Pugh C (n = 265) (overall significance, P < 0.001), suggesting that Child–Pugh stage is closely associated with OS in HCC patients (Fig. 5). These results also indicate that maintaining liver functional reserve is essential for prolonging OS in HCC patients. As shown in our results, prognosis in HCC patients with Child–Pugh C cirrhosis is extremely poor.33 Thus, in these patients, most of the current HCC practice guidelines recommend LT for patients within the Milan criteria and best supportive care for patients outside the Milan criteria.4,6 However, in Japan, due to the limited number of brain death donors and advanced age in HCC patients, the Japan Society of Hepatology recommends non-transplant therapies such as transcatheter arterial chemotherapy and ablative therapies even in HCC patients with Child–Pugh C cirrhosis.34 However, whether HCC patients with Child–Pugh C cirrhosis treated with non-transplant therapies could obtain survival benefit remains unclear.33 To confirm these results, well-characterized studies will be needed in the future.
Figure 5.
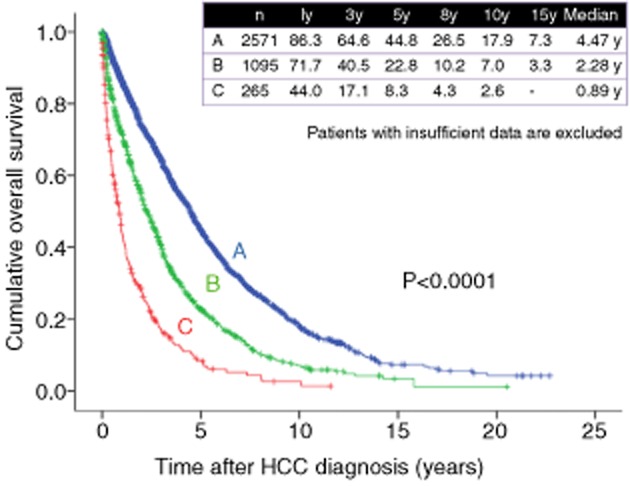
Cumulative overall survival according to Child–Pugh stage (Osaka Red Cross Hospital, Japan). HCC, hepatocellular carcinoma; y, years.
Figure 6.
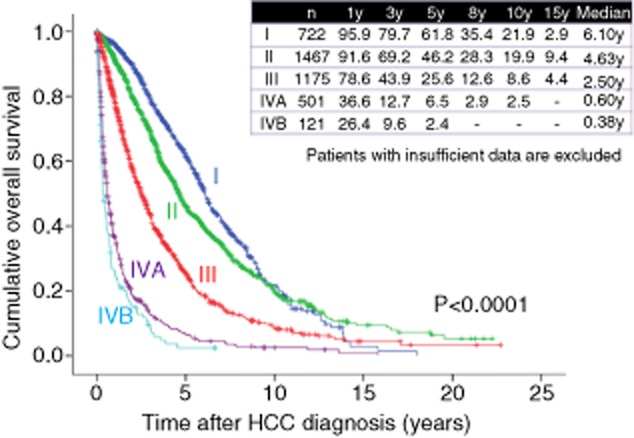
Cumulative overall survival according to hepatocellular carcinoma (HCC) stage (Osaka Red Cross Hospital, Japan). y, years.
On the other hand, as for HCC stage, 1-, 3- and 5-year survival rates are 95.9%, 79.7% and 61.8%, respectively, in patients with HCC stage I (n = 722), 91.6%, 69.2% and 46.2%, respectively, in patients with HCC stage II (n = 1467), 78.6%, 43.9% and 25.6%, respectively, in patients with HCC stage III (n = 1175), 36.6%, 12.7% and 6.5%, respectively, in patients with HCC stage IVA (n = 501), and 26.4%, 9.6% and 2.4%, respectively, in patients with HCC stage IVB (n = 121) (overall significance, P < 0.001), demonstrating that HCC stage is also closely associated with OS in HCC patients (Fig. 6). A disease staging system is particularly essential for the management of HCC as it helps to predict prognosis. As shown in Figure 6, patients with advanced stage of HCC have extremely poor prognosis. In this regard, a more adequate surveillance program for early detection of HCC development will be necessary.35
As for causes of liver disease, 1-, 3- and 5-year survival rates are 74.3%, 50.7% and 39.1%, respectively, in patients with hepatitis B virus (HBV)-related HCC (n = 460), 85.1%, 61.0% and 41.1%, respectively, in patients with HCV-related HCC (n = 2734), 78.9%, 56.3% and 38.6%, respectively, in patients with NBNC HCC (n = 551) and 72.2%, 52.3% and 35.5%, respectively, in patients with HBV and HCV-related HCC (n = 83) (overall significance, P = 0.620), demonstrating that prognosis for HCC patients is not affected by causes of liver disease (Fig. 7).
Figure 7.
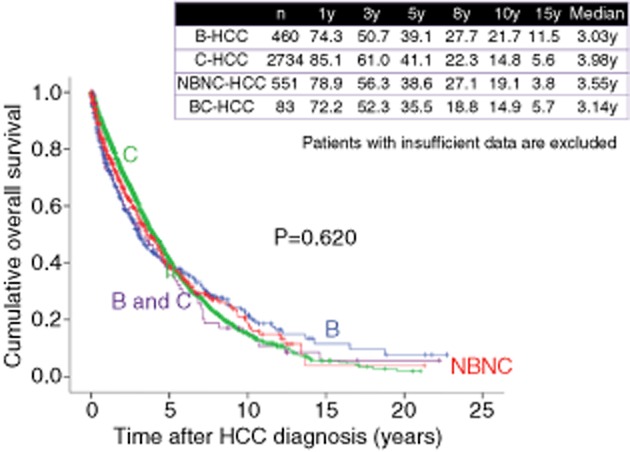
Cumulative overall survival according to causes of liver disease (Osaka Red Cross Hospital, Japan). HCC, hepatocellular carcinoma; NBNC, non-B, non-C; y, years.
