Abstract
Fullerenes are carbon molecules that form polyhedral cages. Their bond structures are exactly the planar cubic graphs that have only pentagon and hexagon faces. Strikingly, a number of chemical properties of a fullerene can be derived from its graph structure. A rich mathematics of cubic planar graphs and fullerene graphs has grown since they were studied by Goldberg, Coxeter, and others in the early 20th century, and many mathematical properties of fullerenes have found simple and beautiful solutions. Yet many interesting chemical and mathematical problems in the field remain open. In this paper, we present a general overview of recent topological and graph theoretical developments in fullerene research over the past two decades, describing both solved and open problems. WIREs Comput Mol Sci 2015, 5:96–145. doi: 10.1002/wcms.1207
Conflict of interest: The authors have declared no conflicts of interest for this article.
For further resources related to this article, please visit the WIREs website.
INTRODUCTION
Buckminsterfullerene C60 - Ih is a convex and ‘spherical’ molecule (in the sense that the atoms lie on the surface of a sphere) with a highly symmetric icosahedral structure. It was originally conjectured independently by Ōsawa in 19701,2 and Stankevich, Bochvar, and Galpern in 1973,3,4 discovered by mass spectrometry in 1985 by Kroto et al. through laser evaporation of graphite,5–8 and synthesized in larger amounts by Krätschmer et al.9 in 1990 (the first mass-spectroscopic studies on small carbon cluster were performed by Mattauch et al.10 in 1943). Fullerenes are the polyhedral analogs to the two-dimensional graphene sheets.a They occur in nature and have been detected in interstellar space, albeit in minute amounts.11–14 In general, (classical) fullerenes are cage-like, hollow molecules of pseudo-spherical symmetry consisting of pentagons and hexagons only, resulting in a trivalent (and in the most ideal case) convex polyhedron with exactly three edges (bonds) joining every vertex occupied by carbon, idealized as sp2 hybridized atoms.15 In graph theoretical terms, fullerenes belong to the class of cubic, planar, three-connected, and simple graphs, consisting of 12 pentagons only (the 12 Pentagon Theorem) and F6 hexagons. This gives the general formula of  (F6 ≥ 0 and F6 ≠ 1) for carbon fullerenes, with the number of hexagonal faces F6 = 20 for C60. The smallest possible fullerene is C20 (F6 = 0), a dodecahedron consisting of 12 connected pentagons, and the only Platonic solid in the family of fullerene polyhedra.16 The truncated icosahedron C60 belongs to the class of Archimedean solids.17 C60 - Ih is the minimal arrangement such that no two pentagons share an edge (isolated pentagons): The edges of each pentagon join only hexagons, and the edges of each hexagon alternately join pentagons and hexagons. Thus, it is the smallest member in the class of fullerenes fulfilling the so-called isolated pentagon rule (IPR), which brings thermodynamic stability to a fullerene cage.18
(F6 ≥ 0 and F6 ≠ 1) for carbon fullerenes, with the number of hexagonal faces F6 = 20 for C60. The smallest possible fullerene is C20 (F6 = 0), a dodecahedron consisting of 12 connected pentagons, and the only Platonic solid in the family of fullerene polyhedra.16 The truncated icosahedron C60 belongs to the class of Archimedean solids.17 C60 - Ih is the minimal arrangement such that no two pentagons share an edge (isolated pentagons): The edges of each pentagon join only hexagons, and the edges of each hexagon alternately join pentagons and hexagons. Thus, it is the smallest member in the class of fullerenes fulfilling the so-called isolated pentagon rule (IPR), which brings thermodynamic stability to a fullerene cage.18
There are infinitely many fullerenes, the number of isomers growing as  for N carbon atoms as shown in Figure 1.19 The reason for the N9 growth comes from algebraic geometry, and is discussed below. These isomers come in many different shapes depending on the distribution of the pentagons as shown in Figure 2.b As experimental techniques advance to synthesize new fullerene isomers21 with many interesting chemical and physical applications,22–25 see for example the recent bottom-up synthesis of C60 by Scott et al.26 and Kabdulov et al.,27 one naturally wants to know more about their thermodynamic stability and electronic properties.28 As the isomer space rapidly grows into the millions and beyond, this becomes a daunting task for larger fullerenes. Even when the search is restricted to fullerenes that have isolated pentagons, known to yield the best stability, the number of candidates for most thermodynamically stable isomers is huge, many orders of magnitude too large for quantum chemical methods to be a viable means of analyzing stability. But, remarkably, the fullerene graphs contain all the information we need, and we are able to sort through the millions of isomers, finding a few candidates for the most stable, by way of simple, easily computed topological indicators,29,30 such as the neighbor indices for pentagons or hexagons,31 or the incremental assignment of heat of formations to certain face patches.32,33
for N carbon atoms as shown in Figure 1.19 The reason for the N9 growth comes from algebraic geometry, and is discussed below. These isomers come in many different shapes depending on the distribution of the pentagons as shown in Figure 2.b As experimental techniques advance to synthesize new fullerene isomers21 with many interesting chemical and physical applications,22–25 see for example the recent bottom-up synthesis of C60 by Scott et al.26 and Kabdulov et al.,27 one naturally wants to know more about their thermodynamic stability and electronic properties.28 As the isomer space rapidly grows into the millions and beyond, this becomes a daunting task for larger fullerenes. Even when the search is restricted to fullerenes that have isolated pentagons, known to yield the best stability, the number of candidates for most thermodynamically stable isomers is huge, many orders of magnitude too large for quantum chemical methods to be a viable means of analyzing stability. But, remarkably, the fullerene graphs contain all the information we need, and we are able to sort through the millions of isomers, finding a few candidates for the most stable, by way of simple, easily computed topological indicators,29,30 such as the neighbor indices for pentagons or hexagons,31 or the incremental assignment of heat of formations to certain face patches.32,33
Figure 1.

Number of distinct (non-isomorphic) fullerene isomers CN (with and without fulfilling the IPR) with increasing number of carbon atoms N up to N = 400 (double logarithmic scale). (Data taken from the House of Graphs Ref 34)
Figure 2.
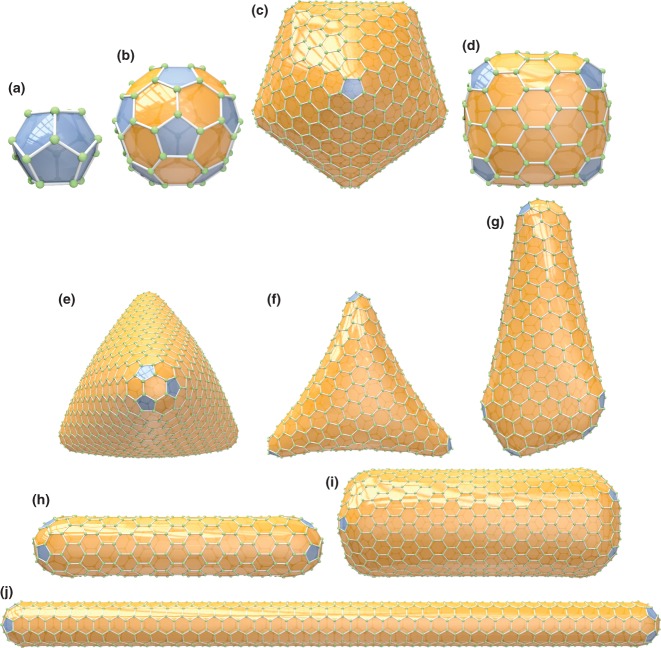
A selection of different 3D shapes for regular fullerenes (distribution of the pentagons DP are set in parentheses). ‘Spherically’ shaped (icosahedral), for example, (a) C20-Ih, (b) C60-Ih, and (c) C960-Ih (DP = 12 × 1); barrel shaped, for example, (d) C140-D3h (DP = 6 × 2); trigonal pyramidally shaped (tetrahedral structures), for example, (e) C1140-Td (DP = 4 × 3); (f) trihedrally shaped C440-D3 (DP = 3 × 4); (g) nano-cone or menhir C524-C1 (DP = 5 + 7 × 1); cylindrically shaped (nanotubes), for example, (h) C360-D5h, (i) C1152-D6d, (j) C840-D5d (DP = 2 × 6). The fullerenes shown in this figure and throughout the paper have been generated automatically using the Fullerene program.35
There has been an intense activity in the field of topological and graph theoretical descriptions of molecules such as fullerenes29,31,36–40 over the past 20 years, to the extent that it has become a major sub-discipline within mathematical chemistry. It comes perhaps as a surprise that graph theory applied to molecular structures has not yet made it into main-stream university teaching. Furthermore, since the publication of the standard reference work in this area, An Atlas of Fullerenes by Fowler and Manolopoulos,31 there has not been a comprehensive review on this subject highlighting the many activities in recent times. In contrast, there are excellent reviews and books available on the chemistry and physics of fullerenes22,23,41–47 or on their electronic structure calculations.28 In this overview we aim to close this gap and report on the many new and exciting developments in the topological and graph theoretical treatment of fullerenes, which have taken place over the past decade or so.c
TOPOLOGICAL PROPERTIES OF FULLERENES
A Short Introduction to Graph Theory and Embeddings
A graph is a pair  , where
, where  defines a set of vertices, and
defines a set of vertices, and  is a set of edges, each an unordered pair connecting two vertices. If a graph can be drawn in the two-dimensional plane without edge crossings, then it is called planar, and in this case it can also be drawn using only straight lines. A planar embedding is a map
is a set of edges, each an unordered pair connecting two vertices. If a graph can be drawn in the two-dimensional plane without edge crossings, then it is called planar, and in this case it can also be drawn using only straight lines. A planar embedding is a map  assigning a 2D-coordinate to each vertex such that straight-line drawings of the edges do not cross. For planar graphs in general, planar embeddings are not unique. But whenever at least three vertices must be removed before the graph separates into two disconnected components, the graph is called three-connected, and there is essentially only one wayd to embed it in the plane.48 Because of this, a three-connected planar graph has a well-defined set of faces,
assigning a 2D-coordinate to each vertex such that straight-line drawings of the edges do not cross. For planar graphs in general, planar embeddings are not unique. But whenever at least three vertices must be removed before the graph separates into two disconnected components, the graph is called three-connected, and there is essentially only one wayd to embed it in the plane.48 Because of this, a three-connected planar graph has a well-defined set of faces,  . We can therefore represent a three-connected planar graph as
. We can therefore represent a three-connected planar graph as  without ambiguity, and will do so where convenient.
without ambiguity, and will do so where convenient.
Whenever a graph is planar, it can also be embedded onto a surface of a sphere without edge crossings, and vice versa. Once again, this embedding is unique (in the same sense as for a planar embedding) if and only if the graph is three-connected, and we obtain the same faces. In fact, the graphs describing three-dimensional polyhedra are exactly the three-connected planar graphs,49 and are therefore called polyhedral graphs.
The number of neighbors to a vertex v, that is, the number of edges incident to v, is called its degree, abbreviated as deg(v). If every vertex of a graph G has the same degree k, then G is called k-valent or k-regular. The special case of three-valent graphs is called cubic, and has a host of attractive mathematical properties, not least of which is their deep connections to algebraic geometry.19
Fullerenes have the neat property that the graphs formed by their bond structure are both cubic, planar, and three-connected, for which all faces are either pentagons or hexagons. Because of this, the mathematics describing them is in many cases both rich, simple, and elegant. We are able to derive many properties about their topologies, spatial shapes, surface, as well as indicators of their chemical behaviors, directly from their graphs.
Planar connected graphs fulfil Euler's polyhedron formula,
| 1 |
with  being the number of vertices (called the order of the graph), E = |
being the number of vertices (called the order of the graph), E = | | the number of edges, and F = |
| the number of edges, and F = | | the number of faces (for fullerenes these are hexagons and pentagons). The hand-shaking lemma in graph theory tells us that
| the number of faces (for fullerenes these are hexagons and pentagons). The hand-shaking lemma in graph theory tells us that
| 2 |
Since for fullerenes deg(vi) = 3 for all vertices, we obtain50
| 3 |
Hence, for fullerenes and fulleroidse one has an even number of vertices. The total number of faces is F = F5 + F6, where F5 and F6 are the number of pentagons and hexagons respectively. From this we derive from Eq 3
E = 3F5 + 3F6 − 6 and N = 2F5 + 2F6 − 4. On the other hand, each pentagon (hexagon) has five (six) edges, which gives E = (5/2)F5 + 3F6. From these equations together with Eq 1 we obtain F5 = 12 for regular fullerenes (the ‘12 Pentagon Theorem’ for fullerenes), which gives the number of hexagons as F6 = (N − 20)/2 with N ≥ 20, and the general formula  for fullerenes. Hence, from the fact that we only allow for cubic planar graphs consisting of pentagons and hexagons, we restrict the number of pentagons to exactly 12 (no more and no less!), and every addition of a hexagon adds two new vertices. Moreover, N = 22 with just 1 hexagon and 12 pentagons is not valid, but every number of hexagons two or greater yields at least one fullerene.51 The most stable C22 molecule is, however, a cage-like fulleroid with a four-membered ring.52
for fullerenes. Hence, from the fact that we only allow for cubic planar graphs consisting of pentagons and hexagons, we restrict the number of pentagons to exactly 12 (no more and no less!), and every addition of a hexagon adds two new vertices. Moreover, N = 22 with just 1 hexagon and 12 pentagons is not valid, but every number of hexagons two or greater yields at least one fullerene.51 The most stable C22 molecule is, however, a cage-like fulleroid with a four-membered ring.52
In this article, we will often work with the dual graph. The dual graph G* of a planar connected graph G has a vertex corresponding to each face of G, and an edge joining two neighboring faces in G. By this process, the old vertices become the new faces. The dual of a planar graph is itself a planar graph, and for fullerenes it is a triangulation with 12 vertices of degree 5 and the remaining of degree 6. For planar graphs in general, the dual is not unique, but as discussed above, it is unique for three-connected graphs such as fullerenes, and hence the dual operation is well defined.48 The dual operation is an involution, that is, (G*)* = G, so we can think of the dual as just another representation of the same graph. Figure 3 shows the duals of two representative fullerenes with their corresponding 2D graph representation discussed in the next section.
Figure 3.

Planar embeddings of fullerene graph and dual (blue color and dotted lines for the dual representation), and 3D embeddings of the duals: (a) C20-Ih, for which the dual is the icosahedron; (b) C60-Ih, for which the dual is the pentakis-dodecahedron.
A useful representation of a graph is the adjacency matrix Aij well known to chemists, as it is widely used in Hückel theory of conjugated organic molecules. The adjacency matrix is a symmetric matrix defined by Aij = 1 if the graph G contains an edge vi − vj, and Aij = 0 otherwise. It is equivalent to the edge-set representation, and is much easier to work with in many settings. As the number of edges for fullerenes is E = 3N/2, the adjacency matrix is very sparse. If two graphs G1 and G2 are isomorphic, the corresponding adjacency matrices A1 and A2 are related by vertex permutation, and thus share the same set of eigenvalues and eigenvectors. Matrices play a special role in chemical graph theory, and the reader is referred to Ref 53 for more details.
Drawing Fullerene Graphs: Methods for Planar Embedding
Planar embeddings can be drawn on paper and give us a way to visualize the structures of planar graphs. However, not all choices of embeddings are equally informative. We want the drawings to be simple, not too cluttered, and to expose as much as we can of the graph's structure and symmetry. In general the full symmetry group can not be realized in two dimensions, but we can find embeddings that show as much of it as possible. Figure 4 shows planar drawings of three icosahedral fullerenes, C20 - Ih, C60 - Ih, and C540 - Ih, exposing 10-fold D5-symmetry out of the full Ih group. In this section, we will discuss methods for constructing good planar embeddings.
Figure 4.

Planar embeddings (no crossing edges) for three different fullerenes obtained from a perspective projection. (a) icosahedron C20-Ih; (b) truncated icosahedron C60-Ih; (c) C540-Ih.
While a small graph can easily be drawn by hand, larger graphs such as C540 shown in Figure 4(c) require a computer algorithm and a drawing program. The first method we will discuss is called the Schlegel projection, and requires first an embedding of the graph as a three-dimensional polyhedron. In 1886, Victor Schlegel showed that a planar graph can be obtained from a three-dimensional polyhedron by projection in the following way: A point  is placed slightly above one of the faces, and a plane P is chosen to lie below the polyhedron. Lines are then drawn from
is placed slightly above one of the faces, and a plane P is chosen to lie below the polyhedron. Lines are then drawn from  through each of the polyhedron's vertices. The intersection of each line
through each of the polyhedron's vertices. The intersection of each line  with P defines the 2D coordinate of the vertex v. If
with P defines the 2D coordinate of the vertex v. If  is chosen sufficiently close to the face, and if the points of the polyhedron lie on a sphere, the resulting 2D embedding will be planar. While Schlegel only proved that the projection produces a planar layout when the vertices lie on a sphere, it still works in many cases where this is not the case, and the method is widely used to the extent that planar graph embeddings sometimes (incorrectly) are called Schlegel diagrams. However, Schlegel diagrams are specifically the embeddings that result from the Schlegel projection. The Schlegel projection is illustrated in Figure 5(a).
is chosen sufficiently close to the face, and if the points of the polyhedron lie on a sphere, the resulting 2D embedding will be planar. While Schlegel only proved that the projection produces a planar layout when the vertices lie on a sphere, it still works in many cases where this is not the case, and the method is widely used to the extent that planar graph embeddings sometimes (incorrectly) are called Schlegel diagrams. However, Schlegel diagrams are specifically the embeddings that result from the Schlegel projection. The Schlegel projection is illustrated in Figure 5(a).
Figure 5.