Cumulative Overall Survival in the 1980s, 1990s and 2000s
As shown in Figure 8, 1-, 3-, 5-, 8- and 10-year survival rates were 55.7%, 24.7%, 10.9%, 6.5% and 4.2%, respectively, in HCC patients in the 1980s (n = 474), 78.5%, 53.2%, 33.7%, 18.1% and 12.3%, respectively, in HCC patients in the 1990s (n = 1270), and 86.2%, 64.1%, 47.4%, 29.2% and 20.8%, respectively, in HCC patients in the 2000s (n = 2421) (overall significance, P < 0.001), showing that the prognosis for HCC patients has significantly improved in these three decades. As for HCV-related HCC, 1-, 3-, 5-, 8- and 10-year survival rates were 72.1%, 40.1%, 18.4%, 9.5% and 4.8% respectively, in the 1980s (n = 149), 81.1%, 56.0%, 35.4%, 17.6% and 11.4%, respectively, in the 1990s (n = 946), and 88.8%, 66.6%, 48.5%, 28.8% and 20.7%, respectively, in the 2000s (n = 1639) (overall significance, P < 0.001) (Fig. 9). As for HBV-related HCC, 1-, 3-, 5-, 8- and 10-year survival rates were 65.9%, 40.7%, 26.7%, 17.9% and 15.6% respectively, in the 1980s and 1990s (from 1981 to 1999) (n = 178), and 80.0%, 57.8%, 48.8%, 36.5% and 24.0%, respectively, in the 2000s (n = 282) (P < 0.001) (Fig. 10). As for NBNC HCC, 1-, 3-, 5-, 8- and 10-year survival rates were 72.5%, 49.7%, 30.8%, 24.0% and 16.5%, respectively, in the 1980s and 1990s (from 1981 to 1999) (n = 126), and 81.0%, 58.8%, 42.6%, 27.1% and 20.6%, respectively, in the 2000s (n = 425) (P = 0.074) (Fig. 11). Significant improvement of survival in hepatitis virus-related HCC may be partly attributed to the progress in antiviral therapies such as nucleoside analogs for hepatitis B and interferon (IFN) therapy for hepatitis C.36–39 Furthermore, detecting populations at high risk populations for development of HCC and close surveillance for HCC occurrence of these populations may contribute to the prolongation of survival in these patients. On the other hand, as compared with hepatitis virus-related HCC, the prognosis in NBNC HCC patients has modestly improved. Possible reasons for this are: (i) detecting populations at high-risk of development HCC and close surveillance for HCC occurrence in patients with NBNC liver disease are difficult; and (ii) performing antiviral therapies is impossible in NBNC HCC patients.22,23
Figure 8.
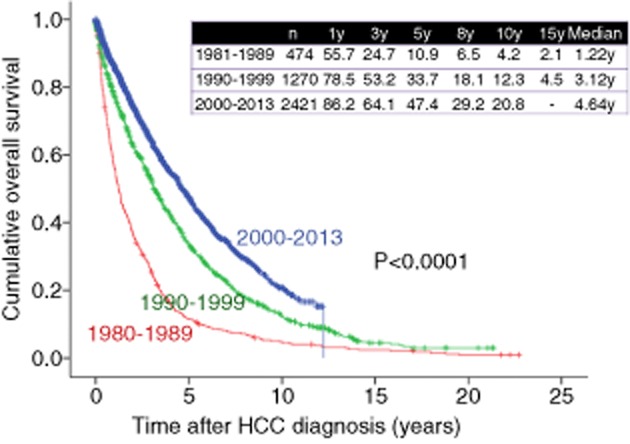
Cumulative overall survival for cases in the 1980s, 1990s and 2000s (Osaka Red Cross Hospital, Japan). HCC, hepatocellular carcinoma; y, years.
Figure 9.
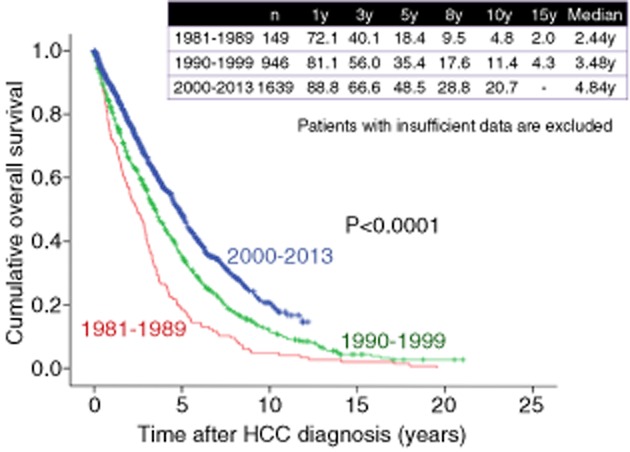
Cumulative overall survival for patients with hepatitis C virus-related hepatocellular carcinoma (HCC) in the 1980s, 1990s and 2000s (Osaka Red Cross Hospital, Japan). y, years.
Figure 10.
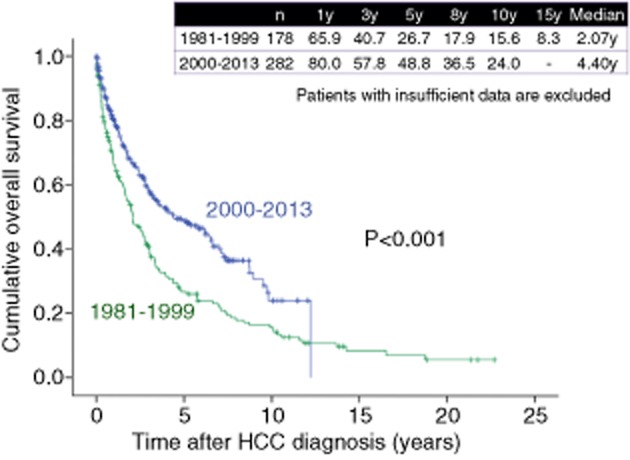
Cumulative overall survival for patients with hepatitis B virus-related hepatocellular carcinoma (HCC) in the 1980s, 1990s and 2000s (Osaka Red Cross Hospital, Japan). y, years.
Figure 11.
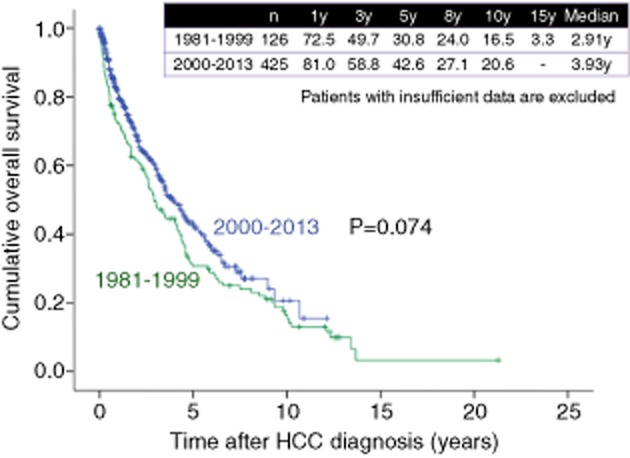
Cumulative overall survival for patients with non-B, non-C (NBNC)-related hepatocellular carcinoma (HCC) in the 1980s, 1990s and 2000s (Osaka Red Cross Hospital, Japan). y, years.
HCC Therapy: Changes for the Last Few Decades
SR
Surgical resection for hcc has been performed for more than 40 years. Although several treatment modalities have been proposed, SR is still considered the first-line therapeutic option for the majority of early stage HCC with well-preserved liver function.40 According to the European Association for the Study of the Liver (EASL) guidelines, SR is indicated in patients with a single tumor not exceeding 2 cm in diameter, performance status (PS) 0, Child–Pugh A and no portal hypertension.41 In Japan, however, SR is considered in patients with three or less tumors within 3 cm in diameter, no vascular invasion, Child–Pugh A or B, and expected tolerance to surgery, or even in those with four or more tumors larger than 3 cm and vascular invasion if they are expected to tolerate the surgery and that the treatment may improve the patient's prognosis.34 In our country, large tumor size of HCC is not considered to be an absolute contraindication for SR, although the risk of vascular invasion and dissemination increases with tumor size.34 For the last few decades, prognosis in HCC patients treated with SR has significantly improved. The establishment of operative guidelines for HCC patients with poor hepatic reserve, improved perioperative management and advances in surgical techniques have reduced the risk of postoperative mortality.42 Taura et al. reported that the OS rate in HCC patients treated with SR between 1991 and 2000 (n = 398) was significantly better than that in HCC patients treated with SR between 1985 and 1990 (n = 212) (58.0% vs 39.1% at 5 years, P < 0.0001).43 However, in our experience in HCC patients treated with SR (n = 887), 1-, 3-, 5-, 8- and 10-year survival rates have been 81.3%, 61.2%, 44.5%, 31.9% and 22.3% respectively, in the 1980s and 1990s (from 1981 to 1999) (n = 339), and 91.2%, 70.6%, 54.5%, 36.1% and 25.7%, respectively, in the 2000s (n = 548) (P = 0.020) (Fig. 12).
Figure 12.
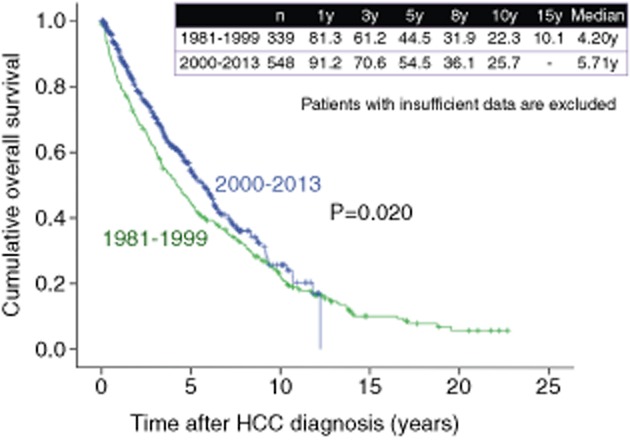
Cumulative overall survival for hepatocellular carcinoma (HCC) patients treated with surgery in the 1980s, 1990s and 2000s (Osaka Red Cross Hospital, Japan). y, years.
Traditional surgical management for HCC is open hepatectomy (OH). Laparoscopic hepatectomy (LH), first reported in 1993 as a newly developed procedure, has been performed around the world and has been established as a safe and feasible option for malignant liver tumors. A number of advantages such as less operative morbidity, reductions in postoperative pain and shorter length of hospitalization have been identified when comparing LH to OH from case-matched analyses and case series.44,45 Advances in surgical techniques are pushing the boundaries of SR for localized disease. On the other hand, several investigators reported that anatomical resection for patients with HCC could improve survival compared with non-anatomical resection.40,46
LT
Among therapies for HCC, the most effective curative option is LT.4,6 However, LT is not appropriate for all patients, and sufficient preoperative evaluation is needed to prudently allocate the scarce resources available.4,6 In an attempt to identify the most appropriate patients to undergo LT, the Milan criteria which consider both tumor number and tumor size (a single tumor <5 cm or ≤3 tumors of ≤3 cm) emerged as the international standard by which potential transplant candidates are evaluated in 1990s and this criteria has been validated in several studies.47,48 In HCC patients within the Milan criteria treated with LT, 5-year survival rates after LT are reported to range 70–80%, and HCC recurrence rates are approximately 10%.47,48
On the other hand, several investigators have examined the effect of expanding the Milan criteria on survival, primarily by liberalizing the restrictions on tumor size. Yao et al. demonstrated that using University of California San Francisco (UCSF) criteria (single nodule ≤6.5 cm or ≤3 nodules each ≤4.5 cm, with total combined tumor diameter ≤8 cm), the 5-year survival rate after LT in patients within UCSF criteria was 75%, while Kaido et al. reported that using the Kyoto criteria (a combination of tumor number ≤10, maximal diameter of each tumor ≤5 cm, and serum des-γ-carboxy prothrombin levels ≤400 mAU/mL), the 5-year survival rate after living donor LT in HCC patients within the Kyoto criteria was 82%, indicating that studies of these expanding criteria showed promising results.49,50 However, due to the limited number of donors and the scarcity of sufficient available data, current guidelines do not recommend LT for HCC patients outside the Milan criteria.34,41
TACE
Transcatheter arterial chemoembolization is a procedure whereby an embolic agent is injected into the tumor-feeding artery to deprive it of its major nutrient source by means of embolization; this results in ischemic necrosis of the targeted tumor.11,51 Differences in blood supply to HCC tumors and the liver form the theoretical basis of transcatheter arterial therapy for HCC.11,51 Transcatheter arterial embolization was initially used to treat HCC by Doyon et al. in 1974 and was applied to most unresectable HCC using gelatin sponge particles and anticancer agents by Yamada et al. in Japan.52,53 In the 1980s, TACE was the only non-surgical therapy for unresectable HCC until the introduction of PEI therapy for HCC. In the mid-1990s, lipiodol was newly introduced to enhance mainly the therapeutic effect. It is a substance which is selectively retained within the tumor and increases chemotherapeutic exposure as a drug carrier.54,55 Thereafter, TACE using lipiodol emulsion for unresectable HCC has been spread rapidly. The survival benefit of TACE for unresectable HCC was established in two randomized controlled trials (RCT) and in one meta-analysis.56–58 Thus, TACE plays an important role in treating unresectable HCC. It is clearly defined as a first-line therapy with an improved 2-year survival rate as compared with conservative therapy.59 The EASL guidelines recommend TACE for unresectable, Child–Pugh A or B multiple HCC with no vascular invasion while in Japan the therapy is recommended even for HCC with vascular invasion if it is Vp1 or Vp2.34,41
Takayasu et al. conducted a nationwide survey in 8510 HCC patients treated with TACE and reported 1-, 3- and 5-year survival rates of 82%, 47% and 26%, respectively, while in our data, as shown in Figure 13, in 765 HCC patients treated with TACE, 1-, 3- and 5-year survival rates are 72.5%, 36.4% and 17.5%, respectively, which means our results are worse than their results.60 This is probably due to the differences in baseline tumor characteristics between these two studies. Their study included 927 patients (13%) with stage I HCC and 501 patients (7%) with stage IVA HCC, whereas our study included only 32 patients (4.2%) with stage I HCC and 177 patients (23.1%) with stage IVA or stage IVB HCC.60
Figure 13.