(a) Schlegel projection of C60-Ih. (b) Cone projection of disk-shaped C72-D6d.
A problem with the Schlegel projection is that it often fails, and produces crossing edges, when used with many non-spherical polyhedra or when the parameters are not well chosen. The cone-projection method, shown in Figure 5(b), which projects the vertices out on an enveloping cone of the fullerene polyhedron and then down onto a plane, seems to be more robust.35
Both the cone-projection and the Schlegel projection require first a polyhedral embedding of the graph, and on top of that often necessitate bit of hand-tuning to produce a planar embedding. The third method we mention is called the Tutte embedding,54 and is guaranteed to always yield a planar embedding for three-connected planar graphs. In the Tutte embedding, one fixes the coordinates of a single, outer face, and solves a sparse linear system
| 4 |
such that in the result, every vertex is placed in the barycenter of its neighbors. For fullerenes, Tij = δij − Aij/3 and  for every i where vi is not a fixed vertex, and for the vertices vi on the outer face, Tij = δij and
for every i where vi is not a fixed vertex, and for the vertices vi on the outer face, Tij = δij and  is the fixed coordinate. While the Tutte embedding is guaranteed to be planar, the solution to the linear system results in exponential crowding of the vertices when embedding large graphs, making the result difficult to interpret. Hence, it is useful to follow the Tutte embedding by an optimization to make the face sizes more even.35 We note that there are many different graph drawing algorithms available, each with advantages and disadvantages, but none that are guaranteed to always yield good results.54–57
is the fixed coordinate. While the Tutte embedding is guaranteed to be planar, the solution to the linear system results in exponential crowding of the vertices when embedding large graphs, making the result difficult to interpret. Hence, it is useful to follow the Tutte embedding by an optimization to make the face sizes more even.35 We note that there are many different graph drawing algorithms available, each with advantages and disadvantages, but none that are guaranteed to always yield good results.54–57
The Large Isomer Space
How does the number of CN-isomers grow as we increase the number of vertices N? Naively one might assume a hypothetical sphere of ‘connected’ hexagons (which is of course impossible because of Euler's theorem) and substitute 12 hexagons by pentagons to obtain a set containing all possible fullerene isomers fulfilling Euler's theorem (just like playing Lotto), we obtain an upper bound for the number of isomers of the order  . This is, however, three orders of magnitude too large, but the proof is nontrivial. In 1998, William Thurston showed that all the triangulations of the sphere with vertex degree at most 6 form a lattice of integer points in a complex hyperbolic space C(1, 9) through a parameterization as described in Ref 19. The triangulations with N vertices or less correspond to the lattice points inside the intersection between a certain cone and a ball of radius
. This is, however, three orders of magnitude too large, but the proof is nontrivial. In 1998, William Thurston showed that all the triangulations of the sphere with vertex degree at most 6 form a lattice of integer points in a complex hyperbolic space C(1, 9) through a parameterization as described in Ref 19. The triangulations with N vertices or less correspond to the lattice points inside the intersection between a certain cone and a ball of radius  in this space. Hence their number is roughly proportional to N10, and the number of triangulations with exactly N vertices is roughly proportional to
in this space. Hence their number is roughly proportional to N10, and the number of triangulations with exactly N vertices is roughly proportional to
| 5 |
or, in short,  . Because they are the largest class of the finitely many triangulation classes with vertex degree six or less, the fullerenes have the same asymptotic count. Hence, the number of CN isomers grows as
. Because they are the largest class of the finitely many triangulation classes with vertex degree six or less, the fullerenes have the same asymptotic count. Hence, the number of CN isomers grows as  . We note in passing that the connection of fullerenes to combinatorial manifolds is a deep one and gives rise to many of the beautiful mathematical properties exhibited by fullerenes.
. We note in passing that the connection of fullerenes to combinatorial manifolds is a deep one and gives rise to many of the beautiful mathematical properties exhibited by fullerenes.
The high order polynomial growth of fullerene isomer counts makes it difficult to establish a complete database for fullerene graphs up to high vertex numbers.34 Moreover, the proportion of IPR isomers to the total number of isomers grows with increasing N, gradually approaching 1 with increasing N, as is shown on Figure 4.
Face Spiral Representations of Fullerene Graphs
One of the first methods for encoding fullerene graphs was the face spiral algorithm by Manolopoulos et al.,58 which unwinds all faces of a fullerene ‘like an orange peel’ (the Manolopoulos face spiral algorithm). More specifically, starting with a sequence of three mutually adjacent faces, new faces are added to the string such that the next face is adjacent to the previous one and the one that was added to the string earliest, and that has neighboring faces left which are not part of the spiral string (yet). As the result, one obtains a string of length F of 12 fives and F − 12 sixes, corresponding to the sizes of faces.
There are 6N possible spiral starts resulting in up to 6N spirals per fullerene graph. In most fullerenes, some spirals cannot be completed because the two rules for choosing the next face cannot be both fulfilled. Since a fullerene spiral always consists of 12 fives and N/2 − 10 sixes, this spiral string can be abbreviated as a list of the 12 pentagon positions, called the face spiral pentagon indices (FSPI) {Sn|n = 1, …, 12}. The lexicographically smallest of all successful spirals is the canonical spiral representation of the graph. For example, for C60 we have the canonical FSPI of {1, 7, 9, 11, 13, 15, 18, 20, 22, 24, 26, 32} as shown in Figure 6.31
Figure 6.

(a) Canonical face spiral for C60-Ih. (b) The first fullerene (C28-D2) with a failing face spiral.
While it was initially conjectured that every fullerene can be unwound into a face spiral58 (for good reason: the first counterexample comes after over 1012 isomers) there is an exceedingly small proportion of fullerene graphs59 (but infinitely many in total) that do not admit a face spiral,60 that is, where all 6N spiral starts fail. See Figure 6 for a case where a face spiral fails. The smallest of these unspiralable fullerenes is C380 - T.61 Manolopoulos et al.,60 Fowler et al.,62 Yoshida et al.,63 and Brinkmann et al.64 compiled lists of non-spiral fullerenes with up to 1000 atoms and selected point groups (all T+, all isolated pentagon triple (IPT) fullerenes with D2 and T symmetry, all D3 +, and all truncated IPT fullerenes respectively). Two of those are shown in Figure 7.
Figure 7.

B3LYP optimized structures for the two smallest non-spiralable fullerenes (a) C380-T and (b) C384-D3 (see Ref 59 for details).
In order to encode any fullerene graph, it is necessary to extend the face spiral algorithm to a general version in which the spiral may ‘jump’ from one face to a non-adjacent face. Both Brinkmann65 and Fowler et al.66 published general face spiral algorithms for cubic polyhedral graphs, including fullerene graphs. Fowler's algorithm extends the two above mentioned rules such that the subgraph that has not been added to the spiral yet never gets disconnected, and as a result the spiral never gets stuck. Brinkmann's algorithm, in contrast, adds faces as defined by the non-general version, but defines how to proceed if all neighbors of the current face have been added to the spiral already. Both algorithms yield the same spiral string as the non-general algorithm if no jump is required.
For the non-general as well as both general algorithms for finding the canonical face spiral of a graph, there is an inverse algorithm that efficiently regenerates the graph from the (general) spiral string representation. With the extension to general spirals, all fullerene isomers with any vertex number can be generated. In addition, all the information of the fullerene graph is contained in a general face spiral consisting of 12+ numbers (12 for most fullerenes). This makes it rather convenient for database storage as the face spiral uniquely defines the fullerene.
In a similar way to face-spirals we can introduce vertex spirals (or Hamilton spirals) useful for identifying locations of substituents (such as hydrogen) or reaction centers67 (for details see Ref 68). For fullerenes with no vertex spirals the scheme can be extended to general spirals with jumps similar to general face spirals.
Generation of Fullerene Graphs
In order to explore chemical, physical, or graph theoretical properties for a wide range of fullerenes, it is important to have access to a list of (stored) fullerene graphs. For this, one requires an exhaustive and efficient generator for all fullerene isomers of a given vertex number N. The general face spiral algorithm is well suited for compactly storing and recreating specific fullerene graphs. It also allows for sorting all isomers of CN for a given vertex number N according to their FSPIs including information about jumps if required. It is, however, extremely inefficient to generate all isomers CN directly through a face spiral algorithm. In the non-general case one attempts to create graphs from  spiral strings while the number of non-isomorphic graphs only grows ∼ N9. A large and quickly growing portion of all potential spiral strings are either non-canonical spirals, duplicates or are not successful for coding a fullerene. Including general spirals increases the overhead by two orders of magnitude, as jumps could be inserted at any step and with arbitrary lengths l < N.
spiral strings while the number of non-isomorphic graphs only grows ∼ N9. A large and quickly growing portion of all potential spiral strings are either non-canonical spirals, duplicates or are not successful for coding a fullerene. Including general spirals increases the overhead by two orders of magnitude, as jumps could be inserted at any step and with arbitrary lengths l < N.
A different approach to generating fullerene graphs is by adding faces to an existing graph, while considering different sites for addition at each step. Liu et al.69 introduced a method which grows a graph starting with a single face and thus only reaches a fullerene graph in the last step. Brinkmann et al.70 use patches, rather than single faces, as building blocks. These patches can be obtained by subdividing existing fullerene graphs.
Further development in efficient graph generation came from transforming an existing fullerene graph into a new, larger one by adding faces. Using C24 as a ‘seed’, Brinkmann et al.71 were able to generate almost all fullerene graphs up to C200. However, three graphs were not accessible by the applied set of transformations. To cure this shortcoming, Hasheminezhad et al.72 defined a set of patch replacements (or growth operations) which, starting from either C20 or C28 - Td, yield every fullerene in a systematic way. The set consists of one single operation that elongates a nanotube with minimal caps, one class of linear generalized Stone-Wales transformations73,74 that depend on one parameter, and one class of bent generalized Stone-Wales transformations that depend on two parameters. Brinkmann et al.75 used this set of growth operations to define a fast and complete algorithm that recursively generates all fullerene isomers up to a given maximal N. The algorithm prunes the recursion tree in such a way that the only one representative of each isomorphism class is ever considered. Without such a scheme, exhaustively generating all fullerene graphs would succumb to combinatorial explosion.76 The result is an incredibly efficient algorithm which has been used to generate an exhaustive database up to C400, which is available at the House-of-Graphs website.34 Sometimes one is not interested in generating exhaustive isomer lists (which are huge for large N), but rather to generate in a directed fashion fullerenes that have specific properties. For this, one may start with one specific isomer CN and derive others by certain transformations. This will be discussed in the following section.
Transformation of Fullerene Graphs
Transformations of fullerene graphs can be divided into local transformations that leave all but a certain region of the graph unchanged, and global transformations such as the Goldberg-Coxeter transformation.77,78 A planar graph can be transformed locally by replacing one fullerene patch74,79–84 by either a different patch or the same patch in a different orientation. A patch is a set of faces that is bounded by a simple cycle,82,85 that is, a cycle that traverses no vertex or edge twice. Some authors relax this condition and allow cycles that are non-selfintersecting, but are permitted to traverse any edge at most twice. This definition includes patches that have bridges, and is used in the complete characterization of fullerenes by Hasheminezhad et al.72
Two patches can be exchanged if they share the same boundary code. The boundary code of a patch is the sequence of free valencies of the vertices that lie on the boundary (in case of cubic graphs, a sequence of zeros and ones). A few patch replacements are shown in Figure 8.
Figure 8.

(a) Endo-Kroto (EK) 2-vertex insertion. (b) Stone-Wales (SW) transformation. (c) Example of an extended Stone-Wales transformation. (d) A patch replacement which introduces one heptagon.
Replacing patches of equal size can be understood as a formal isomerization, while replacement by larger or smaller patches, referred to as vertex insertion or deletion, or growth operations, formally derives a molecular graph of a different size. Two fullerene graph patches with the same boundary code contain the same number of pentagons, as can be seen from Euler's polyhedron formula. The most prominent example for formal isomerizations is the Stone-Wales (or pyracylene) transformation (SW),86 which can be generalized73,87 by allowing a variable distance between the two pentagons. Astakhova et al.88 extended this rearrangement mechanism even further to higher numbers of pentagons. Brinkmann et al.83 generated a catalog of isomerization pairs with up to five pentagons.
Isomerization operations can be reformulated as sequences of vertex-pair switches, called 2-switches.89 According to Berge's switching theorem,90 the whole isomer space of a given CN is accessible by repeated application of 2-switches. However, intermediate structures of this procedure might have faces different from pentagons and hexagons, or result even in non-planar graphs.
Growth operations can be classified according to the number of pentagons and the number of vertices that are added. There are no growth operations for fullerene graphs that involve no or only one pentagon.81 The smallest example is the Endo-Kroto transformation (EK),91 which involves two pentagons and adds two vertices. Additional noteworthy examples are the addition of four and six vertices at a patch that contains three pentagons and has C3 symmetry.92 Brinkmann et al.84 compiled an extensive list of growth pairs. The three classes of patch replacements defined by Hasheminezhad et al.72 form the basis of the currently fastest fullerene graph generator, as discussed in a previous section.75
With respect to the polyhedral representation of a fullerene graph, growth operations can be understood as the capping of a domain by additional vertices. As a result the pentagons in that domain move toward each other. Patches in which all pentagons are fused cannot be capped. Conversely, the inverse of a growth operation corresponds to the truncation of a domain of high curvature: The distances between the affected pentagons increase.
While patch replacements are useful from a graph theoretical point of view to obtain new fullerene isomers, the EK C2 insertion and the SW transformation have also been suggested to resemble viable reaction pathways.86,91,93–95 As Stone and Wales pointed out in their initial article, a concerted mechanism for the SW transformation ([2+2]) is Woodward-Hoffmann forbidden in the electronic ground state.86 Other mechanisms that involve the breaking of one bond93,96,97 or the catalysis by additional carbon atoms98 are therefore more likely. For a discussion of different mechanisms see Ref 93. For every suggested pathway, however, the activation barrier is so high that SW transformations are only feasible at very high temperatures.93,94 The SW transformation is believed to be one of the main mechanisms by which fullerene cages equilibrate during formation to form the most stable isomer.99,100 Starting at C60 - Ih, 1709 out of the 1811 other isomers are accessible by consecutive SW transformations.101
Endo and Kroto proposed a concerted mechanism for what has since been known as the EK C2 addition.91 However, more recent experimental and theoretical investigations show, that the concerted reaction leads to an unstable adduct and is followed by C2 ejection.102 For a summary of alternative mechanisms of the C2 addition to fullerene cages see Ref 95.
Given any fullerene, it is possible to construct an infinite series of larger ones with (essentially) the same three-dimensional shape. This is possible through the Goldberg-Coxeter transformation, named after an infinite series of icosahedral-symmetry polyhedra described by Goldberg in 1937.77 By superimposing a hexagonal mesh on the surface of the dodecahedron, as shown in Figure 9(a), a new polyhedron is obtained with the same number of pentagons and an increased number of hexagons. The number of vertices in the new polyhedron is k2 + kl + l2, where k and l are integers describing the scale and orientation of the mesh, as shown for the dual in Figure 9(b). The construction used by Goldberg was discovered independently, and applied to the shapes of vira, by Caspar and Klug in the 1960s, and later popularized by Coxeter.78
Figure 9.

Illustration of GC-transform. (a) Original figure of a hexagonal sheet from Goldberg's paper.77 (b) The GC-transform acting on a face in the fullerene dual for various values of k and l.
While originally only defined for C20, yielding exactly all the fullerenes of icosahedral symmetry, Dutour and Deza have shown that it is well defined for all cubic planar graphs.103 However, it is not trivial to determine how to automatically perform the general transform, the main difficulty lying in how to ‘glue’ the graph back together when the new edges cross the transformed triangles in complex ways. The general construction by Dutour and Deza is quite algebraically heavy handed, and is not easily understood, nor lends itself easily to implementation. In the case of fullerenes, however, it is quite easy to give a procedural description of the transformation in terms of the fullerene dual: Because a fullerene dual is a triangulation of the sphere with no vertices of degree more than 6, it can be unfolded onto the plane of equilateral triangles, called the Eisenstein plane. The unfolded surface forms a polygon in the Eisenstein plane with every degree 5 vertex on the polygon periphery, and with each edge on the periphery appearing twice, once in each direction.
The vertices in the Eisenstein triangulation is a sub-ring of the complex plane, and we can write
| 6 |
Because it is a sub-ring, multiplication by Eisenstein integers (a, b) are exactly the operations that bring (1, 0) into every other vertex point. Because ω2 = ω − 1, we get the multiplication rule
| 7 |
and the magnitude of (a, b) is (a + bω)(a + bω− 1) = a2 + ab + b2. Hence, if T is an equilateral triangle with unit area, the area of (k, l)T is k2 + kl + l2. This is no coincidence: multiplication in the Eisenstein ring exactly corresponds to the Goldberg-Coxeter transformation in the dual formulation. This yields a simple and practical formulation of the Goldberg-Coxeter transform of any triangulation of the sphere with deg(v) ≤ 6:
| 8 |
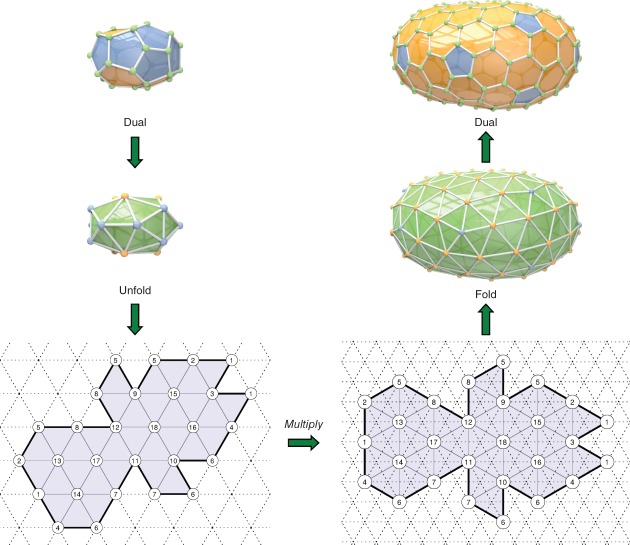
The procedure is illustrated in Figure 10. Details for how to efficiently perform the fold and unfold operations are given in Avery (unpublished manuscript). Note that two successive GC-transformations on a graph G* can be expressed as a single GC-transformation by multiplying the two Eisenstein numbers together. Similarly, a GC-transformation can be inverted by dividing instead of multiplying. For any particular fullerene, we can even use Euclid's algorithm to find out whether it is a GC-transform of a smaller fullerene, and to find the smallest such ‘parent’ fullerene.59
Figure 10.
The steps in the GC2,1 Goldberg-Coxeter transformation from C32-D3h(5) to C224-D3h. The transform and the diagrams of the unfolded fullerenes were automatically generated using the program Fullerene.35
Notice, that if one were to cut out the two diagrams in Figure 10, gluing together the edges so that the numbers on the vertices match, one obtains the three dimensional structure of the given C32 and C224 fullerenes. The reader is invited to do so.
There are two special cases of the Goldberg-Coxeter transformation that warrant closer study: the case l = 0, named the halma transform (or k-inflation) for the construction's similarity to the game board of halma, or ‘Chinese Checkers’; and the case k = l = 1, called the leapfrog transform. Both of these types of Goldberg-Coxeter transformations are simple to understand: every triangle is subdivided and reconnected the same way, as illustrated on Figure 11. While the GC-transform in general can introduce, reverse, or remove chirality (but otherwise preserves symmetry), both the halma and leapfrog transformation always preserve symmetry.
Figure 11.

Subdivision of a triangle in the dual graph; (a) for the leapfrog transformation (k = l = 1), and (b) for the lowest few halmas (l = 0).
Geometry of Fullerenes
Many of the beautiful properties of fullerenes derive from their relation to algebraic and differential geometry. This relation is mostly out of scope for this review, but in this section we will touch on the subject informally. The subject is treated in great depth by Thurston19 and others.
An important quantity for understanding the geometry and shapes of fullerenes is the Gaussian curvature. The Gaussian curvature is the product of the two principal curvatures, which for each point on the surface are the maximal and minimal curvatures in any direction through that point. By the Bertrand-Diquet-Puiseux theorem, the Gaussian curvature in a point is the same as the difference between 2π and the angle required to make a circle in a neighborhood around this point. Figure 12 illustrates three categories of surfaces with zero, positive, and negative Gaussian curvature respectively. If the Gaussian curvature is zero in a point, the surface only bends in one direction around that point. A surface that has zero Gaussian curvature everywhere is characterized by being ‘flat’ in the sense that it can be unwrapped onto a plane without tearing. Positive Gaussian curvature bends the same way in all directions. A positive curvature surface can be cut open and unwrapped onto a plane. Finally, negative curvature around a point constitutes a saddle point, yielding a wobbly pringle-like surface. Negative curvature surfaces cannot be unwrapped onto a plane, because there is no room: around a negative curvature point, the angle of a circle is greater than 2π. If one were to make a cut in a negative curvature surface, it could only be flattened out by allowing parts of it to overlap itself.
Figure 12.

Examples of surfaces with zero (a), positive (b), and negative (c) Gaussian curvature around a point.
The Gaussian curvature only depends on the topology of the surface, and is independent of how it is isometrically embedded in space. In the same way, the surface metric (or Riemann metric), which determines the geodesics and distances between points along the surface, can be derived directly from the graph (Avery et al., unpublished manuscript), and is also independent of the spatial embedding. However, while we do not require a 3D embedding to analyze these surface properties, they themselves do determine the possible ways that the surface can embed isometrically into space, and what is its ‘natural’ three-dimensional shape.
Fullerenes are all closed surfaces with non-negative Gaussian curvature everywhere. Surprisingly, this is simply due to them having faces no larger than hexagons! In fact, the slightly larger class of three-connected cubic planar graphs with largest face size 6, and their dual triangulations, are in a sense all the positive curvature genus 0 surfaces.f
It is most easy to understand the geometry of fullerenes when considering their duals. These are equilateral triangulations of a closed surface. The equilateral triangle plane, also called the Eisenstein plane, is the dual of a hexagonal mesh. Hexagon planes (like graphene) and their dual degree-six triangulations are flat in the sense that they have Gaussian curvature 0 everywhere: any surface of equilateral triangles with vertex degree only six can be unwrapped into the plane without stretching or tearing. What happens now if we set the degree to 5 of a single vertex in the plane? To do this, we must cut out an infinite triangular wedge of angle 2π/6. Gluing together the edges forms an infinite cone with Gaussian curvature zero everywhere except the degree 5 vertex at the cone's apex, which introduces the positive curvature 2π/6. Every time we introduce a vertex of degree 5 (or a pentagon in the hexagonal plane), it introduces a surface cut that is glued together to locally introduce the positive Gaussian curvature 2π/6 at the vertex. After 12 pentagons we reach 4π, the Gaussian curvature of the sphere, closing the surface.g Unfolding again along the 12 cuts would result in a polygon similar to the diagrams shown in Figure 10. Had we instead introduced, for example, degree 4 vertices, each would contribute with curvature 2π/3, requiring only 6 to close the surface. The positions at which we placed the 12 pentagons determine where and how strongly the surface bends, and through that the natural shape of the fullerene, giving rise to the many interesting polyhedral shapes shown in Figure 12.
Because fullerenes always have exactly 12 curvature-2π/6 bends, with the remaining surface of Gaussian curvature zero, they adopt polyhedral shapes rather than being spherical in nature (the latter defined as atoms lying on a sphere), and the most symmetric shapes that they can achieve have icosahedral symmetry. The non-spherical nature of fullerenes was pointed out already by Bakowies and Thiel in 1995.104,105
The curvature of cubic graph surfaces also provides an intuitive understanding of why fullerenes, having only hexagon and pentagon faces, would be more prevalent in nature than other polyhedral carbon structures: smaller face sizes introduce much sharper bends, reducing stability. Heptagons by themselves introduce no sharper bends than pentagons, but each heptagon necessitates an extra pentagon to balance out the negative Gaussian curvature and reach a closed surface, increasing the total number of bends by two. However, with the right distribution of heptagons and pentagons, it is possible to construct quite smoothly curving near-spherical fulleroid surfaces that could potentially be stable in nature. We discuss fulleroids, a generalization of fullerenes that allow arbitrary face sizes, in the final section.
Generating Accurate 3D Geometries
The Cartesian coordinates for the ideal molecular geometry of small highly symmetric fullerenes such as C20 - Ih or C60 - Ih can be calculated directly using basic geometry.106 This is not the case for fullerenes in general: They come in many different shapes, most of which do not have a simple mathematical formula. However, we do know that the ideal geometry (in which the faces of the dual polyhedron are equilateral triangles) can be derived directly from the fullerene graph. This is because of their special properties discussed above. However, while we know that a fullerene graph determines the ideal 3D structure as a conformal, isometric embedding of the surface into space, it is not yet known how to compute it directly. However, we can use numerical optimization methods to obtain good 3D structures—both efficiently and with results that are very close to the physical molecular geometry.
Generating Initial Structures
Before optimizing a fullerene structure by for example a force-field method, we need a reasonable initial structure. Specifically, we need an embedding of the graph as a polyhedron (i.e., no crossing edges) such that its faces are the same ones as in the final polyhedron, and such that the magnitude of the force experienced by the vertices does not vary too greatly, causing slow convergence or numerical instability. We here outline two methods for obtaining initial geometries that are suitable for input to the fullerene force-field optimization.
The Fowler-Manolopoulos 3D embedding algorithm (also called Adjacency Matrix Eigenvector or AME algorithm) diagonalizes the N × N adjacency matrix Aij of the fullerene graph to obtain eigenvectors  , of which three ‘P-type eigenvectors’ with one nodal plane are chosen to construct the Cartesian coordinates (termed topological coordinates by Manolopoulus and Fowler) for the initial structure.31,107 This often yields suitable coordinates because fullerenes can be modeled as spherical aromatic systems fulfilling approximately the Laplace differential equation with real spherical harmonics Ylm(θ, ϕ) as eigenfunctions, of which the three Ylm (l = 1) are associated with the coordinates (xi, yi, zi).31,108 Cartesian coordinates constructed in this way already reflect the space symmetry of the fullerene. The P-type eigenvectors can easily be found for near-spherical fullerenes like C20 or C60 - Ih, because the sequence of eigenvalues mimics the spherical harmonics. However, for distorted fullerenes, or for very large fullerenes where the spectrum of the adjacency matrix becomes very dense with quasi-degeneracies, it becomes difficult to find the appropriate eigenvectors, and the assumption that the eigenvectors resemble spherical harmonics becomes decreasingly valid. For example, for large fullerene nano-tubes aligned around the z-axis, such as the one shown in Figure 12, the eigenvalues belonging to the Px- and Py-type eigenvectors do not lie close to the Pz eigenvalue. Hence, one must search for the appropriate set of eigenvectors, and identifying them is not always easy.109–111 Second, the bond lengths tend to vary substantially in the constructed fullerene, leading to unreasonable large bond distances once the minimum bond distance is set to ≈1.4 Å, and a scaling procedure for the eigenvectors may only solve part of this problem.31 Finally, because it is often necessary to search for the right eigenvectors and hence calculate a large number of them, the algorithm scales up to
, of which three ‘P-type eigenvectors’ with one nodal plane are chosen to construct the Cartesian coordinates (termed topological coordinates by Manolopoulus and Fowler) for the initial structure.31,107 This often yields suitable coordinates because fullerenes can be modeled as spherical aromatic systems fulfilling approximately the Laplace differential equation with real spherical harmonics Ylm(θ, ϕ) as eigenfunctions, of which the three Ylm (l = 1) are associated with the coordinates (xi, yi, zi).31,108 Cartesian coordinates constructed in this way already reflect the space symmetry of the fullerene. The P-type eigenvectors can easily be found for near-spherical fullerenes like C20 or C60 - Ih, because the sequence of eigenvalues mimics the spherical harmonics. However, for distorted fullerenes, or for very large fullerenes where the spectrum of the adjacency matrix becomes very dense with quasi-degeneracies, it becomes difficult to find the appropriate eigenvectors, and the assumption that the eigenvectors resemble spherical harmonics becomes decreasingly valid. For example, for large fullerene nano-tubes aligned around the z-axis, such as the one shown in Figure 12, the eigenvalues belonging to the Px- and Py-type eigenvectors do not lie close to the Pz eigenvalue. Hence, one must search for the appropriate set of eigenvectors, and identifying them is not always easy.109–111 Second, the bond lengths tend to vary substantially in the constructed fullerene, leading to unreasonable large bond distances once the minimum bond distance is set to ≈1.4 Å, and a scaling procedure for the eigenvectors may only solve part of this problem.31 Finally, because it is often necessary to search for the right eigenvectors and hence calculate a large number of them, the algorithm scales up to  , which quickly becomes a heavy operation as N grows.
, which quickly becomes a heavy operation as N grows.
We have found it useful to sacrifice some accuracy in the initial geometry for a method that is more stable, and which scales approximately as  . The fullerene force-field optimization is insensitive enough to the starting geometry that the difference is not noticeable. The method starts with any planar embedding of the fullerene graph. We choose the Tutte-embedding, which is guaranteed to be planar, and can be computed in near-linear time by solving two sparse linear systems of equations.54 The planar embedding is then mapped onto the surface of a sphere (or e.g., ellipsoid, if the fullerene is a priori known to be elongated) in the following way: For every vertex v, let the topological distance from the outer face (the ‘depth’ of the vertex) be dv, and its coordinate in the Tutte-embedding be
. The fullerene force-field optimization is insensitive enough to the starting geometry that the difference is not noticeable. The method starts with any planar embedding of the fullerene graph. We choose the Tutte-embedding, which is guaranteed to be planar, and can be computed in near-linear time by solving two sparse linear systems of equations.54 The planar embedding is then mapped onto the surface of a sphere (or e.g., ellipsoid, if the fullerene is a priori known to be elongated) in the following way: For every vertex v, let the topological distance from the outer face (the ‘depth’ of the vertex) be dv, and its coordinate in the Tutte-embedding be  . Let D be the maximum depth, and
. Let D be the maximum depth, and  be the barycenter in the embedding. We assign one angle ϕ(v) = (dv + 1/2)π/D + 1, placing vertex layers equidistantly along the ϕ-angle according to their depth. The second angle θ(v) is the angle of
be the barycenter in the embedding. We assign one angle ϕ(v) = (dv + 1/2)π/D + 1, placing vertex layers equidistantly along the ϕ-angle according to their depth. The second angle θ(v) is the angle of  around
around  . Then assigning the angles v ↦ (θ(v), ϕ(v)) yields an embedding on the sphere with no crossings, since the Tutte-layout is guaranteed to be planar, but which avoids the exponential crowding of the planar Tutte-embedding, such that the vertices are distributed in a reasonable manner across the surface of the sphere.h It remains, however, a challenge to embed the fullerene graph on a more realistic surface.
. Then assigning the angles v ↦ (θ(v), ϕ(v)) yields an embedding on the sphere with no crossings, since the Tutte-layout is guaranteed to be planar, but which avoids the exponential crowding of the planar Tutte-embedding, such that the vertices are distributed in a reasonable manner across the surface of the sphere.h It remains, however, a challenge to embed the fullerene graph on a more realistic surface.
Fullerene Force-Field
This section describes how to obtain good results for the molecular geometry numerically by way of specially tailored force-field optimization methods. This yields geometries that are very close to the ones obtained by long and computationally heavy quantum chemical calculations, as we shall see.
It is common for molecular force-field optimizations to distinguish between single and double bonds, even introducing different types of single and double bonds depending on atoms and groups in close proximity. For each bond, that is, edge in the graph, one sums over all the Kekulé structures and assigns fractional double bond character to each edge. This strategy is not practical for fullerenes, even for small cages like C60,112,113 due to exponential growth in perfect matchings and hence Kekulé structures, as discussed below. Force fields for fullerenes should therefore be designed in a way that avoids explicit treatment of double bonds. As a good approximation it is assumed that the properties of bonds, angles, and dihedral angles in a fullerene are determined by the size of their neighboring faces alone. The force fields discussed below are specifically designed for fullerenes based on this assumption.
The first force field tailored to fullerenes by Wu et al.114 was designed for C60 - Ih and uses harmonic force field terms. It distinguishes between two bond types: bonds adjacent to two hexagons, and bonds adjacent to a pentagon and a hexagon, which are optimized for bond lengths r0 with values of 1.54 Å and 1.41 Å, respectively. Angles are either part of a pentagon (θ0 = 3/5 π) or a hexagon (θ0 = 2/3 π). The force constants k1 through k4 are given (in 105 dyn/cm) as 11.0, 10.0, 1.0, and 1.0. No further parameters are taken into account.
In the following years a number of extensions and modifications to the Wu force field were published, taking into account an increased number of parameters. These extensions include improved force constants,115 torsions,116 angles and distances to second and third neighbors,117 mixed terms between distances and angles,118,119 the volume of the tetrahedron defined by an atom and its three neighbors,118 a complete force field for C60 - Ih,120 and a strategy for defining complete force fields for any deltahedron or trivalent polyhedron using mixed terms of distances and angles.121 Except for the force field by Ceulemans et al.,121 all above mentioned force fields have been exclusively designed for and applied to C60 - Ih.
The program Fullerene35 includes a 22-parameter force field with explicit dihedral angles. It takes into account three types of distances (bonds that are part of 0, 1, or 2 pentagons), two angles (part of a pentagon or a hexagon) and four types of improper dihedral angles, defined between a vertex and its three neighbors (where the starting vertex is part of 0, 1, 2, or 3 pentagons). It is applicable to all fullerenes and yields structures that are in very good agreement with DFT optimized structures. For example, for C380 - T, the rms error between the B3LYP/SVP and the force field optimized structure is only 0.038 Å. For many purposes, the force-field optimized molecular geometry, which is computed in milliseconds, is good enough to be used instead of a more computer time intensive quantum chemical optimization (although semiempirical methods including density functional based tight-binding are also very computer time efficient and have yielded good results122).
Fullerene Symmetry
There are two symmetry groups associated with a fullerene: the ideal or topological symmetry group of the fullerene graph, and the real or physical symmetry group of the molecule in 3D space. The physical symmetry group is a subgroup of the ideal one, due to (first or second order) Jahn-Teller distortions, variation of bond-lengths, or other effects from external perturbations. But because the shape is often only mildly altered from the ideal shape, the ideal symmetry group carries useful information about the physical system.
A surprising theorem by Mani123 shows that any three-connected cubic planar graph can be embedded in space as a convex polyhedron, the point group of which realizes the full symmetry group of the graph. That is: every graph automorphism of a fullerene is also a rotation or reflection of its ideal polyhedral shape. In addition, it can be shown124 that the point group for a fullerene must be one of the 28 point groups listed in Table1. The largest group is the icosahedral group Ih of order 120, and each of the 27 remaining attainable point groups are subgroups of Ih.
Table 1.
The 28 Possible Point Groups for Fullerenes Sorted According to Their Order  of the Group
of the Group  31
31
| Order | Point Groups | Order | Point Groups | Order | Point Groups |
|---|---|---|---|---|---|
| 120 | Ih | 60 | I | 24 | Td, Th, D6h, D6d |
| 20 | D5h, D5d | 12 | T, D6, D3h, D3d | 10 | D5 |
| 8 | D2h, D2d | 6 | D3, S6, C3h, C3v | 4 | D2, S4, C2h, C2v |
| 3 | C3 | 2 | C2, Cs, Ci | 1 | C1 |
Can we automatically determine the symmetry group for the fullerene directly from the graph, without referring to spatial coordinates? (The latter requires first finding a maximally symmetric 3D embedding) For general graphs, this is a difficult problem. However, in the case of cubic polyhedral graphs, for which general face spirals determine isomorphism, it is both simple and efficient to do so.
Before moving on, we take note of an ambiguity in terminology. The terms symmetry group and automorphism group often refer simply to the group formed by operations that leave the system invariant. In the group theoretical sense, a group is fully defined by its multiplication table. Point groups, however, are not just groups, but are isometries of space and carry additional information about how they transform space. For example, the point groups D6, C6v, D3d, and D3h are all the exact same group, but differ as point groups. It is unfortunate for this reason that ‘point groups’ are called ‘groups’. To make the distinction explicit, we will use the term abstract group for the symmetry group in the group theoretical sense, and either point group or symmetry group for the isometries.
Assume that we are given the fullerene graph dual G*, constructed from a generalized spiral S = (d1, d2, …, dF), and wish to compute the automorphism group  of G*. Since G* is constructed from S, the entries in S are the degrees of vertex number 1, 2, …, F in G*. For every vertex v of degree d1, we have 2d1 different spiral starts: d1 for clockwise and d1 for counter-clockwise traversal. If a spiral start (f1, f2, f3) unwinds G* to the input spiral S, there is an automorphism of G* that maps (1, 2, 3) ↦ (f1, f2, f3). These are all the automorphisms, and the number of starts that unwind to S is the order
of G*. Since G* is constructed from S, the entries in S are the degrees of vertex number 1, 2, …, F in G*. For every vertex v of degree d1, we have 2d1 different spiral starts: d1 for clockwise and d1 for counter-clockwise traversal. If a spiral start (f1, f2, f3) unwinds G* to the input spiral S, there is an automorphism of G* that maps (1, 2, 3) ↦ (f1, f2, f3). These are all the automorphisms, and the number of starts that unwind to S is the order  of the automorphism group. This construction lets us easily obtain the actual group: The spiral start determines the entire spiral, and by simply writing down the vertex names in G* while unwinding the spiral, we obtain a permutation representation of the group element
of the automorphism group. This construction lets us easily obtain the actual group: The spiral start determines the entire spiral, and by simply writing down the vertex names in G* while unwinding the spiral, we obtain a permutation representation of the group element
| 9 |
This is a faithful (but not irreducible) representation of the group, and if we wish, we can easily build the multiplication table by composing all pairs of the permutations. Similarly, we can calculate characters, irreducible representations, and all other properties, and we can identify the group. There are a number of ways to make this efficient. In Wirz et al. (unpublished manuscript), we present a general, but efficient,  -algorithm that works for all cubic polyhedral graphs, including all fullerenes and fulleroids.
-algorithm that works for all cubic polyhedral graphs, including all fullerenes and fulleroids.
The permutation representation πF allows us to not only find the abstract symmetry group, but contains enough information to identify the point group, that is, the isometries of the three-dimensional fullerene polyhedron. Fowler and Manolopoulos published a program for identifying the point group of any spiralable fullerene in Ref 31, based on site symmetries and counting group orbits. The symmetry points of interest in a fullerene are the vertices, midpoints of edges, the barycenter of the polygons and the whole cage, the latter having the full symmetry of the point group. They have certain site symmetries according to the rotational axes or mirror planes going through these symmetry points,31 which are collected in Table2. The full isometry group  , which correspond to the rotations, reflections, roto-inversions, and inversions that leave the ideal polyhedron invariant, also act as permutations of the symmetry points,107,125,126 the action always being a subgroup of
, which correspond to the rotations, reflections, roto-inversions, and inversions that leave the ideal polyhedron invariant, also act as permutations of the symmetry points,107,125,126 the action always being a subgroup of  . The permutations of the symmetry points in fact completely determine the point group symmetry, and we can find the full point group of the graph G (or, equivalently, its dual G*) as follows:
. The permutations of the symmetry points in fact completely determine the point group symmetry, and we can find the full point group of the graph G (or, equivalently, its dual G*) as follows:
Table 2.
The Site Symmetries of the Local Symmetry Points in a Fullerene31
| Symmetry Points | Site Symmetries (Order) |
|---|---|
| Vertices | C3v(6), C3(3), Cs(2), C1(1) |
| Edge centers | C2v(4), C2(2), Cs(2), C1(1) |
| Pentagon centers | C5v(10), C5(5), Cs(2), C1(1) |
| Hexagon centers | C6v(12), C6(6), C3v(6), C3(3), C2v(4), C2(2), Cs(2), C1(1) |
| Cage center | Full point group |
The order of the site-symmetry group is given in parentheses (the maximum value of any site symmetry group order is 12).
In step 1, one computes the face permutation representation πF of the abstract group, as described above. From this one derives vertex and edge permutation representations πV and πE by acting with every group operation on the dual graph.
In step 2 one computes the vertex, edge, and face group orbits by acting with the permutation representations on every vertex, edge, and face.127 Each orbit belongs to a certain site-symmetry group as shown in Table2. The site-symmetry groups are determined by the orbit sizes. For example, the site-symmetry group of the face fi has order  . By counting the number of sites belonging to each site-symmetry group, we obtain a signature that uniquely identifies the point group:
. By counting the number of sites belonging to each site-symmetry group, we obtain a signature that uniquely identifies the point group:
 |
10 |
The information can be condensed to a site-symmetry count for each site-group order:
| 11 |
In the final step, the point group is determined by the site-symmetry counts by the decision tree structure shown in Figure 13. The method can be extended to any fullerene and fulleroid by using general spirals. However, for every possible point group, the site-symmetry signature must be worked out and added to the decision tree.
Figure 13.