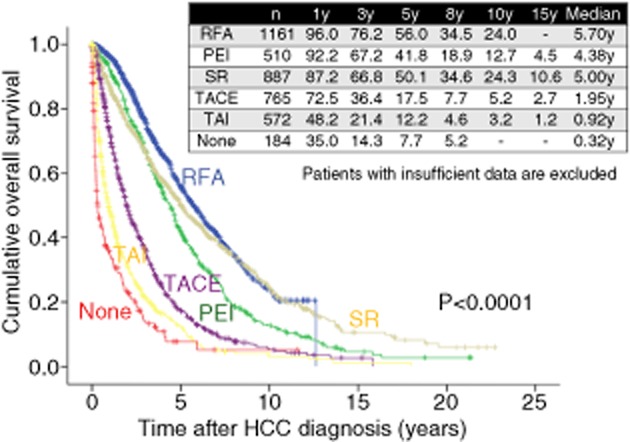
Cumulative overall survival according to treatment modality at initial hepatocellular carcinoma (HCC) therapy (Osaka Red Cross Hospital, Japan). PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; SR, surgical resection; TACE, transcatheter arterial chemoembolization; TAI, transcatheter arterial infusion chemotherapy; y, years.
Although the administration of a chemotherapeutic drug and lipiodol emulsion followed by embolic agents has been the most popular TACE procedure, the recent introduction of an embolic drug-eluting bead (DEB) has provided an attractive alternative to conventional regimens.2 While several reports have consistently shown a clinical benefit from conventional TACE, its significant adverse effects related to the administrated chemotherapeutic regimens have prevented the development of a clear consensus with regard to the type of chemotherapy that should be used or the optimum frequency of treatment sessions.2 Clinical trials have demonstrated that DEB loaded with doxorubicin has a safe pharmacokinetic profile with lower systemic drug exposure and significantly reduced liver toxicity in comparison with conventional TACE.61–63 In Japan, TACE with DEB will likely replace conventional TACE in the near future.
RFA and PEI
Percutaneous ethanol injection, which involves the injection of absolute ethanol directly into targeted tumors through fine needles under guidance of ultrasonography, has been widely used as a standard local ablative therapy for small HCC since its development in Japan in the late 1980s.64–68 However, in many cases its treatment efficacy is unpredictable because the spread of injected ethanol within the targeted tumor is largely affected by the capsule or septa of the targeted tumor.64–68 On the other hand, RFA therapy is an alternative technique to PEI that was introduced in Japan in 1999 and RFA therapy heat generated around the electrode tip distributes homogenously in all directions.64–79 An area of 3 cm or less in diameter can be ablated with a single application of RFA.64–68 The higher level of local tumor control achieved using RFA as compared with PEI seems to be due to the more expansive coagulative effects of thermal ablation on the targeted HCC nodules and microsatellites surrounding the nodules.65–68 The survival rate data indicated a significant benefit for RFA over PEI.65–68 This higher survival rate may be due to the higher rate of complete tumor response using RFA than using PEI; an initial complete response is an independent predictive factor linked to survival.80 In our experience, as shown in Figure 13, 1-, 3-, 5-, 8- and 10-year survival rates are 92.2%, 67.2%, 41.8%, 18.9% and 12.7% respectively, in HCC patients treated with PEI (n = 510) and 96.0%, 76.2%, 56.0%, 34.5% and 24.0%, respectively, in HCC patients treated with RFA (n = 1161) (P < 0.001). The EASL guidelines recommend percutaneous RFA for HCC with PS 0–2, Child–Pugh A or B, and three or less unresectable tumors of 3-cm diameter or less.41 In Japan, percutaneous RFA is generally indicated for patients with Child–Pugh A or B and three or less unresectable tumors of 3 cm diameter or less. Even in patients with unresectable tumors larger than 3 cm, percutaneous RFA in combination with TACE is recommended to expand the ablated area.34 Furthermore, a bipolar RFA device (CelonPOWER System; Celon Medical Instruments, Teltow, Germany) has been recently introduced in Japan.81 Unlike a monopolar RFA system which had been already approved in Japan, the primary characteristic of this novel device is its bipolar feature. That is, two electrodes are located on the same RFA needle, allowing electricity flow only between the electrodes at the treatment site of RFA without the need for a grounding pad and the danger of skin burns.81 We previously demonstrated that using the CelonPOWER System, tumors larger than 3 cm in diameter can be ablated safely.81 More recently, several investigators have used RFA to treat selected patients with resectable HCC with favorable clinical outcomes, and RFA is gradually gaining popularity in the treatment of resectable HCC in many countries, in addition to Japan.82,83
RFA versus surgery
A continuous improvement of survival outcomes after SR and RFA for HCC was achieved.7,84–86 Thus, an open question is whether or not RFA can compete with SR as a first-line therapy for patients with small HCC. However, no definite consensus has been reached as to which of these two therapies is the best for small HCC eligible for surgery. Three RCT and several non-RCT that have compared RFA with SR have been reported.69–79,87–89
Feng et al. reported in their latest RCT that in patients with small HCC with nodular diameters of less than 4 cm and up to two nodules (n = 168), percutaneous RFA may provide therapeutic effects similar to those of SR (cumulative OS rates at 1 and 3 years, 96.0% and 74.8% for SR and 90.6% and 76.7% for RFA [P > 0.05]; cumulative recurrence free survival (RFS) rates at 1 and 3 years, 93.1% and 61.1% for SR and 86.2% and 49.6% for RFA [P > 0.05]).89 Conversely, Huang et al. reported in their RCT that SR may provide better survival and lower recurrence rates than RFA for patients with HCC conforming to the Milan criteria (cumulative OS rates at 1, 3 and 5 years, 98.26%, 92.17% and 75.65% for SR, and 86.96%, 69.57% and 54.78% for RFA [P = 0.001]; cumulative RFS rates at 1, 3 and 5 years, 85.22% 60.87% and 51.30% for SR, and 81.74%, 46.08% and 28.69% for RFA [P = 0.017]).88 Zhou et al. conducted a meta-analysis to evaluate the efficacy of RFA and SR for the treatment of HCC.79 They concluded that SR was superior to RFA for patients with small HCC tumors of more than 3 cm that were eligible for surgery; however, for tumors of 3 cm or less, survival levels did not differ significantly between SR and RFA. Furthermore, Cho et al. reported that using Markov model analysis, SR was preferable to RFA in terms of OS.90 Recently, a Japanese nationwide survey revealed that SR (n = 5361) results in longer OS and time to recurrence than either RFA (n = 5548) or PEI (n = 2059) in patients with HCC who had no more than three tumors (≤3 cm) and liver damage of