The decision tree for determining the symmetry point group for any fullerene from the group order and orbit counts.
Once the point group has been determined, the number of infra-red and Raman active lines, as well as the 13C NMR pattern can be derived.31,107 Moreover, point groups lacking an inversion center are further divided into polar and chiral point groups. A chiral point group is one without any roto-inversion symmetry elements, and a polar point group allows for the fullerene to have a dipole moment. A point group with an inversion center or a mirror plane perpendicular to the axis of rotation cannot be polar. The nine chiral point groups for fullerenes are I, T, D6, D5, D3, D2, C3, C2, and C1.31 The polar fullerenes belong to either of the point groups C3v, C3, C2v, C2, Cs or C1.31 For larger fullerenes, the fraction of low-symmetry to high-symmetry isomers grows rapidly, and the C1 point group increasingly dominates. This can be intuitively understood combinatorially from distributing pentagons on a sphere. Already at C100, more than 99% of the isomers are C1.
The separation of spiral starts into equivalence classes by their spirals yields an interesting relation:
| 12 |
where  is the number of symmetry distinct (general) spirals. For Ih symmetry we have |Ih| = 120, which gives
is the number of symmetry distinct (general) spirals. For Ih symmetry we have |Ih| = 120, which gives  . Hence, Ih-fullerenes can only occur when N is a multiple of 20. In a similar way, for I symmetry we have
. Hence, Ih-fullerenes can only occur when N is a multiple of 20. In a similar way, for I symmetry we have  , and for Td, Th, D6h, or D6d we obtain
, and for Td, Th, D6h, or D6d we obtain  . This explains why some of these high symmetry groups are not found in certain isomer lists.
. This explains why some of these high symmetry groups are not found in certain isomer lists.
In the case of icosahedral symmetry, we have a full characterization of when they occur: As described by Dutour and Deza,103 every fullerene of Ih or I symmetry is a Goldberg-Coxeter transform of C20.77 This means that they occur exactly when N = 20(k2 + kl + l2) for integers k and l. It is also possible to determine when these are of Ih and when they are of I symmetry. Halma and leapfrog transformations, which correspond to Goldberg-Coxeter transforms of l = 0 and k = l, respectively, both preserve symmetry.128 Hence, there is a fullerene with Ih symmetry at N = 20j2 and N = 20(3j2) for every j ∈ ℕ, corresponding to a single Halma or leapfrog transformation on C20. Consecutive application of the two shows that isomers with Ih symmetry are found for every N = 20(3ij2) (j ∈ ℕ and i = 0, 1). Moreover, general Goldberg-Coxeter (k, l) transforms with k ≠ l and l ≠ 0 (k > 0) break horizontal mirror plane symmetry.
Shapes: Volume and Surface Area, Sphericity, and Convexity
For ideal C60 - Ih, where all edges are of equal length, the geometric volume V, and surface area A can be obtained through simple algebraic and geometric considerations. The volume and surface are of a regular 20-sided polyhedron (icosahedron, see Figure 3) made out of equilateral triangles with edge length a is,
| 13 |
Cutting out 12 pentagonal pyramids at length a/3 gives the ideal truncated icosahedron C60 - Ih with
 |
14 |
where re = a/3 is the length of the edge (C–C bond length). This gives the ratios  and
and  between the two volumes and surface areas. C60 has, however, two different bond lengths as the crystal structure shows,129
r5 = 1.455 Å (edge at pentagon-hexagon fusion) and r6 = 1.391 Å (edge at hexagon-hexagon fusion). A smaller bond distance in the hexagon implies that more of the original icosahedron is cut off,
between the two volumes and surface areas. C60 has, however, two different bond lengths as the crystal structure shows,129
r5 = 1.455 Å (edge at pentagon-hexagon fusion) and r6 = 1.391 Å (edge at hexagon-hexagon fusion). A smaller bond distance in the hexagon implies that more of the original icosahedron is cut off,
 |
15 |
For r5 = r6 we obtain Eq 14. If r6 = 0 (r5 = a/2) the hexagons become equilateral triangles and for r5 = 0 we just get a hollow icosahedral C12 cluster. For C20 (equal edge lengths) the vertices lie in the center of each face of an icosahedron, and the volume and surface area are easily obtained,
| 16 |
How can we get the volume V and surface area A for any fullerene isomer? As fullerene cages are not guaranteed to have planar faces, their volume or surface area are only approximately defined. There are, however, a number of definitions according to which we can express both quantities. We could triangulate all faces by adding a barycenter  to each face with lines to each vertex of that face. We call the polyhedron obtained in this way a triangulated face polyhedron, TFP. For a fullerene, the graph representing the TFP is identical to the dual structure of its leap-frog transform. The total surface area A is obtained by summing over all areas Ai of the triangles obtained, which works even for faces where the vertices do not lie on a plane. Using Gauss' theorem (divergence theorem), the volume V is found by summing over the face normals,
to each face with lines to each vertex of that face. We call the polyhedron obtained in this way a triangulated face polyhedron, TFP. For a fullerene, the graph representing the TFP is identical to the dual structure of its leap-frog transform. The total surface area A is obtained by summing over all areas Ai of the triangles obtained, which works even for faces where the vertices do not lie on a plane. Using Gauss' theorem (divergence theorem), the volume V is found by summing over the face normals,
| 17 |
where  is any point on face i, and Ai is the area of the face.
is any point on face i, and Ai is the area of the face.
The different volumes and surfaces areas can be used for calculating some important measures for fullerenes, such as the sphericity S (how spherical a fullerene is) and convexity C (how convex a fullerene is). The surface of a triangulated fullerene may not be convex. We can measure the non-convexity by comparing to the convex hull of the fullerene cage, which is the smallest convex polyhedron that contains all the points. The convex hull (CH) is uniquely defined, and there are several algorithms available, such as the incremental 3D convex hull algorithm.130 A measure for convexity C is obtained from the proportion of ‘empty space’ between the non-convex polyhedron and the convex hull by comparing the volumes or surface areas, that is, CA = A/ACH and CV = V/VCH.
The simplest measure of sphericity is the isoperimetric quotient qIPQ,131 defined for a polyhedron as
 |
18 |
where qIPQ = 1 for an ideal sphere and DIPQ is a measure of the deviation from an ideal spherical shape. DIPQ is shown for several fullerenes in Figure 14.
Figure 14.
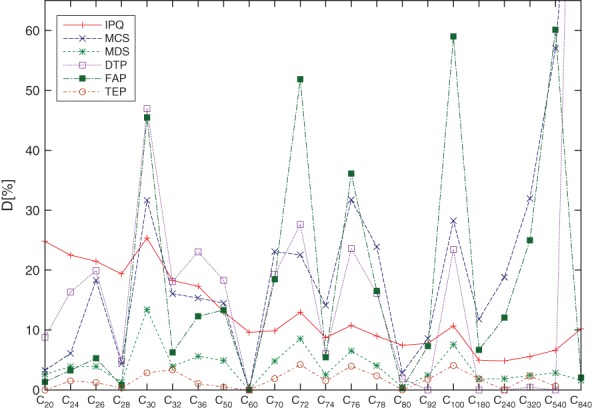
Various deformation parameters D (in percent) for a series of fullerenes selected according to stability. For larger fullerenes, Goldberg-Coxeter transformed structures of C20 were chosen. Geometries were obtained from DFT (up to C540) or force field optimizations. IPQ: isoperimetric quotient; MCS: Minimum covering sphere; MDS: Minimum distance sphere; DTP: Diaz-Tendero parameter; FAP: Fowler asymmetry parameter; TEP: (1 − ρ) from the topological efficiency parameter ρ.
We usually regard C20 - Ih and C60 - Ih as almost spherical because all vertices lie on a (covering) sphere. We may therefore analyze whether or not the vertices of a given fullerene lie on a sphere. For this, we define the minimum covering sphere as a sphere of minimum radius that encloses all vertices in the polyhedral embedding (J. J. Sylvester, 1857).132,133 Mathematically, this translates into finding the solution to the problem134,135
| 19 |
where ‖ · ‖ denotes the Euclidian norm,  is the location of the vertex vi in 3D space, and
is the location of the vertex vi in 3D space, and  is the center of the MCS with radius R and is usually close to the barycenter of the fullerene. The MCS is uniquely defined134,135 and can be obtained using an efficient algorithm, such as the one proposed by Yıldırım.136 The convex hull of a 3D embedding of a graph G is contained entirely within the MCS. For fullerenes with an ellipsoidal shape like C72 - D6d, or with a cylindrical shape such as a nanotube137 (see Figure 2), it is more appropriate to use a minimum covering ellipsoid or cylinder instead of a sphere. The ellipsoidal problem has been addressed recently, and is known as the minimum volume axis-aligned ellipsoid problem (MVAE).138–141
is the center of the MCS with radius R and is usually close to the barycenter of the fullerene. The MCS is uniquely defined134,135 and can be obtained using an efficient algorithm, such as the one proposed by Yıldırım.136 The convex hull of a 3D embedding of a graph G is contained entirely within the MCS. For fullerenes with an ellipsoidal shape like C72 - D6d, or with a cylindrical shape such as a nanotube137 (see Figure 2), it is more appropriate to use a minimum covering ellipsoid or cylinder instead of a sphere. The ellipsoidal problem has been addressed recently, and is known as the minimum volume axis-aligned ellipsoid problem (MVAE).138–141
We can now define a number of useful measures for sphericity. The MCS distortion (normalized to the smallest C–C distance in the fullerene) is given by,
| 20 |
The MCS definition for the distortion is biased to the case of few atoms sticking out on a sphere and another measure may therefore be more appropriate. We define the minimum distance sphere as
| 21 |
with
| 22 |
A similar definition that uses the mean deviation from the average distance taken from the barycentric point has been introduced by Nasu et al.142
Some other useful sphericity parameters are the Diaz-Tendero (DTP) parameter,143,144
| 23 |
where a ≥ b ≥ c are the rotational constants, and the Fowler asymmetry parameter (FAP),
| 24 |
where Ri is the radial distance of atom i from the barycenter, and Rav is the average distance.145 If all atoms lie on a sphere, we have λFAP = 0.
Figure 4 shows a comparison of sphericity parameters for a number of stable fullerenes. As C20 is slightly deformed due to Jahn-Teller distortion, the vertices do not lie exactly on a sphere anymore, and DMDS ≠ 0. In contrast, the purely topological efficiency parameter (1 − ρ) (see discussion in the topological indicator section) is exactly zero for C20 (and for C60, C80, and C240). For C60, all vertices lie on a sphere and all deformation parameters are zero, except for the IPQ, which except for its simple definition is perhaps not the best measure for sphericity. All deformation parameters reveal the highly deformed fullerenes (spikes in Figure 4).
Toward the Solid State: Hilbert's Problem and Space Fillings
Solid C60 - Ih packs in a rotationally disordered face centered cubic (fcc) structure with a lattice constant of afcc = 14.17 ± 0.01 Å,129,146 which according to Fischer et al. implies a close packing of spheres of a diameter of 10.02 Å.146 C60 undergoes a phase transition below 255 K to a simple cubic (sc) structure, from which one can infer a lattice parameter of asc = 14.04 ± 0.01 Å.147,148 The only other solid state fullerene obtained by experiment is that of C70 - D5h, which adopts a closed packed structure of either hcp or fcc symmetry138,149 with a fcc lattice constant of afcc = 14.96 Å. In the solid state, the interactions between the fullerenes are of Van der Waals type. Note that the polarizability and therefore the Van der Waals coefficients grow linearly with increasing number of carbon atoms in the fullerene cage,150 which should converge toward the graphene limit for the per-atom value. Experimentally, the solid state behavior of C60 has been studied in great detail. One interesting fact is that the bulk moduli of C60 (B = 6.8 GPa for the simple cubic phase and 8.8 GPa for the fcc phase) and C70 (11 GPa for the rhombohedral phase) are quite small as applied pressure will just squeeze out the compressible Van der Waals space.151–153 These bulk moduli are only twice as large compared to the rare gas solids,154 and much smaller than that of diamond (442 GPa155). However, once the Van der Waals space is squeezed out and the fullerene cages touch, the bulk modulus increases substantially, as the fullerene cage is not easily compressible, similar to the high in-plane stiffness of graphite or graphene.156 We mention that it requires high pressures above 20 GPa to turn C70 into graphite (using step-like shock-wave compression).157 For overviews on solid-state properties of fullerenes, see Zettl and Cumings153 or Fischer et al.146
From the experimental data of C60 - Ih, we can adjust the Van der Waals radius of the C60 cage such that the experimental lattice constant is obtained, that is, we use a hard sphere model for periodic packing (fcc is the closest packing available for hard spheres as proven by computer by Hales in 2000158,159). We can do this by using the MCS introduced in Eq 19, but we have to add the carbon Van der Waals radius (rVdW(C) = 1.415 Å) to the MCS radius,
| 25 |
Figure 15 shows lattice constants for a number of fullerenes using this hard sphere model, which can be taken as an upper bound to experimental lattice constants. For the other experimentally known fullerene crystal structure, C70, the fcc lattice constant afcc of 14.96 Å is in reasonable agreement with the hard-sphere value of 15.78 Å, considering the fact that the IPR C70 isomer is slightly prolate and distorted. The dependence of the afcc lattice constant on the vertex number can be expressed as  , where the shift follows from the geometry of the fcc cell and the
, where the shift follows from the geometry of the fcc cell and the  -term represents the dependence of the fullerene radius on N. In the hard sphere model we have afcc ∼ R, and the cage radius of the fullerene is R ∼ A1/2 ∼ N1/2 (A being the surface area of the MCS).
-term represents the dependence of the fullerene radius on N. In the hard sphere model we have afcc ∼ R, and the cage radius of the fullerene is R ∼ A1/2 ∼ N1/2 (A being the surface area of the MCS).
Figure 15.
fcc lattice constant afcc for a hard sphere model as a function of the number of carbon atoms N.
For distorted fullerenes, the packing problem becomes far more complicated. For example, we could add the Van der Waals radius to every carbon atom and take the convex hull around this Van der Waals layer. We are then faced with close packing of complicated polyhedral structures, which is a difficult, unsolved problem. However, there exist algorithms for packing arbitrary polyhedra in finite spaces,160,161 and it is possible that one could approximate properties of the (periodic) close packing solution by making the finite cell large enough. For some fullerenes, ellipsoidal, or cylindrical covers are more appropriate as already mentioned.141,162 The close packing of ellipsoids was recently investigated by Donev et al, who showed that the maximum packing density can exceed that of a close sphere packing.163 Nevertheless, the question still remains how fullerenes of a certain topology pack in 3D space. This is related to one of Hilbert's fundamental problems: How can one arrange most densely in space an infinite number of equal solids of given form, e.g., spheres, with given radii, that is, how can one so fit them together that the ratio of the filled to the unfilled space may be as great a possible? A more rigorous mathematical proof (beside Hales' complete computer algorithm158,159) for fcc packing of hard spheres as the most dense packing is still missing, and packing any other deformed spheres remains an open area of research. One possible approximation is to expand the shape of a fullerene in terms of multipole deformations and study the 3D packing of such smooth topologies.
Topological and Chemical Indicators
Chemists can deduct many useful properties of a chemical system just by looking at its structure. For fullerenes, the distribution of the 12 pentagons on a surface, for example, a sphere, can tell us qualitatively how stable the fullerene is or how it would pack in the solid state. Moreover, the symmetry of the underlying structure determines many useful spectroscopic properties. It is perhaps a realistic goal to connect the graph theoretical properties of a fullerene directly with its physical properties by mapping the fullerene graph G into a number describing that property. In more general terms, we define a topological indicator as a map τI from the graph G into a finite series of numbers,
| 26 |
where the numbers xi are called topological indices and can be chosen to be integers, rationals, or reals. A topological index is called a chemical index if it is related to a chemical (or physical) property. Topological indicators may be placed in the same category as crude chemical bonding models, except that they can be strictly defined in graph theoretical terms and sometimes have interesting mathematical properties. There is no restriction that the mapping between fullerene graphs and any topological indicator be one-to-one, and indeed, most of the commonly used topological indicators are the same for many different isomers. Of course, any chemical or physical property is ruled by its underlying electronic structure governed by the Schrödinger equation. However, solving the electronic structure problem for any large fullerene is a daunting task and therefore, topological indices that are easily obtained can be very useful as we shall see.
To give some (trivial) examples, we can list all the possible point groups for fullerenes and define xi = 0 for the ideal point group not matched by the fullerene graph, and xi = 1 if the point group is. The order of the ideal point group  as shown in Table1 is also a topological index. Another example for a topological index is the 12 face spiral pentagon indices as described above.
as shown in Table1 is also a topological index. Another example for a topological index is the 12 face spiral pentagon indices as described above.
The first topological index of chemical relevance was introduced by Wiener in 1947.164 He defined a so-called path number (Wiener index) as the sum of the entries in the topological distance matrix dij, consisting of the length of the shortest path between every pair of vertices in the chemical graph representing the non-hydrogen atoms in the molecule,
| 27 |
where dii = 0. The Wiener index provided a good measure of compactness for acyclic alkanes and gave a reasonable correlation to boiling points.164 The first application of the Wiener index to fullerenes came from Ori and co-workers, who obtained W = 8340 for C60 - Ih.165 For fullerenes, low Wiener indices provide a measure of high compactness of the cage. This can be seen from the scaling law, which has been found to behave like  for ‘spherical’ fullerenes166 and
for ‘spherical’ fullerenes166 and  for fullerene nanotubes. For carbon nanotubes, analytical formulae in terms of polynomials in the vertex number are available, for example, for the smallest D5h/D5d nanotubes we have W(k) = 1/3(100k3 + 1175k − 2010) with N = 10k and k ≥ 5,167 and for the smallest D6h/D6d nanotubes we have W(k) = 12(4k3 + 69k − 136) with N = 12k and k ≥ 7.168 It is conjectured that the Wiener index grows like
for fullerene nanotubes. For carbon nanotubes, analytical formulae in terms of polynomials in the vertex number are available, for example, for the smallest D5h/D5d nanotubes we have W(k) = 1/3(100k3 + 1175k − 2010) with N = 10k and k ≥ 5,167 and for the smallest D6h/D6d nanotubes we have W(k) = 12(4k3 + 69k − 136) with N = 12k and k ≥ 7.168 It is conjectured that the Wiener index grows like  (N2 + 1/d), where d describes the dimensionality of the system (the Wiener dimensionality).166 The almost cubic growth with increasing number of vertices can make the Wiener index unwieldy for larger fullerenes. Therefore, Ori and coworkers defined a topological efficiency index ρ, derived from the Wiener index169,170
(N2 + 1/d), where d describes the dimensionality of the system (the Wiener dimensionality).166 The almost cubic growth with increasing number of vertices can make the Wiener index unwieldy for larger fullerenes. Therefore, Ori and coworkers defined a topological efficiency index ρ, derived from the Wiener index169,170
| 28 |
which has the advantage that, unlike the Wiener index, it does not grow to large numbers. Small values of ρ ≥ 1 indicate topologically efficient structures, for example, both C20 - Ih and C60 - Ih have ρ = 1.168 It can be seen as a measure of sphericity that does not involve 3D Cartesian coordinates, as shown nicely in Figure 4. Vukičević et al. showed that among the 4478 isomers of C66, the joint information of the Wiener index and the topological efficiency index correctly identifies the C66 - C2v(11) as the most stable molecule169 (as detected experimentally in endohedral Sc2@C66171).
Many different topological indices have been introduced and studied since, mainly for the structural and statistical analysis of molecules, polyhedra, and graphs in general, often yielding interesting mathematical properties (for a list of topological indices see for example Ref 172). The distance matrix dij gives rise to a number of very useful topological indices including the topological radius R and diameter D,173 Hosoya polynomials174 and related Wiener indices, the Szeged index Sz,175 and the Balaban index J,29,176 to name but a few (for fullerenes one has W ∼ Sz and W ∼ − J).29,177 There are also a number of topological indices connected to the adjacency matrix (or Hückel matrix), for example Hückel orbital energies and related properties (occupation numbers, band gap,31 spectral moments178 etc.),31 and the Estrada index.179,180 For a more detailed discussion of topological indices see Refs 29,89,173, and 181.
Some of these topological indices can directly be related to the stability of the fullerene cage. For example, the Fowler-Manolopoulos pentagon indices {pn|n = 0, …, 5} define the number of pentagons attached to n other pentagons (n is called the neighbor index),31 that is, for IPR fullerenes we have p0 = 12 and pn = 0 for n ≥ 1. For any fullerene, we of course must have p1 + … + p5 = 12. Fowler-Manolopoulos hexagon indices are similarly defined and useful for IPR fullerenes, that is, {hi|i = 0, …, 6}, where hn defines the number of hexagons with neighbor index n. In an IPR fullerene every hexagon is adjacent to a minimum of three others and we can restrict the list of hexagon neighbor indices for example to (h3, h4, h5, h6).31
We may contract the neighbor indices to one useful topological index describing the stability of fullerenes.31 The single pentagon signature P1 is defined as
| 29 |
max(P1) = 30 (for C20 only), and for IPR fullerenes such as C60 we have P1 = 0. For IPR fullerenes a more useful single topological index is defined through the hexagon signatures. The standard deviation σh of the hexagon neighbor index distribution is defined as
| 30 |
where
 |
31 |
We call the topological index Hn the n-th moment hexagon signature. For general fullerene isomers, low P1 values and high Hi values correlate with high thermodynamic stability.31 It turns out, however, that H1 stays constant for a given vertex count in IPR fullerenes, as this index is related to certain face patterns as we shall see. Fowler et al. advocated the use of the index σh instead, as low values of σh are better predictors for stability, and the strain is minimized when all hexagon neighbor indices are as similar as possible.31 For example, for C60 we have {hi} = {0, 0, 0, 20, 0, 0, 0} giving H1 = 60, H2 = 180, and σh = 0. Fowler et al. devised the following scheme for estimating the internal order among fullerenes sorted by stability:173 First order the isomers according to lowest P1-value (each pentagon-pentagon fusion carries a penalty of about 20–25 kcal/mol). If multiple IPR fullerenes are present, that is, P1 = 0, we order these first by largest H2-value, and finally by lowest Wiener index W (which indicates high compactness of the fullerene cage). The result is an efficient but effective screening method to find the most stable fullerene isomers.
Ju et al. presented a relationship between the Fowler-Manolopoulos hexagon indices and a particular hexagon structure count to provide a graphical interpretation.182 The complete list of different pentagon and hexagon combinations up to three connected faces are shown in Figure 16. Note that for connecting two or three faces we have
 |
32 |
where the notation is described in Figure 6. For example n556 is the sum of all occurring ring patterns containing the combination of two pentagons and one hexagon. Ju et al. showed that for the second moment hexagon signature one gets182
| 33 |
and
| 34 |
Figure 16.

The 15 basic shapes for the two- and three-ring (face) adjacencies on the surface of a fullerene; (l) denotes linear, (b) bent, (o) open, and (c) closed ring patterns (see Ref 183).
Hence for IPR fullerenes, σh and H2 are related through the simple equation
| 35 |
and one requires only H2 to discuss the stability of IPR fullerenes. Stevanović extended these relations to general fullerenes (IPR or not),184
| 36 |
and
| 37 |
This not only illustrates nicely the current development in the area of topological indices, it also suggests that the stability of fullerenes can be approximated by counting different face patterns.183
Indeed, Cioslowski et al.'s incremental scheme for the heat of formation  for IPR fullerenes33 uses 25 linearly independent face patterns (structural motifs) of up to 13 connected hexagons and pentagons to calculate the energy EFP, including an additional curvature term EC,
for IPR fullerenes33 uses 25 linearly independent face patterns (structural motifs) of up to 13 connected hexagons and pentagons to calculate the energy EFP, including an additional curvature term EC,
 |
38 |
where mi stands for the total count of a specific face pattern (FP) that appears in the fullerene, εi is the FP energy contribution, and the second term in Eq 38 is the curvature term (C), which is repulsive for C20 and approaches zero for N → ∞. For example, for C60 - Ih we just have one pattern in Cioslowski et al.'s scheme consisting of a hexagon surrounded by 3 pentagons and 3 hexagons. There are 20 of these in C60, and with ε = 44.281 kcal/mol, we obtain ΔHf = 616.814 kcal/mol for C60.33 This compares rather well with the estimated NIST listed heat of formation for C60 (612 ± 25 kcal/mol), although this value should probably be corrected downwards to a value of 602.7 kcal/mol as recently pointed out by Karton et al. using high-level theoretical procedures.185 The rms error for the standard enthalpies of formation for the 115 IPR isomers chosen was 4.0 kcal/mol compared to B3LYP/6-31G* calculations.
Alcami et al. devised a similar scheme for general fullerenes.32 By analyzing the most stable structures from B3LYP-DFT calculations of C20 to C72, and using only connections between four faces with varying number of pentagons with a total of 9 different motifs, an expression similar to Cioslowski et al. was used for EFP, but without the curvature term EC. This scheme gives ΔHf = 654.0 kcal/mol for C60 - Ih. For all fullerenes studied the rms error is 15.4 kcal/mol for the enthalpy of formation compared to DFT calculations, or 0.31 kcal/mol per carbon atom.
The question is: How well are topological indices suited to describe fullerene stability? Table3 shows a comparison of stabilities for all C34 isomers obtained from DFT geometry optimizations (using the PBE functional186 and a def2-SVP basis set for carbon187) in comparison with several topological indicators.35 According to the pentagon signature, isomer C34 - C2(5) has the lowest P1 value, but with a very small HOMO-LUMO gap obtained from Hückel theory. This is indeed the case as the DFT results show. Alcami et al.'s stability indicator correctly predicts the most stable (P1 = 14) and the least stable isomer (P1 = 17). For the four isomers with pentagon signature P1 = 15, the two results (ΔEDFT and ΔEA) do not agree in their sequence (note that Alcami et al.'s scheme was not designed to distinguish between different isomers of a fixed vertex count). The different fullerene isomers are all very similar in surface area and volume. Concerning the HOMO-LUMO gap, the most stable isomer does not correspond to the one with the largest HOMO/LUMO gap. Moreover, these values are very sensitive to the method applied, and a better measure for the gap given by the singlet-triplet separation. The fullerenes with the smallest HOMO-LUMO gaps (isomers 2 and 6) prefer in fact the triplet ground state. The Hückel HOMO/LUMO gap on the other hand is only good for a qualitative discussion. We note that the two different fullerene isomers 3 and 5 have identical Wiener indices, and the isomer 6 with the smallest Wiener index is not the most stable isomer.
Table 3.
Stability and Topological Indicators for the Six Isomers of C34
| LI | PG | P1 | ΔEDFT | ΔEA |  |
 |
 |
A | V | W | NHC | NPM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C2 | 17 | 74.93 | 13.5 | 0.069 | 0.424 | 0.0267 | 81.41 | 60.30 | 1978 | 28 | 212 |
| 2 | Cs | 15 | 28.10 | 4.2 | 0.120 | 0.150 | −0.153 | 81.27 | 61.70 | 1975 | 58 | 219 |
| 2(T) | Cs | 15 | 24.55 | 4.2 | — | — | — | 81.32 | 61.69 | 1975 | 58 | 219 |
| 3 | Cs | 15 | 31.48 | 4.0 | 0.368 | 0.406 | 0.0945 | 81.42 | 61.80 | 1973 | 52 | 196 |
| 4 | C2 | 15 | 15.19 | 5.5 | 0.343 | 0.619 | 0.2229 | 81.32 | 62.06 | 1976 | 33 | 229 |
| 5 | C2 | 14 | 0 | 0 | 0.006 | 0.480 | 0.1333 | 81.28 | 62.27 | 1973 | 42 | 204 |
| 6 | C3v | 15 | 33.70 | 7.8 | 0 | 0.225 | −0.094 | 81.42 | 61.79 | 1971 | 66 | 195 |
| 6(T) | C3v | 15 | 31.54 | 7.8 | — | — | — | 81.40 | 61.75 | 1971 | 66 | 195 |
LI is the numbering scheme according to the lexicographically ordered face spiral pentagon indices, PG the ideal point group, P1 the pentagon signature, energy differences (in kcal/mol) ΔEDFT the DFT(PBE) energy difference to the most stable isomer (kcal/mol), ΔEA the Alcami energy difference to the most stable isomer (kcal/mol), energy gaps (in eV)  the Hückel HOMO-LUMO gap,
the Hückel HOMO-LUMO gap,  the PBE HOMO-LUMO gap,
the PBE HOMO-LUMO gap,  the singlet-triplet gap (in eV), A the surface area (Å2), V the volume (Å3), W the Wiener index (see Ref 188), NHC the number of Hamiltonian cycles, and NPM the number of perfect matchings. The symbol (T) for isomers 2 and 6 in the first column indicates that the triplet electronic state is taken instead of the singlet state.
the singlet-triplet gap (in eV), A the surface area (Å2), V the volume (Å3), W the Wiener index (see Ref 188), NHC the number of Hamiltonian cycles, and NPM the number of perfect matchings. The symbol (T) for isomers 2 and 6 in the first column indicates that the triplet electronic state is taken instead of the singlet state.
The first halma transform of C20 is C80 and therefore of Ih symmetry. There are seven IPR isomers out of a total of 31,924 isomers as possible candidates for the energetically most stable one. Table4 shows the properties of all IPR isomers of C80. With larger fullerenes the band gap becomes very small (graphene is a semi-metal or zero-gap semiconductor189), and one has to check for states of higher spin multiplicity. For C80, Hückel theory indicates an open-shell case for the isomers 6 and 7. In fact, the results show that four of the isomers have a triplet ground state, with the singlet and triplet states for C80 - Ih being quasi-degenerate. The DFT calculations further show that the most stable fullerene is not the high symmetry C80 - Ih isomer, but the first one in the IPR isomer list, which is of D5d symmetry and has the largest H2 value (previously, isomer 2 has been predicted to be the most stable isomer190). The results show that the first three isomers are very close in energy and it requires perhaps a more sophisticated electron correlation treatment to sort out the sequence in stability. Isomer 1 also has the highest count in perfect matchings among all IPR isomers (although the non-IPR C60 - D3d(4) nanotube has a much higher perfect matching count of NPM = 524, 250). The volumes and surface areas are all very similar with the highest symmetry isomer C80 - Ih being largest.
Table 4.
Stability and Topological Indicators for the Seven IPR Isomers of C80
| LI | PG | H2 | ΔEDFT | ΔEA | ΔEC | ΔEHRE |  |
 |
 |
A | V | W | NHC | NPM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D5d | 500 | 0 | 0 | 4.34 | 2.12 | 0.220 | 0.355 | 0.168 | 203.70 | 255.16 | 17340 | 10450 | 270153 |
| 2 | D2 | 496 | 2.63 | 3.0 | 0.75 | 0.0 | 0.528 | 0.460 | 0.293 | 203.85 | 257.72 | 17352 | 10642 | 237585 |
| 3 | C2v | 488 | 1.81 | 10.5 | 0.04 | 14.7 | 0.102 | 0.009 | 0.091 | 203.92 | 260.80 | 17412 | 9918 | 201623 |
| 4 | D3 | 492 | 7.03 | 7.5 | 7.17 | 6.70 | 0.408 | 0.098 | −0.036 | 203.87 | 258.71 | 17368 | 9906 | 222588 |
| 4(T) | D3 | 492 | 6.21 | 7.5 | 7.17 | — | — | — | — | 203.85 | 258.40 | 17368 | 9906 | 222588 |
| 5 | C2v | 484 | 6.33 | 16.5 | 2.92 | 24.7 | 0.298 | 0.038 | −0.090 | 203.99 | 262.48 | 17454 | 9004 | 182555 |
| 5(T) | C2v | 484 | 4.25 | 16.5 | 2.92 | — | — | — | — | 203.98 | 262.41 | 17454 | 9004 | 182555 |
| 6 | D5h | 480 | 6.81 | 22.5 | 0 | 37.7 | 0 | 0.081 | −0.002 | 204.01 | 263.50 | 17500 | 10970 | 169375 |
| 6(T) | D5h | 480 | 6.38 | 22.5 | 0 | — | — | — | — | 204.00 | 263.49 | 17500 | 10970 | 169375 |
| 7 | Ih | 480 | 17.49 | 30.0 | 14.2 | 63.4 | 0 | 0.115 | −0.003 | 204.02 | 263.61 | 17600 | 10500 | 140625 |
| 7(T) | Ih | 480 | 17.43 | 30.0 | 14.2 | — | — | — | — | 204.02 | 263.63 | 17600 | 10500 | 140625 |
LI is the numbering scheme according to the lexicographically ordered IPR face spiral pentagon indices, PG the ideal point group, H2 the second moment hexagon signature, energy differences (in kcal/mol) ΔEDFT the DFT(PBE) energy difference to the most stable isomer, ΔEA the Alcami energy difference to the most stable isomer, ΔEC the Cioslowski energy difference to the most stable isomer, ΔEHRE the Hückel resonance energy (taking the resonance value as β = –60 kcal/mol), energy gaps (in eV)  the Hückel HOMO-LUMO gap,
the Hückel HOMO-LUMO gap,  the PBE HOMO-LUMO gap,
the PBE HOMO-LUMO gap,  the singlet-triplet gap (in eV), A the surface area (Å2), V the volume (Å3), W the Wiener index, NHC the number of Hamiltonian cycles, and NPM the number of perfect matchings. The symbol (T) indicates that the triplet electronic state is taken instead of the singlet state.
the singlet-triplet gap (in eV), A the surface area (Å2), V the volume (Å3), W the Wiener index, NHC the number of Hamiltonian cycles, and NPM the number of perfect matchings. The symbol (T) indicates that the triplet electronic state is taken instead of the singlet state.
The results shown here clearly demonstrate that the topological indicators help enormously to sort out the most stable isomers. The situation is often quite complicated as seen from the DFT calculations here, or for example from the work of other authors who compared stabilities within a list of isomers.191–193 In the next section we introduce topological indicators that are connected to π-electron resonance structures (Kekulé structures) in fullerenes.
Kekulé Structures and Perfect Matchings, Clar and Fries Structures, and the Pauling Bond Order
Every chemist learns how to draw double bonds into an aromatic system called a Kekulé (or resonance) structure, sometimes also called a benzenoid graph.194 For benzene there are only two possible Kekulé structures with three double bonds as we all know, but for C60 there are as many as 12,500 Kekulé structures with 30 double bonds (a fullerene has exactly nDB = N/2 double bonds), of which only 158 are symmetry distinct, that is, non-isomorphic.195–197 A Kekulé structure is the same as a perfect matching in the chemical graph  , where the edges of the matching correspond to double bonds. We write
, where the edges of the matching correspond to double bonds. We write  for the set of all perfect matchings of the graph, and the number of different Kekulé structures
for the set of all perfect matchings of the graph, and the number of different Kekulé structures  is called the Kekulé number. In graph theory, a perfect matching is a selection of edges such that every vertex of the graph G is part of exactly one edge in the matching. The edges of the matching correspond to the double bonds.
is called the Kekulé number. In graph theory, a perfect matching is a selection of edges such that every vertex of the graph G is part of exactly one edge in the matching. The edges of the matching correspond to the double bonds.
Chemists know from basic Hückel theory that for two different benzenoid graphs B1 and B2, K(B1) > K(B2) implies that B1 is more stable than B2 because of resonance stabilization. Schmalz et al. calculated the Kekulé number for several small fullerenes up to C84.50 Their work showed that for C60, the least stable isomer C60-D5h(1) has K = 16,501, while for the most stable isomer C60-Ih(1812) we have K = 12,500.50 In fact, Austin et al. showed that 20 isomers of C60 have a higher Kekulé numbers than C60-Ih.198 The Kekulé number is therefore not a good indicator for fullerene stability,198 but useful for rationalizing the different bond lengths in fullerenes as we shall see.
All cubic graphs have exponentially many perfect matchings and hence exponentially many Kekulé structures.199 While the theoretical lower bounds proved in Refs 113,199 have small exponents, the actual exponential behavior kicks in rapidly, as seen in Figure 17: The mean number of perfect matchings for a CN isomer is approximately 20 · 2(N − 20)/4. In general, calculating the number of perfect matchings for graphs is intractable,i but for planar graphs (such as fullerenes) the Kekulé number can be computed in  time using the Pfaffian matrix (the Fisher-Kasteleyn-Temperley algorithm).200
time using the Pfaffian matrix (the Fisher-Kasteleyn-Temperley algorithm).200
Figure 17.