class A or B.91 In view of these results it is therefore still unclear whether or not SR can achieve higher survival rates than RFA for patients with resectable HCC, although the majority of previous reports indicated that SR had a superior efficacy over RFA in selected HCC patients. In addition, all of the previous three RCT were Chinese based.87–89 In comparing the results of these RCT with those from Japanese-based studies, the mean age of the Chinese patient population was approximately 10 years younger than that of the patients in the Japanese studies.75,78,79,87–89,91 In the etiology of liver disease in the Chinese studies, patients with chronic hepatitis B were in the majority.87–89 However, in the Japanese studies, patients with chronic hepatitis C were in the majority; hence, the study results did not reflect the actual situation in Japan where Japanese HCC patients consist of many aged patients, and the etiology of background liver disease involves chronic hepatitis C, which accounts for approximately 80% of Japanese HCC patients.75,78,79,91 We also believe that antiviral therapy for background liver diseases (i.e. nucleotide analog therapy for hepatitis B and IFN therapy for hepatitis C) and nutritional supporting therapy for improving liver function such as branched-chain amino acid therapy, as well as tumor-related factors such as HCC stage, tumor size and tumor number should be taken into account when assessing clinical outcomes after initial therapy for HCC.38,39,92–94 Therefore, caution should be exercised for interpreting these study results. In our country, an RCT (SURF trial) comparing survival between surgery and RFA for patients with resectable HCC of 3 cm or less in size and up to three nodules is underway.95
In our experience, in LC patients with HCC with three nodules or less and up to 3 cm in diameter, the 3- and 5-year OS rates in the SR group (n = 207) were 76.3% and 55.8%, respectively, and the corresponding OS rates in the RFA group (n = 666) were 77.2% and 55.5%, respectively (P = 0.767) (Fig. 14). Furthermore, after using propensity score matching (adjusted for possible variables associated with long-term survival of HCC patients: age, sex, tumor number, maximum tumor size, cause of liver disease and Child–Pugh classification), the 3- and 5-year OS rates in the SR group (n = 179) were 75.9% and 56.8%, respectively, and the corresponding OS rates in the RFA group (n = 179) were 78.6% and 60.6%, respectively (P = 0.266) (Fig. 15). Our study results indicate that in LC patients with HCC with three nodules or less and up to 3 cm in diameter, patients treated with RFA at initial therapy had prognoses comparable with those treated with SR.
Figure 14.
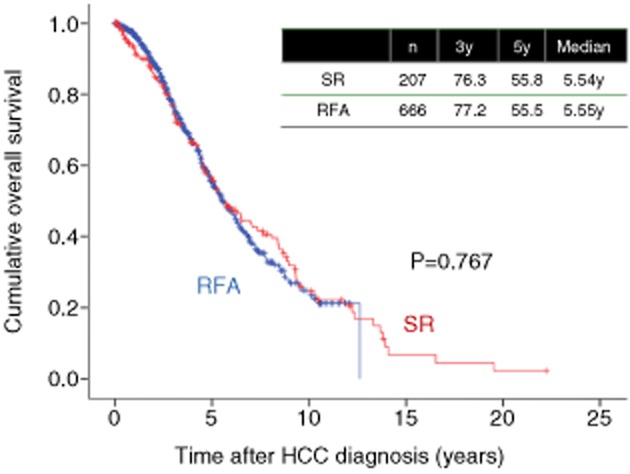
Cumulative overall survival in hepatocellular carcinoma (HCC) patients with liver cirrhosis, tumor size within 3 cm and up to three nodules treated with radiofrequency ablation (RFA) (n = 666) and surgical resection (SR) (n = 207). y, years.
Figure 15.
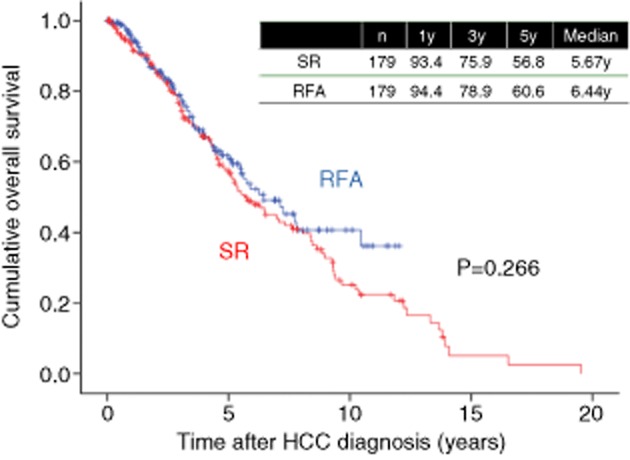
Cumulative overall survival in hepatocellular carcinoma (HCC) patients with liver cirrhosis, tumor size within 3 cm and up to 3 nodules treated with radiofrequency ablation (RFA) (n = 179) and surgical resection (SR) (n = 179) after propensity score matching. y, years.
Chemotherapy
Although systemic chemotherapy such as doxorubicin was not demonstrated to be effective for the treatment of advanced HCC for several decades, two randomized studies showed that sorafenib therapy obtained survival benefit over the placebo group for patients with unresectable HCC, and molecular-targeted therapy with sorafenib is now approved for use as first-line systemic chemotherapy in these patients.5,96–98 Sorafenib is a multikinase inhibitor that blocks tumor growth and cell proliferation.5,96,97 Several clinical trials of molecular targeting agents other than sorafenib have been performed. However, no trials to date have shown that any of these agents have superior efficacy compared with sorafenib for the treatment of unresectable HCC.99–101 The EASL guidelines recommend sorafenib for unresectable, advanced, Child–Pugh A or B HCC with PS 0–2 and vascular invasion or distant metastasis.41 According to the Japanese guidelines, on the other hand, sorafenib is recommended for unresectable, advanced, Child–Pugh A HCC with vascular invasion or distant metastasis as well as for patients intolerant to TACE or in whom the procedure is anatomically unsuitable.34
Although sorafenib has a significantly higher survival benefit for patients with advanced HCC, the response to sorafenib remains low and the median OS is only extended by approximately 3 months.5,96–101 Furthermore, predictive factors of responders to sorafenib in patients with HCC have not been well established.102 To optimize the beneficial effects of sorafenib, combination or sequential therapies comprising sorafenib and other therapies for HCC have been examined. The limited effects of single therapy indicated that the combination therapy would enhance the overall treatment effect. A recent systematic review suggested that sorafenib-based combination with some anticancer agent could be a more effective and tolerable treatment for advanced HCC in the future.103 Sequential sorafenib therapy after TACE for unresectable HCC is also promising. Sansonno et al. reported that a conventional TACE procedure followed by sorafenib therapy resulted in a significantly longer time to progression in patients with intermediate stage HCV-related HCC, with no unexpected side-effects.104 On the other hand, a clinical trial to study the recurrence-preventing efficacy of sorafenib by administration of it after curative therapy such as SR or RFA is underway (STORM trial).105
The success of sorafenib has spurred an explosive increase of clinical studies testing novel molecular targets and other agents for the treatment of HCC. They included sunitinib, brivanib, foretinib, TSU-68, erlotinib, AZD6244, linifanib, regorafenib tivantinib and monoclonal antibodies such as bevacizumab and glypican-3.106,107 If favorable results are obtained by these trials, the treatment strategy for HCC will be drastically changed.