Minimum, median, and maximum perfect matching count for all isomers of CN up to C120. (a) When looking at the plot for all N, we notice three distinct series depending on the value of N mod 6. (b) For each of the three series, both the maximum and minimum counts follow a simple exponential function (shown as dashed lines). Here, the ‘peak series’ at N mod 6 = 2 is shown. (c) The bounds for the IPR isomers (solid curves) are shown together with the bound for all isomers (dashed curves) for the series N mod 6 = 0. The other two series behave similarly.
Pauling, Brockway, and Beach introduced bond orders derived from Kekulé structures in order to rationalize the differences in the C–C bond lengths encountered for polycyclic aromatic compounds.201 The Pauling bond order (PBO) Pij of an edge vi − vj is defined as the sum over all appearances of double bonds in perfects matchings
| 39 |
where βij = 1 if the edge vi − vj has a double bond in the perfect matching M, otherwise βij = 0. This gives 0 ≤ Pij ≤ 1, with 0 being a pure C–C single bond and 1 a pure C=C double bond. In C60-Ih there are two different type of bonds and therefore two different PBOs, depending if the edge shares two hexagons (Phh = 11/25 = 0.44) or a hexagon and a pentagon (Php = 7/25 = 0.28).202 Note that the Hückel bond orders derived from the eigenvectors of the adjacency matrix are generally larger with Phh = 0.6010 and Php = 0.4758 for C60-Ih.203
Narita et al. derived a linear relationship between the PBO and the bond distance in a fullerene obtained from X-ray diffraction,202
| 40 |
Figure 18 shows an AFM picture of one of the symmetry equivalent hexagons in C60 clearly showing the two different types of bonds. The measured bond lengths are rhh = 1.38(2) Å and rhp = 1.454(12) Å,204 in excellent agreement with the values obtained from Eq 40 (rhh = 1.378 Å and rhp = 1.442 Å).
Figure 18.

Atomic force microscopy (AFM) image for C60 by Gross and co-workers204 at a tip height of z = 3.3 Å showing the different bond orders of individual carbon-carbon bonds in a hexagon. (Copyright © 2012, American Association for the Advancement of Science)
Kekulé structures containing benzenoid moieties are of special interest. These are the Fries and Clar structures. Fries structures are Kekulé structures with maximum number of benzenoid hexagons. The Fries number Fries(G) of a benzenoid graph is the maximum number of benzenoid hexagons over all of its Kekulé structures, and a Fries structure is a perfect matching that realizes this maximum (there could be many). Similarly, the Clar number Clar(G) is the largest number of independent sets of benzenoid faces (separated from each other) over all Kekulé structures, and a Clar structure is a perfect matching that realizes this (there could be many). It is clear that a Clar structure localizes benzenoid structures in fullerenes. For C60-Ih we have Fries(G) = 20 and Clar(G) = 8, and an example for a Fries and Clar structure of C60-Ih is shown in Figure 19. One might naively assume that a set of Clar structures form a subset of Fries structures, but this is generally not the case for fullerenes.205
Figure 19.

One of the Fries structures of C60, and superimposed the structure with highest Clar number (Clar(C60) = 8). Double bonds are shown in red and isolated aromatic hexagons are shaded in.
We expect maximum stability for fullerenes with the highest Clar number. For example, C60-Ih is unique among all other isomers in that it has a Fries structure where all hexagons contain three double bonds and all pentagons none. This is also seen as a reason for the unique stability of C60. However, finding the Clar number is not a trivial problem as it is computationally NP-hard.206 Ye and Zhang recently showed that for C60 there are exactly 18 fullerenes with maximal Clar number of 8.207 Some of them contain as many as 6 fused pentagons. Hence the Clar number alone is not a good measure for stability. However, out of the 18 fullerenes with Clar number 8, C60 has the largest Kekulé count.208 Fowler showed that leapfrog transforms of fullerenes not only are closed shell, but also have the maximum proportion of benzenoid hexagons.209
Figure 17(c) shows that the expected number of perfect matchings is no different for IPR fullerenes than it is for all fullerenes, except for small fullerenes for which it is larger than average. However, the maximum number is always significantly lower for IPR fullerenes. One could assume that the larger the Kekulé number is the more Clar sextets can be found and the more stable the fullerene is. While this has not been explored in detail, Table4 shows that for C80 the most stable D5h isomer has the lowest perfect matching count. There are, however, some good lower and upper bounds of these topological indices known. Došlić showed that the number of Kekulé structures grows exponentially with a lower bound of 2N/8.210 This is not a very tight bound, however, as for C60 it predicts at least 181 Kekulé structures, and there are as many as 12,500. The upper bound for Fries numbers is well known, Fries(G) ≤ N/3, which is seen to be the graphenic limit. Fries structures with a maximum N/3 Fries number are called complete Fries structures211 or perfect Clar structures. This happens exactly for Clar structures that use every vertex. In that case, the Clar and the Fries structure is the same, and corresponds to a ‘soccer ball coloring’ of the faces. An interesting result comes from Pisanski who proved in a paper with Fowler that the fullerenes with maximum Fries number N/3 are exactly the class of leapfrog fullerenes.212 Given a leapfrog, we can find a perfect Clar structure, and given a perfect Clar structure, we can derive an inverse leapfrog transformation. This gives an easy way to test whether a particular isomer admits a perfect Clar structure: simply test whether the inverse leapfrog operation is successful. An upper bound for Clar numbers has been obtained by Zhang and Ye, Clar(G) ≤ (N/6 − 2).213 Both IPR fullerenes, C60-Ih, and C70-D5h with Clar(C70) = 9 achieve this upper bound. Such fullerenes are called extremal fullerenes.213
Finally we mention that the Kekulé structures can be used to determine the resonance stability of fullerenes. To start with a very simple model, Randić et al. took the Kekulé structures of fullerenes ranging from C20 to C72, and calculated the π-content Pπ of hexagons and pentagons, which is obtained by summing all the Pauling bond orders in a specific ring.197 For C60-Ih this gives  for a hexagon, and
for a hexagon, and  . High hexagon π-count together with low pentagon π-count gives stability to a fullerene. High hexagon π-count increases the aromaticity in the system (for more details see Ref 197). Klein et al. applied Herndon's resonance theory214,215 using counts of 2m-resonance cycles to obtain the resonance energy.195 They correctly pointed out that the reduced pπ-orbital overlap in strongly curved fullerenes will reduce the resonance energy and has to be taken into account.195 Here we should mention again that obtaining all Kekulé structures for a fullerene is an exponential problem, and it is more advantageous to apply methods that scale polynomially in time, such as Hückel or more sophisticated (semi-empirical) theories.
. High hexagon π-count together with low pentagon π-count gives stability to a fullerene. High hexagon π-count increases the aromaticity in the system (for more details see Ref 197). Klein et al. applied Herndon's resonance theory214,215 using counts of 2m-resonance cycles to obtain the resonance energy.195 They correctly pointed out that the reduced pπ-orbital overlap in strongly curved fullerenes will reduce the resonance energy and has to be taken into account.195 Here we should mention again that obtaining all Kekulé structures for a fullerene is an exponential problem, and it is more advantageous to apply methods that scale polynomially in time, such as Hückel or more sophisticated (semi-empirical) theories.
Hamiltonian Cycles, IUPAC Rules, and Naming Fullerene
For naming an alkene, the IUPAC rules state that one has to find the longest continuing carbon chain containing as many double bonds as possible. For a cyclic system one chooses the longest cyclic chain, and if there are multiple longest cycles, one must choose the one that maximizes the number of double bonds along the chain. This is related to finding a Hamilton cycle, which is a closed path in a graph that visits every vertex exactly once. If at least one such cycle exists, we say that the graph is Hamiltonian. In this case, the Hamilton cycles are the longest carbon chains, and the optimal one must be chosen among these.
It is an open problem whether every fullerene has a Hamilton cycle.j In practice, however, we find that fullerenes are not only Hamiltonian, but admit exponentially many Hamilton cycles. The mean number of Hamilton cycles taken over all CN-isomers grows approximately as 8 · 2(N − 20)/5.8 for N mod 4 = 0 and as 12 · 2(N − 20)/6 for N mod 4 = 2 (Schwerdtfeger et al., unpublished manuscript). Because of this, naming fullerenes according to the IUPAC alkene rules is a computationally heavy task: we must search through all the exponentially many Hamiltonian cycles (main rings) to find the one in which the secondary bridges are labeled in the lexicographically smallest way. This is computationally feasible only for small fullerenes such as the ones shown in Figure20.
Figure 20.

One of the Hamiltonian cycles out of the many for (a) C60-Ih (NHC = 1090), and (b) the C60-D5h carbon nanotube (NHC = 3040).
In addition to being infeasible to calculate, as N grows, the name resulting for a fullerene from the IUPAC alkene rules rapidly becomes long and unmanageable. Even for a relatively small fullerene such as C60-Ih, the namek is already unreadable, and the name length keeps growing with the fullerene size.216 The IUPAC alkene name for fullerenes is now seen mostly as a curiosity—and a good example of how a well meant rule has is limits.
IUPAC employs an alternative naming scheme for fullerenes: CN-PG[5, 6]fullerene, where PG stands for the (ideal) point group, and [5, 6] denotes a polyhedral cage with only pentagon and hexagon faces. For example, the IPR C60 fullerene has the official IUPAC name C60-Ih[5, 6]fullerene. While computing the name of a fullerene according to this scheme is easy and efficient, and contrary to the alkene naming scheme is short and easy to understand, we find that this scheme is not much better than the IUPAC alkene nomenclature. On the one hand it is unnecessarily long: ‘fullerene’ implies ‘[5, 6]’, and vice versa. On the other hand, it does not uniquely specify a fullerene, since many isomers share the same vertex count and symmetry. For example, while C120-Td[5, 6]fullerene happens to be unique, C120-C2[5, 6]fullerene refers to 10,787 different fullerene isomers, and C120-C1[5, 6]fullerene refers to no less than 1,660,007 different molecules. We therefore advocate the use of one of the following two names to uniquely specify fullerenes, based on the (generalized) canonical face spiral pentagon indices (FSPI), see the chapter on face spirals above. Both schemes uniquely and compactly identify a specific fullerene molecule.
For moderate values of N, we can specify the fullerene by its canonical index, which is the lexicographic number of its FSPI among the isomers. For example, the IPR C60 molecule can be written as C60-Ih(1812) or C60-Ih(IPR−1), as it is the last fullerene of the 1812 C60 isomers in the lexicographically ordered FSPI list.
For large CN, for which it is not feasible to compile databases for all isomers, we annotate instead with the FSPI. For the vast majority of fullerenes, this is a list of 12 small integers. For the extremely rare cases where jumps are necessary (there are only two non-spiral fullerenes out of the 2.6 × 1012 fullerenes up to C400), two extra integers are required per jump. No currently known case requires more than one jump. With this scheme, C60-Ih(IPR−1) is written C60-Ih{1, 7, 9, 11, 13, 15, 18, 20, 22, 24, 26, 32}, and the unspiralable C380 is written C380-T{110, 2; 45, 70, 71, 82, 83, 110, 119, 120, 144, 184, 185, 192}. In the latter example, the two numbers before the semicolon denote a cyclic shift of length 2 before adding face number 110.59
The canonical FSPI notation is especially advantageous for more reasons than being compact and complete without needing to refer to a precomputed database. The procedure to construct a fullerene graph from the FSPI (the ‘windup’ operation) is geometrically intuitive—visualized like peeling an orange—and the algorithm is so simple that it can be performed with pen and paper. Thus, one can even reconstruct fullerenes of moderate sizes from the FSPI by hand, without the help of a computer.
The algorithm for the windup operation is  , and the inverse operation, unwind, takes
, and the inverse operation, unwind, takes  expected time to find a single generalized spiral, and
expected time to find a single generalized spiral, and  to find the canonical one.59 The canonical FSPI constitutes a canonical labeling of fullerene graphs up to isomorphism. Hence one can check whether two fullerene graphs are isomorphic simply by testing whether they have the same canonical FSPI, and the canonical FSPI gives us a unique graph representative of each isomorphism class via the windup procedure. As discussed later, the FSPI representation makes it easy to directly compute the ideal symmetry group of the fullerene. Because the generalized face spiral algorithm is complete for all connected planar cubic graphs, a compressed form of the face spiral similar to the FSPI can be used in general for fulleroids, to be introduced in the last chapter.
to find the canonical one.59 The canonical FSPI constitutes a canonical labeling of fullerene graphs up to isomorphism. Hence one can check whether two fullerene graphs are isomorphic simply by testing whether they have the same canonical FSPI, and the canonical FSPI gives us a unique graph representative of each isomorphism class via the windup procedure. As discussed later, the FSPI representation makes it easy to directly compute the ideal symmetry group of the fullerene. Because the generalized face spiral algorithm is complete for all connected planar cubic graphs, a compressed form of the face spiral similar to the FSPI can be used in general for fulleroids, to be introduced in the last chapter.
Thermodynamic Stability and the Graphene Limit
The spherical shape of C60-Ih with no adjacent pentagons is seen as the main reason for its unusual stability,217 which underlines the importance of Kroto's isolated pentagon rule (IPR).18 For example, Nagase and co-workers investigated the stability of potential candidates for the lowest energy structure of C74, with the sole IPR D3h isomer being 16 kcal/mol lower in energy than the C2 isomer containing two fused pentagons.218 It is therefore convenient to compare the stability of a fullerene to C60-Ih (relative fullerene stability, RFS) by using the isodesmic reaction C60/60 → CN/N,
| 41 |
where E[CN] is the total energy for a CN fullerene. For N → ∞ we obtain the graphene limit, which can be estimated to be approximately ΔERFS(∞) = − 9 kcal/mol.
As E ∼ N, E/N approaches a constant value for infinite systems, for example, the graphene limit if the pentagons are as separated as possible from each other, so that the polyhedral surface is mainly constituted of flat graphene sheets. Alternatively, for nanotube fullerenes with pentagon caps, the infinite particle limit is just the corresponding infinite nanotube. For the graphene limit, the next dominant term in a 1/N expansion will be the surface tension or curvature term, which is ∼ N− 1 as for example used by Cioslowski et al.,33 see Eq 38 (although a N− 1/2 law has been also suggested before59). Indeed, as Figure 21 shows, the stability follows approximately a linear behavior in our 1/N fit (we set ΔERFS = 0 for N = 60),
| 42 |
that is fullerenes become more energetically stable with increasing vertex number N. It was already shown experimentally that C70 is more electronically stable than C60.219 Alcami et al.'s scheme predicts the correct graphene limit (N → ∞), and Cioslowski's scheme performs well for medium sized IPR fullerenes, but has an incorrect convergence behavior toward the graphene limit.32,33 C20 is the smallest member of the fullerene family, and as seen from Figure 21 also the least electronically stable one. It has been detected in 2000 by Prinzbach16 (see also Ref 220), and a current review on the state of affair concerning C20 is given by Fei et al.221
Figure 21.

The stability of fullerenes CN in comparison to C60 obtained from density functional calculations up to the graphene limit (N → ∞). Topological stability indices from resonance energies222 (TRE using β = − 216 kcal/mol) or ring patterns by Alcami32 and Cioslowski33 are also shown. The graphene limit is estimated from the heat of formation of C60.185
It becomes more and more difficult to extract the most stable isomers from the huge isomer space as the size of the fullerene increases. Besides using topological indicators, Hückel theory can be used to determine the resonance stability of a fullerene. It requires diagonalization of the adjacency matrix Aij (the Hückel matrix is defined as Hij = αδij + βAij, where α is the Coulomb and β the resonance integral, and δij is the unit matrix), and the occupied Hückel orbitals determine the Hückel resonance stability (HRS) of a fullerene (in units of β),
 |
43 |
where ni = 0, 1, 2 are the occupation numbers, εi the orbital energy for Hückel orbital i, and the last term comes from subtracting the non-resonant ethene value (single double bond). This gives EHRS = 33.161β for C60. Per π-electron this gives a resonance energy of 0.5527β much higher compared to benzene with exactly β/3. This should indicate that C60 is more aromatic than benzene, which has been a matter of some debate in the past (see the review by Bühl and Hirsch223). The problem is that the π-overlap in non-planar systems is not ideal and there is substantial mixing (hybridization) with the pσ orbitals of carbon, thus questioning the σ-π-separability in Hückel theory. Hence there should be a correction accounting for the curvature at each carbon atom. Such curvature corrections, for example through using the π-orbital axis vector method (POAV), have been considered before with some success,224,225 and the different approaches are discussed in detail by Bakowies and Thiel.226 For example, Haddon et al. showed that C240 is significantly more stable than C60,225 which is indeed the case as Figure 1 shows. Clearly, as the fullerene system becomes larger, the curvature term becomes smaller. Moreover, for similar shaped fullerenes the Hückel resonance energy might still be a good approximation. Indeed, as Table4 for C80 shows, the first two isomers are the most stable ones predicted by the simple Hückel resonance energy term in agreement with DFT calculations, and isomer 7 is the least stable one. The Hückel method only requires a diagonalization of the adjacency matrix, and for obtaining bond orders and resonance energies it is computationally more efficient than sorting out Kekulé structures.
We mention that the Hückel spectrum {εi} for fullerenes shows some interesting mathematical properties.227,228 The largest eigenvalue (which translates into the lowest occupied level as the resonance integral β is negative) in a fullerene is always εmax = + 3. The smallest eigenvalue (highest unoccupied level) is largest for C20 with  , and for the IPR isomers C60 we have
, and for the IPR isomers C60 we have  . As a geometric consequence, the famous golden ratio
. As a geometric consequence, the famous golden ratio  appears here as it does in the volume and surface area calculations discussed above. We also mention that in general, different graphs can have the same Hückel spectrum,229 and this is the case also for fullerenes: the eigenvalues do not uniquely determine the fullerene graph.108
appears here as it does in the volume and surface area calculations discussed above. We also mention that in general, different graphs can have the same Hückel spectrum,229 and this is the case also for fullerenes: the eigenvalues do not uniquely determine the fullerene graph.108
It is well known that the Hückel scheme for canonical resonance structures is of rather limited use, and Aihara230 and Gutman et al.231 therefore introduced a different reference system by defining the topological resonance energy (TRE) for a graph G as
| 44 |
where the reference energy EMP(G) is the sum of all roots given by the matching polynomial, which for a fullerene is
| 45 |
Here the polynomial coefficients c(G, k) define the number of ways of choosing k non-adjacent edges from the graph G. Balasubramanian gave polynomial coefficients c(G, k) for a number of fullerenes up to C50,232 Babić determined the coefficients for C70,216 and Salvador for C60 and C70 to C100.233 The first few polynomial coefficients are known as they are independent of the isomers for a specific vertex count, that is, c(G, 0) = 1, c(G, 1) = − 3N/2, c(G, 2) = 3N(3N − 10)/8, c(G, 3) = − (9N3 − 90N2 + 232N)/16.232 The last coefficient c(G, N/2) is just the number of perfect matchings in the graph. The computation of these coefficients soon becomes computationally intractable.233 Babić et al. found, however, a good correlation between EMP and Eπ values for fullerenes, and the Eπ value suffices to calculate the topological resonance energy approximately as (in units of β),222
| 46 |
In order to give reasonable results for the isodesmic reaction energy (Eq 41), a rather low value of β = − 216 kcal/mol has to be applied. This scaled TRE plot is shown in Figure 21, which is in reasonable agreement with the results obtained from DFT calculations.
The five or six Pauling bond orders in a pentagon or hexagon respectively can be added to give the Pauling ring bond order.234 For C60-Ih this gives 1.40 (5 × 11/25) for a pentagon and 2.16 (3 × 11/25 + 3 × 7/25) for a hexagon compared to 3.00 for benzene. According to Randić this suggests only moderate aromaticity for C60-Ih.234 We are not going into the controversy of aromaticity of fullerenes,235 but refer to a recent paper by Schleyer and co-workers,236 who find that both C20-Ih and C60-Ih are not spherically π aromatic237 but spherically π anti-aromatic, which for C60 explains the large heat of formation. In this respect it is interesting that Schleyer and co-workers conclude that fullerenes are not highly stable molecules,236 which perhaps is in line with the data shown in Figure 1 showing no ‘magic’ stability for C60 compared to the other fullerenes. We finally mention that fullerene cage abundance is not only guided by thermodynamics, but mostly by kinetic stability. For example, the relative isomer abundance of fullerenes and carbon nanotubes correlates well with kinetic stability.238
Electronic Aspects to Structure and Stability
So far we have discussed topological aspects to determine the structure and stability of fullerenes and basic Hückel theory gives us a first insight into the electronic structure.239 For example, Fowler and co-workers have shown that leapfrog fullerenes adopt a closed-shell structure,212,240,241 and have equal numbers of positive and negative eigenvalues.242 For a more detailed discussion on this subject see Ref 243. However, as we have already seen, more detailed quantum chemical calculations are required to describe the bonding in fullerenes accurately (see also the work by Thiel and co-workers244–246). For example, the IPR isomer C80-C2v(5) shown in Table4 prefers a triplet ground state (at the DFT-PBE level of theory) contrary to Hückel theory, which predicts a singlet state. This is perhaps not surprising as already Hückel theory predicts a small HOMO/LUMO gap. As the band gap closes further with an increasing number of vertices (see for example the recent paper by Noël et al.247), we cannot expect anymore to predict the correct spin ground state from simple topological arguments. Moreover, the system becomes more multi-reference in nature, making even a quantum theoretical treatment difficult. As an example, C50-D5h(271) was investigated by Lu et al. who showed that two singlet states with different HOMO symmetries are quasi-degenerate.248
In addition, some fullerenes may undergo first- or second-order Jahn-Teller (JT) distortions (which could, however, be very small and almost undetectable for larger fullerenes), that is, they can distort to subgroups of the ideal point group symmetry given by the fullerene topology.249,250 For example, C20 is well known to distort away from the ideal Ih symmetry, which has led to much discussion and debate over the correct electronic ground state.251 The JT distortion in C20 is a consequence of two electrons being distributed over four energetically degenerate gu-orbitals, resulting in a rather complicated topology of the JT energy hypersurface.248,250,252–254 JT distortions for highly symmetric open- or closed-shell fullerenes may require a multi-reference treatment. It is therefore often difficult to predict the correct electronic ground state and corresponding physical point group symmetry of a fullerene.
The Gas Phase Formation of Fullerenes
Since the discovery of fullerenes almost 30 years ago, there has been considerable activity, both from the experimental and theoretical side, to gain a detailed understanding of fullerene formation in the gas phase. However, the formation mechanism and especially the high yield of C60-Ih and C70-D5h remains elusive and somewhat controversial.
Fullerenes can be produced by (a) evaporating a carbon target (graphite, amorphous carbon, fullerenes), optionally with addition of metal oxides with a laser,5 (b) an electric arc between carbon electrodes,9 or (c) by partial combustion of carbon rich organic compounds.255 Each of these methods can be adjusted by several experimental parameters (gas pressure, carrier gas, rod feeding rate, amount of oxygen, etc., and therefore indirectly the carbon vapor concentration, expansion rate, annealing time, etc.). Furthermore, fullerenes are found in space,14 at meteor impact sites, after lightnings and bush-fires, and soot from household candles.99 These conditions are similar in that carbon vapor is formed at very high temperatures with a deficit of possible reactants like oxygen or hydrogen. The distribution of yielded fullerene cage sizes depends on the production method and the above-mentioned experimental parameters; however, C60-Ih and C70-D5h are always among the most abundant species.
A large number of formation mechanisms have been proposed.99 First, inspired by their resemblance to graphene sheets or nanotubes, it was suggested that graphene curls or nanotubes break apart to form fullerene cages, followed by various bottom-up strategies that suggest the successive addition of faces (party line256,257, pentagon road257) or polyyne rings (ring stacking258,259) before the cage is closed, the folding of chains to form a cage (ring fusion zipper260), or the growth of already existing cages (fullerene road261, closed network growth98), and many others. The currently most widely accepted mechanism comes from Irle, Zheng, Wang, and Morokuma, and is called the ‘shrinking hot giant road’.99 It is based on the concept of self organizing structures under non-equilibrium conditions, backed by extensive QM/MD simulations.
This mechanism can be divided into five phases: First, linear polyyne chains and cycles form. In the second stage (nucleation), entangled carbon chains rehybridize and form faces. It is to be noted, that pentagons and hexagons are close in energy at the given temperatures,99 however, smaller and larger faces are formed as well. Third, more carbon dimers attach to the side chains of an existing nucleus (growth), allowing for the formation of additional faces. Fourth, after the formation of sufficiently many faces of sizes <6, the cage may close spontaneously (cage closure), leaving a carbon cage with face sizes not restricted to 5 and 6 and with polyyne side chains attached to it. The final step is the ejection of carbon dimers off side chains and the cage in combination with rapid isomerization of the cage structure resulting in a fullerene without side chains and faces of sizes 5 and 6 only. Fullerenes can not only shrink but also grow in steps of C2,95,98,262 and the existence of C2 and C3 fragments is backed by spectroscopy.263 The addition and ejection of small carbon fragments (especially C2) to and from forming fullerene cages is a fast equilibrium. While the non-cage carbon concentration is high addition prevails; as the carbon vapor expands ejection begins to dominate.95 The first four steps are exothermic, while the last step is endothermic but increases the overall entropy.
As shown in Figure 1, large fullerenes are more electronically stable than small ones, with graphene having a lower energy than any fullerene. In an equilibrium one would therefore anticipate the formation of graphene—the formation of strained cages and especially the high yield of C60-Ih and C70-D5h require further explanation. The experimental conditions of the cooling and expanding carbon vapor are, however, far from an equilibrium, and the whole formation must be understood as a process of self-organization that is governed by kinetics more so than thermodynamics. Once fullerenes have formed they are subject to restricted equilibration only. Curl et al. have shown numerically that exchange of carbon dimers between existing cages is sufficient to explain the high yield of C60-Ih and C70-D5h as well as a broad distribution of larger cages.264 They show, that the driving forces for the C2 exchange are the energy difference between CN and CN ± 2 and the high stability of C60-Ih and C70-D5h relative to their isomers rather than entropy; even at 4000 K entropic effects are not strong enough to explain the existence of small cages.264
The experimental observation of endohedral metallofullerenes supports a top-down formation mechanism in two ways: As any enclosed fragments need to enter the cage before it is closed, fullerenes containing fragments that use up most of the space available in the carbon cage must either be formed top down or their existence implies the breaking and reformation of carbon bonds to open and close the cage after it was formed. Secondly, enclosed metal fragments may stabilize otherwise unstable fullerenes: Zhang et al.265 report the finding of non-IPR M2C2@C84(51383), which they interpret to be a missing link in the top-down road, and which would have ejected further C2 fragments without the stabilizing metal carbide.
How Much Space Is Inside a Fullerene? Endohedral Fullerenes and Buckyonions
We consider only topological aspects here as there are several reviews on the chemistry of endohedral fullerenes available.22,266,267 We only note that endohedral enclosure can change the stability between different isomers.268 For example, Tb3N@C84 has been isolated and shows one fused pentagon pair.269 Rodríguez-Fortea et al. argued that if the enclosed metal atom or cluster donates electrons to the fullerene cage, the negative charge resides mostly at the pentagons.270 As this creates a Coulomb repulsion between the pentagons, one can introduce the inverse pentagon separation index (IPSI),
| 47 |
with Rij being the Euclidian distance between the pentagons (although the topological distance might be used instead). Small IPSI-values are preferred270 as these correspond to a small Coulomb repulsion.
It is clear that the cavity in the fullerene cage should be large enough to encapsulate atoms, molecules, or even smaller fullerenes.22,271–282 The size of the cavity can be estimated from fitting shapes such as spheres or ellipsoids inside a fullerene cage as discussed above.
Conversely to the minimum covering sphere problem (MCS), we wish to find the largest sphere that is fully contained within the polyhedron. This is called the maximal inscribed sphere (MIS), or simply inscribed sphere, and its radius for a given polyhedron P is
| 48 |
where  is constrained to the points interior to P. In the case of convex polyhedra, the MIS is unique, and can be computed directly by finding the Chebyshev centre of the polyhedron.283 The inscribed radius is then determined by a linear programming problem. However, in the non-convex case, the MIS is no longer unique: Consider two overlapping circles in 2D space with the points lying on these circles. We now have two equivalent possibilities for placing our inner circle. The same argument holds for peanut-shaped fulleroids, which are introduced in the last chapter. Hence, in the non-convex case, we only search for one of the possibly many largest spheres contained entirely inside the polyhedron, giving us a numerical optimization problem.
is constrained to the points interior to P. In the case of convex polyhedra, the MIS is unique, and can be computed directly by finding the Chebyshev centre of the polyhedron.283 The inscribed radius is then determined by a linear programming problem. However, in the non-convex case, the MIS is no longer unique: Consider two overlapping circles in 2D space with the points lying on these circles. We now have two equivalent possibilities for placing our inner circle. The same argument holds for peanut-shaped fulleroids, which are introduced in the last chapter. Hence, in the non-convex case, we only search for one of the possibly many largest spheres contained entirely inside the polyhedron, giving us a numerical optimization problem.
Once the MIS has been obtained, we can estimate whether an atom or molecule fits inside the fullerene without coming close to the repulsive wall of the fullerene cage. For example, the space available inside C60-Ih is roughly RMIS(C60) − RVdW(C) ≈ 3.550 − 1.415Å = 2.135Å. If we compare this to the Van der Waals radii for the rare gas atoms, which are 1.40 Å for He, 1.54 Å for Ne, 1.88 Å for Ar, 2.02 Å for Kr, and 2.16 Å for Xe,284 we see that they all fit into C60-Ih as experimentally already verified285–291 (even for the borderline case of Xe as theoretical investigations show292–294). Even molecules like water fit into C60.295,296 If we take C20 as a endohedral host molecule we arrive at a different picture as RMIS(C20) − RVdW(C) = 2.040 − 1.415Å = 0.635Å, and even He does not fit anymore into the C20 cage.297,298 We note the important role of dispersion interactions in endohedral fullerenes.299 Further, C60 is a very stiff molecule,300 and the cage cannot so easily be expanded by endohedral atoms or molecules. In other words, the endohedral system can become repulsive very soon if the size of the endohedral atom or molecule becomes too large.301
As a further example we consider hyperfullerenes (also called buckyonions),271,272,274,302 that is, fullerenes which contain smaller fullerenes inside their cage. They have already been detected and studied by theoretical methods.271,272,274,277–280 In a similar way to the rare gas estimates, we can derive approximately the following (conservative) condition for a non-repulsive enclosing hyperfullerene,
| 49 |
This only holds for ideal spherical systems, but can be seen as a lower limit for the guest CM fullerene. For C20@C60 we get ΔR60,20 = (3.553 − 2.084 − 2 × 1.415) Å = − 1.361 Å and it is therefore predicted to be thermodynamically very unstable. Indeed, DFT(B3LYP) calculations predict C20 @ C60 → C20 + C60 − 1230 kcal/mol (−15 kcal/mol per carbon atom), the high energy required to expand the C60 cage. In fact, analyzing C20@C60 we find that the carbon atoms of C20 close bonds with the carbon of the C60 cage, that is, the structure should be considered as a carbon cluster rather than a hyperfullerene. Moreover, the volume of C20@C60 increases by 22% compared to C60. In contrast, C60 fits nicely into C240, and C240 just into C540 in agreement with the analysis of Bates and Scuseria (see Figure 22).303 Here we get ΔR240,60 = 0.583Å and ΔR540,240 = 0.066Å.
Figure 22.