On the other hand, hepatic arterial infusion chemotherapy (HAIC) for advanced HCC was originally developed in Japan.98 Because no RCT regarding efficacy of HAIC for advanced HCC has been conducted and its use is based solely on empirical clinical data, HAIC for advanced HCC is not appreciated in Western countries.98 However, there are several encouraging reports from Japan. Ando et al. reported that in 48 advanced HCC patients with portal vein tumor thrombosis (PVTT) treated with HAIC using low-dose 5-fluorouracil (5-FU) and cisplatin (low-dose FP), the response rate was 48%.108 Ueshima et al. demonstrated that in advanced HCC patients with vascular invasion treated with HAIC using low-dose FP, the response rate was 38.5%, the median time to progression was 4.1 months and the median survival time was 15.9 months, which are superior to those in the SHARP study.5,109 Obi et al. reported that in 116 advanced HCC patients with portal venous invasion treated with combination therapy of intra-arterial 5-FU and systemic IFN-α, 19 patients (16%) showed complete response and 42 (36%) showed partial response.110 HAIC for advanced HCC may have potential benefit for overcoming drawbacks in sorafenib therapy for advanced HCC, although further well-characterized studies are necessary.
Radiotherapy
Although radiotherapy for advanced HCC is not recommended in current guidelines, it can be a promising therapy.34,41,111,112 Xi et al. demonstrated that in 41 advanced HCC patients with PVTT or inferior vena cava invasion treated with stereotactic body radiotherapy, 15 (36.6%) achieved complete response, 16 (39.0%) achieved partial response.111 Nakazawa et al. compared the survival benefits of sorafenib (n = 40) versus radiotherapy (n = 57) in HCC patients with PVTT using propensity score-matching analysis and concluded that radiotherapy is a better first-line therapy than sorafenib in advanced HCC patients with PVTT.112 However, further prospective studies are warranted to confirm these results.
Other new emerging therapies for HCC
Recently, new promising therapies for HCC have emerged. For intermediate stage HCC, there is increasing evidence supporting a role for transarterial radioembolization. Radioembolization is a form of brachytherapy in which intra-arterially injected (90)Y-loaded microspheres serve as sources for internal radiation purposes and produces average disease control rates above 80% and is a very well-tolerated therapy in general.113–115
Based on the immune system's antitumoral effect, immunotherapy is also a promising new treatment option for HCC. Actually, specific antitumoral T-cell responses can be detected in HCC patients.13 Clinical trials regarding the effect of the active specific immunotherapy (including dendritic cell vaccine, liver cancer vaccine and HCC genetically engineered vaccine) in HCC patients on survival is still underway.116
On the other hand, gene therapy has the potential to provide therapeutic benefits for patients with HCC and has been the subject of intense clinical research in recent years. miRNA are endogenous single-stranded RNA, approximately 22 nucleotides in length. After the discovery of miRNA in 1993, the considerable extent of the gene regulatory capacity of miRNA has been studied. These investigations have shown that specific miRNA have central roles in critical biological processes such as apoptosis, development, cell proliferation and oncogenesis.117 Clinical studies with regard to the potential clinical uses of miRNA are ongoing, most notably in the early diagnosis and treatment of HCC.118
However, all the evidence that support the use of these therapies in HCC is mainly based on retrospective series or non-controlled prospective studies. Hence, further well characterized RCT will be needed to confirm clinical efficacy of these therapies on survival.
Conclusion
Over the last three decades, prognosis in patients with HCC have markedly improved due to the advances in the treatment for HCC. Furthermore, baseline characteristics in HCC patients have markedly changed. New emerging diagnostic imagings and the adequate selection of high-risk groups for HCC occurrence could enable detection of early stage HCC, potentially improving outcome. Recent new emerging therapies may further improve prognosis in HCC patients.
Finally, we show annual trends for the treatment of HCC in our hospital from 1981 to 2013 in Figure 16.
Figure 16.
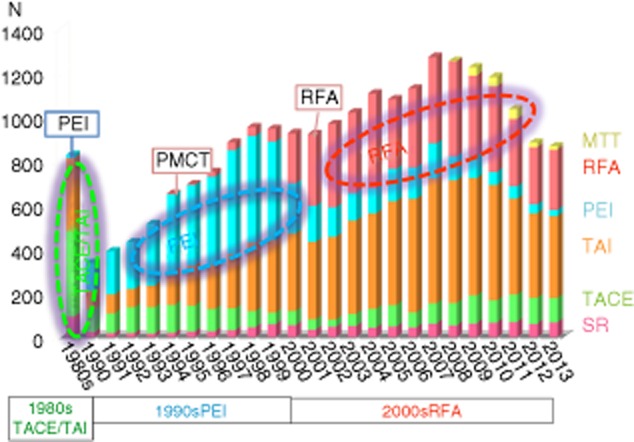
Annual trends for the treatment of hepatocellular carcinoma (1981–2013, Osaka Red Cross Hospital, Japan). MTT, molecular-targeted therapy; PEI, percutaneous ethanol injection; PMCT, percutaneous microwave coagulation therapy; RFA, radiofrequency ablation; SR, surgical resection; TACE, transcatheter arterial chemoembolization; TAI, transcatheter arterial infusion chemotherapy.
Acknowledgments
THE AUTHORS WOULD like to thank Haruko Takada for data collection.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa H, Osaki Y. Non-B, non-C hepatocellular carcinoma. Int J Oncol. 2013;43(5):1333–1342. doi: 10.3892/ijo.2013.2061. (Review). [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM. Update treatment approach to hepatocellular carcinoma. J Gastoenterology. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 7.van Meer S, de Man RA, Siersema PD, van Erpecum KJ. Surveillance for hepatocellular carcinoma in chronic liver disease: evidence and controversies. World J Gastroenterol. 2013;19(40):6744–6756. doi: 10.3748/wjg.v19.i40.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist. 2010;15(Suppl 4):23–33. doi: 10.1634/theoncologist.2010-S4-23. [DOI] [PubMed] [Google Scholar]
- 9.Gish RG, Lencioni R, Di Bisceglie AM, Raoul JL, Mazzaferro V. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6(2):173–185. doi: 10.1586/egh.11.105. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Crocetti L. Image-guided ablation for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:181–194. doi: 10.1007/978-3-642-16037-0_12. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39(4):503–509. doi: 10.1053/j.seminoncol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Duan F, Lam MG. Delivery approaches of gene therapy in hepatocellular carcinoma. Anticancer Res. 2013;33(11):4711–4718. [PubMed] [Google Scholar]
- 13.Miamen AG, Dong H, Roberts LR. Immunotherapeutic approaches to hepatocellular carcinoma treatment. Liver Cancer. 2012;1(3-4):226–237. doi: 10.1159/000343837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankey BF, Ries LA, Kosary CL, et al. Partitioning linear trends in age-adjusted rates. Cancer Causes Control. 2000;11:31–35. doi: 10.1023/a:1008953201688. [DOI] [PubMed] [Google Scholar]
- 15.Nishikawa H, Kimura T, Kita R, Osaki Y. Treatment for hepatocellular carcinoma in elderly patients: a literature review. J Cancer. 2013;4(8):635–643. doi: 10.7150/jca.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health. 2006. Labour and Welfare. Abridged Life Tables for Japan 2006). Available at: http://www.mhlw.go.jp/english/database/db-hw/lifetb06/index.html. Accessed March 20, 2014.
- 17.Kiyosawa K, Tanaka E. Characteristics of hepatocellular carcinoma in Japan. Oncology. 2002;62(Suppl 1):5–7. doi: 10.1159/000048269. [DOI] [PubMed] [Google Scholar]
- 18.Ikai I, Arii S, Okazaki M, et al. The Liver Cancer Study Group of Japan, Kyoto, Japan. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol Res. 2007;37:676–691. doi: 10.1111/j.1872-034X.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. 2010;53(1):39–43. doi: 10.1159/000252782. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Imai Y, Hiramatsu N, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148(11):820–826. doi: 10.7326/0003-4819-148-11-200806030-00004. [DOI] [PubMed] [Google Scholar]
- 21.Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46:1230–1237. doi: 10.1007/s00535-011-0431-9. [DOI] [PubMed] [Google Scholar]
- 22.Kaibori M, Ishizaki M, Matsui K, Kwon AH. Clinicopathologic characteristics of patients with non-B non-C hepatitis virus hepatocellular carcinoma after hepatectomy. Am J Surg. 2012;204:300–307. doi: 10.1016/j.amjsurg.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Abe H, Yoshizawa K, Kitahara T, Aizawa R, Matsuoka M, Aizawa Y. Etiology of non-B non-C hepatocellular carcinoma in the eastern district of Tokyo. J Gastroenterol. 2008;43:967–974. doi: 10.1007/s00535-008-2264-8. [DOI] [PubMed] [Google Scholar]
- 24.Nagaoki Y, Hyogo H, Aikata H, et al. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42:368–375. doi: 10.1111/j.1872-034X.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Ohtake T, Nishiguchi S, et al. The Japan Non-B, Non-C Liver Cirrhosis Study Group. Survey of non-B, non-C liver cirrhosis in Japan. Hepatol Res. 2013;43(10):1020–1031. doi: 10.1111/hepr.12056. [DOI] [PubMed] [Google Scholar]
- 26.Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(7):1712–1723. doi: 10.3748/wjg.v20.i7.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 28.Asselah T, Marcellin P. Interferon free therapy with direct acting antivirals for HCV. Liver Int. 2013;33(Suppl 1):93–104. doi: 10.1111/liv.12076. [DOI] [PubMed] [Google Scholar]
- 29.Kwon H, Lok AS. Does antiviral therapy prevent hepatocellular carcinoma? Antivir Ther. 2011;16(6):787–795. doi: 10.3851/IMP1895. [DOI] [PubMed] [Google Scholar]
- 30.Singal AK, Singh A, Jaganmohan S, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8(2):192–199. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155(4):323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 32.Kudo M. Hepatocellular carcinoma 2009 and beyond: from the surveillance to molecular targeted therapy. Oncology. 2008;75(Suppl 1):1–12. doi: 10.1159/000181865. [DOI] [PubMed] [Google Scholar]
- 33.Nouso K, Ito Y, Kuwaki K, et al. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98(7):1161–1165. doi: 10.1038/sj.bjc.6604282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudo M, Izumi N, Kokudo N, et al. HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 35.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzaferro V, Romito R, Schiavo M, et al. HCC Italian Task Force. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa H, Nishijima N, Arimoto A, et al. Effect of nucleoside analog use in patients with hepatitis B virus-related hepatocellular carcinoma. Hepatol Res. 2013;31:4167. doi: 10.1111/hepr.12169. [DOI] [PubMed] [Google Scholar]
- 38.Toyoda H, Kumada T, Tada T, Sone Y, Fujimori M. Transarterial chemoembolization for hepatitis B virus-associated hepatocellular carcinoma: improved survival after concomitant treatment with nucleoside analogues. J Vasc Interv Radiol. 2012;23(3):317–322. doi: 10.1016/j.jvir.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Kubo S, Takemura S, Sakata C, Urata Y, Uenishi T. Adjuvant therapy after curative resection for hepatocellular carcinoma associated with hepatitis virus. Liver Cancer. 2013;2(1):40–46. doi: 10.1159/000346214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makuuchi M, Donadon M, Torzilli G. Hepatic resection for hepatocellular carcinoma in cirrhosis. Ann Ital Chir. 2008;79(2):111–115. [PubMed] [Google Scholar]
- 41.European Association for The Study Of The Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Ann Surg. 2006;244(2):265–273. doi: 10.1097/01.sla.0000217921.28563.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection – 2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 46.Eguchi S, Kanematsu T, Arii S, et al. Liver Cancer Study Group of Japan. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143(4):469–475. doi: 10.1016/j.surg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 48.Chan SC. Liver Transplantation for Hepatocellular Carcinoma. Liver Cancer. 2013;2(3-4):338–344. doi: 10.1159/000343849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8:765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- 50.Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154(5):1053–1060. doi: 10.1016/j.surg.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 51.Takayasu K, Arii S, Ikai I, et al. Liver Cancer Study Group of Japan. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol. 2010;194(3):830–837. doi: 10.2214/AJR.09.3308. [DOI] [PubMed] [Google Scholar]
- 52.Doyon DMA, Jourde AM, Regensberg C, Frileux C. L'embolisation arterielle hepatique dans les tumeurs malignes du foie. Ann Radiol. 1974;17:593–603. [PubMed] [Google Scholar]
- 53.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148(2):397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 54.Yumoto Y, Jinno K, Tokuyama K, et al. Hepatocellular carcinoma detected by iodized oil. Radiology. 1985;154:19–24. doi: 10.1148/radiology.154.1.2981112. [DOI] [PubMed] [Google Scholar]
- 55.Ohishi H, Uchida H, Yoshimura H, et al. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology. 1985;154:25–29. doi: 10.1148/radiology.154.1.2981114. [DOI] [PubMed] [Google Scholar]
- 56.Llovet JM, Real MI, Montaña X, et al. Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 57.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 58.Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 59.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 60.Takayasu K, Arii S, Ikai I, et al. Liver Cancer Study Group of Japan. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;30(1):474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Lammer J, Malagari K, Vogl T, et al. PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;30(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogl TJ, Lammer J, Lencioni R, et al. Liver, gastrointestinal and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;30(1):W562–570. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 64.Ebara M, Ohto M, Sugiura N, et al. Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol. 1990;5(6):616–626. doi: 10.1111/j.1440-1746.1990.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 65.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 66.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127(6):1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54(8):1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Hong SN, Lee SY, Choi MS, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well preserved hepatic function. J Clin Gastroenterol. 2005;39(3):247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- 70.Montorsi M, Santambrogio R, Bianchi P, et al. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9(1):62–67. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Cho CM, Tak WY, Kweon YO, et al. The comparative results of radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma. Korean J Hepatol. 2005;11(1):59–71. [PubMed] [Google Scholar]
- 72.Ogihara M, Wong LL, Machi J. Radiofrequency ablation versus surgical resection for single nodule hepatocellular carcinoma: long-term outcomes. HPB (Oxford) 2005;7(3):214–221. doi: 10.1080/13651820510028846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu MD, Kuang M, Liang LJ, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86(12):801–805. [PubMed] [Google Scholar]
- 74.Lupo L, Panzera P, Giannelli G, Memeo M, Gentile A, Memeo V. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford) 2007;9(6):429–434. doi: 10.1080/13651820701713758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa K, Makuuchi M, Takayama T, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49(4):589–594. doi: 10.1016/j.jhep.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 76.Guglielmi A, Ruzzenente A, Valdegamberi A, et al. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12(1):192–198. doi: 10.1007/s11605-007-0392-8. [DOI] [PubMed] [Google Scholar]
- 77.Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg. 2008;12(9):1521–1526. doi: 10.1007/s11605-008-0553-4. [DOI] [PubMed] [Google Scholar]
- 78.Ueno S, Sakoda M, Kubo F, et al. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16(3):359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi: 10.1186/1471-230X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40(6):1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 81.Osaki Y, Ikeda K, Izumi N, et al. Clinical effectiveness of bipolar radiofrequency ablation for small liver cancers. J Gastroenterol. 2013;48(11):1308–1309. doi: 10.1007/s00535-012-0685-x. [DOI] [PubMed] [Google Scholar]
- 82.Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98(9):1210–1224. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- 83.Nishikawa H, Osaki Y, Iguchi E, et al. Radiofrequency ablation for hepatocellular carcinoma: the relationship between a new grading system for the ablative margin and clinical outcomes. J Gastroenterol. 2013;48(8):951–965. doi: 10.1007/s00535-012-0690-0. [DOI] [PubMed] [Google Scholar]
- 84.Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253(4):745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 85.Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29(6):558–568. doi: 10.3109/02656736.2013.821528. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz ME, Shrager B. Surgical resection for hepatocellular carcinoma in the noncirrhotic: the Western experience. Recent Results Cancer Res. 2013;190:85–100. doi: 10.1007/978-3-642-16037-0_6. [DOI] [PubMed] [Google Scholar]
- 87.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 89.Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 91.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729. doi: 10.1016/j.jhep.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 92.Osaki Y, Ueda Y, Marusawa H, et al. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol. 2012;47(4):444–451. doi: 10.1007/s00535-011-0505-8. [DOI] [PubMed] [Google Scholar]
- 93.Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138(4):299–306. doi: 10.7326/0003-4819-138-4-200302180-00008. [DOI] [PubMed] [Google Scholar]
- 94.Nishikawa H, Osaki Y, Iguchi E, et al. The effect of long-term supplementation with branched-chain amino acid granules in patients with hepatitis C virus-related hepatocellular carcinoma after radiofrequency thermal ablation. J Clin Gastroenterol. 2013;47(4):359–366. doi: 10.1097/MCG.0b013e31826be9ad. [DOI] [PubMed] [Google Scholar]
- 95.Hasegawa K, Kokudo N, Shiina S, Tateishi R, Makuuchi M. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: start of a randomized controlled trial (SURF trial) Hepatol Res. 2010;40(8):851–852. doi: 10.1111/j.1872-034X.2010.00696.x. [DOI] [PubMed] [Google Scholar]
- 96.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 97.Kudo M. Targeted therapy for liver cancer: updated review in 2012. Curr Cancer Drug Targets. 2012;12:1062–1072. [PubMed] [Google Scholar]
- 98.Nishikawa H, Osaki Y, Kita R, Kimura T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in Japan. Cancers. 2012;4:165–183. doi: 10.3390/cancers4010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 100.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pazo-Cid RA, Lanzuela M, Esquerdo G, et al. Novel antiangiogenic therapies against advanced hepatocellular carcinoma (HCC) Clin Transl Oncol. 2012;14:564–574. doi: 10.1007/s12094-012-0842-y. [DOI] [PubMed] [Google Scholar]
- 102.Takeda H, Nishikawa H, Osaki Y, et al. Japanese Red Cross Liver Study Group. Clinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in Japan. Liver Int. 2014 doi: 10.1111/liv.12591. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Abdel-Rahman O, Fouad M. Sorafenib-based combination as a first line treatment for advanced hepatocellular carcinoma: a systematic review of the literature. Crit Rev Oncol Hematol. 2014;91:1–8. doi: 10.1016/j.critrevonc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 104.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17(3):359–366. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology. 2011;81(Suppl 1):50–55. doi: 10.1159/000333259. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z, Zhang G, Wu J, Jia M. Adjuvant therapy for hepatocellular carcinoma: current situation and prospect. Drug Discov Ther. 2013;7(4):137–143. [PubMed] [Google Scholar]
- 107.Shen YC, Lin ZZ, Hsu CH, Hsu C, Shao YY, Cheng AL. Clinical trials in hepatocellular carcinoma: an update. Liver Cancer. 2013;2(3–4):345–364. doi: 10.1159/000343850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ando E, Tanaka M, Yamashita F, et al. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95(3):588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 109.Ueshima K, Kudo M, Takita M, et al. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology. 2010;78:S148–153. doi: 10.1159/000315244. [DOI] [PubMed] [Google Scholar]
- 110.Obi S, Yoshida H, Toune R, et al. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106(9):1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 111.Xi M, Zhang L, Zhao L, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8(5):e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 14(1):84. doi: 10.1186/1471-230X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol. 2014;56(2):464–473. doi: 10.1016/j.jhep.2011.07.012. 2012. [DOI] [PubMed] [Google Scholar]
- 114.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57(5):1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 115.Moreno-Luna LE, Yang JD, Sanchez W, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(3):714–723. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng JW, Lv Y. New progress of non-surgical treatments for hepatocellular carcinoma. Med Oncol. 2013;30(1):381. doi: 10.1007/s12032-012-0381-y. [DOI] [PubMed] [Google Scholar]
- 117.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 118.Greene CM, Varley RB, Lawless MW. MicroRNAs and liver cancer associated with iron overload: therapeutic targets unravelled. World J Gastroenterol. 2013;19(32):5212–5226. doi: 10.3748/wjg.v19.i32.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]