The buckyonion structure C60@C240@C540.
The buckyonions, which have been considered in the past, are all Goldberg-Coxeter transforms of C20 and have the most spherical appearance as they are of icosahedral symmetry. It has been suggested that the rather high sphericity observed for buckyonions in experiments in contrast to the faceted polyhedral structure predicted (see Figure 22) is due to C2 removal in a [c5666] fragment consisting of 1 pentagon and 3 hexagons (notation of Figure 16 is used here) introducing one heptagon and an additional pentagon, that is, [l575].303 As heptagons introduce local negative Gaussian curvature (see discussion below), the fragment flattens out and the buckyonion becomes more spherical in shape.303
For the buckonions one can establish a simple relationship between the number of vertices N > M of the buckyonions CM@CN. As  (R being the radius of the cage), an analysis of GCk,l-transforms of fullerenes up to k = 6 shows that
(R being the radius of the cage), an analysis of GCk,l-transforms of fullerenes up to k = 6 shows that  . This gives a very simple estimate from Eq 49 for such fullerenes,
. This gives a very simple estimate from Eq 49 for such fullerenes,
| 50 |
and we see that this is fulfilled for the cases already discussed, and for the largest buckyonion C60@C240@C540@C960@C1500 considered by Bates and Scuseria.303 In fact, Casella et al. recently showed by using dispersion corrected DFT that the formation of C60@C180 from the two fullerenes is endothermic while C60@C240 is exothermic, and in the latter case only because dispersion interactions have been accounted for.304 We finally note that (possibly damaged) buckyonions are the main product in most gas phase fullerene generation procedures.262
Weird Fulleroidal Shapes: Generalizing Fullerene Structures
Finally, what happens if we relax the rules a little bit, and allow for other types of three-valent (sp2) carbon frameworks? There are many generalizations that lead to structures of beautiful shapes that have both elegant mathematical theory and physical realizations: allowing for polygons with faces different from pentagons and hexagons; for surfaces other than a sphere (genus 0), such as a torus (genus 1), a Klein bottle or a double torus (genus 2); negatively curved Mackay-Terrones or Vanderbilt-Tersoff surfaces; etc.121,242,305–309
We will discuss mainly two types of carbon frameworks: fulleroids, which are fullerene-like structures, and Schwarzites, which are periodic negative-curvature surfaces. Different authors employ a wide range of working definitions for fulleroids. We advocate the following definition, generalizing fullerenes: A fulleroid graph is a three-connected trivalent polyhedral graph. The definition is identical to that of fullerenes, except that there is no restriction on face sizes. Hence, a fullerene is a fulleroid with only pentagon and hexagon faces. We further generalize to genus-n fulleroids, abbreviated Gn-fulleroids, defined as the three-connected trivalent genus-n graphs.
What kind of fulleroids are allowed? Can we tile a sphere or a torus with heptagons only (the answer is no), or with only pentagons and heptagons? (the answer is yes, with the right number.) And how can we construct such fulleroids?310 In order to answer these questions, we need to generalize Euler's equation to the Euler-Poincaré polyhedral formula for orientablel surfaces,
| 51 |
where g is the genus of the graph,m and the corresponding value χ is called the Euler characteristic.39 The Klein bottle is a non-orientable surface (like the Möbius strip), and the corresponding (non-orientable) genus is g′ = 2 with an Euler characteristic of χ = 0 (here we take χ = 2 − g′ instead).
What happens when we allow vertex degrees r > 3? Consider a possibly irregular genus-n graph with N vertices. Let Nr be the number of vertices with degree r, and Fn be the number of n-gonal faces. Then we trivially have
| 52 |
Combining this with the Euler-Poincaré polyhedral formula, we obtain
| 53 |
If we only allow for three-valent graphs (fulleroids), the first term on the left hand side of Eq 53 conveniently vanishes (hence their special place in graph theory). This gives,311
| 54 |
From this equation we make a number of observations. a) We can play with as many hexagons as we want to build fullerenes or fulleroids, but the number of n-gons with n ≠ 6 is limited by Eq 54 or 53. b) While both equations allow for a large variety of face combinations, certain combinations are not allowed. The hand-shaking lemma (last formula in Eq 52) allows only for an even number of vertices. We already know that there exist no polyhedral structures of the type C22[5,6]. To give another example, for CN[5,7] polyhedra with pentagons and heptagons only (no hexagons) one requires at least two heptagons since, analogous to C22, the combination of 13 pentagons and one heptagon is not valid despite being allowed by Euler's formula. Because of this, the smallest [5,7]-fulleroid is C28-D7d with 14 pentagons and 2 heptagons, as shown in Figure 23.n Structures containing only one heptagon are not forbidden, however, but requires introducing hexagons to form CN[5,6,7]-structures. These can be obtained by replacing a [c5666] fragment by [l575],303 and can even be energetically favorable. For example, Fowler and co-workers showed that C62 consisting of one heptagon, 13 pentagon, and 19 hexagon faces is of lower energy than all the 2385 regular fullerene isomers of C62.312 c) For cubic planar graphs with only one type of face we get F3 = 4 (tetrahedron), F4 = 6 (cube), and F5 = 12 (dodecahedron), which are the three three-valent polyhedra of the five Platonic Solids, the other two, the octahedron and icosahedron, require r = 4 and r = 5, respectively, in Eq 53; d) If we allow for two different n-gons, we arrive at the 13 Archimedean solids including our famous buckyball C60-Ih. e) Of course, we can tile a surface with triangles and the duals of fulleroids are prime examples, but in order to do this beside the tetrahedron, we must allow for different valencies of the vertices. f) We can tile a toroid (g = 1) with hexagons only, consistent with Eq 54, see Figure 24. The torus is the only closed, orientable surface that can be tiled exclusively with hexagons. However, the plane, which is not closed, can be tiled with hexagons as well (graphene), as can the Klein-bottle (which is not orientable). Introducing pentagons into a toroid requires higher n-gons such as heptagons, since its total Gaussian curvature must be 2π(2 − 2 · 1) = 0. The Euler-Poincaré's formula allows to introduce pentagons into toroids if we introduce an equal number of heptagons, for example, we can tile a torus exclusively with pentagons and heptagons. Such ‘defects’ are well known in carbon nanotubes,313 which can be realized as infinite toroids with g = 1 and χ = 0. Tiling in a pattern with alternating pentagons and heptagons yields a toroidal arrangement of azulenes, called azuloids.314
Figure 23.
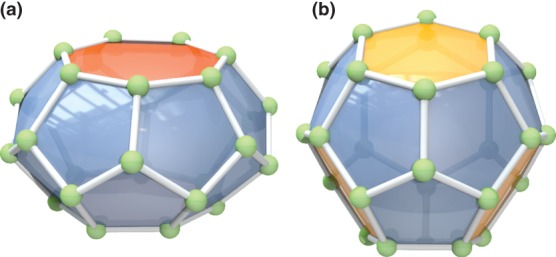
C28[5,7]-D7d, the smallest fulleroid with heptagon extension, compared to the most stable (by ΔE= −102 kcal/mol) C28-Td[5,6] fullerene isomer.
Figure 24.

Torus consisting of hexagons only with 1/3 of the edges tangential (a) and perpendicular (b) to the toroidal direction.
A few examples should be mentioned here. The smaller fullerenes (N < 60) are not very stable compared to C60 because of the energy penalty one has to pay for fusing pentagons. As a result, structures deviating from the classical fullerenes can be more stable energetically or lie close by. For example, for C26, An et al. found six low lying isomers with the lowest structure being the fullerene C26-D3h[5,6], but only 3 kcal/mol lower in energy than the C26-Cs[4,5,6], a fulleroid that contains one square.315 Some combinations with heptagons are, however, disfavored energetically. For example, a Stone-Wales transformationo of four connected hexagons to two pentagons and two heptagons in C540-D2h costs 260 kcal/mol.317
Turning to toroidal fulleroids (also called toroids) with g = 1 and χ = 0, there are different techniques to construct such structures using either combinatorial or geometric approaches.318–320 Like in the nano-tubes, the hexagons can be oriented in different ways around the torus ring. For example, Figure 24(a) shows hexagons with 1/3 of the edges aligned tangential to the toroidal direction. We could also chose the hexagons to be aligned with 1/3 of the edges perpendicular to the toroidal direction (Figure 24(b)), and similar to nanotubes there are also chiral alignments depending on the chirality vector. Kirby and Pisanski showed how 2D graph drawings of toroids can be obtained.314 Borštnik and Lukman321 as well as Diudea and Kirby322 considered the structure and stability of such toroids using simple molecular mechanics. Faghani analyzed the symmetry of toroidal fulleroids.126 Kang analyzed the band gap in such toroids by graph theoretical means.323 Deza et al. pointed out that leapfrog toroids and Klein-bottles have equal numbers of positive and negative eigenvalues, but with 4 and 2 eigenvalues being zero respectively.242 Interestingly, the leapfrog transformation performed on toroids (such as the one shown in Figure 24) results in an open shell toroid.324
As discussed earlier, the positive curvature in fullerenes originate from the pentagons, because sheets of hexagons like to be planar. Introducing heptagons or even octagons into a fullerene requires additional faces of size less than 6 to outweigh them, and results in a highly non-convex structure with often strong negative local Gaussian curvature,325 introducing a saddle-type topology to the structure. Two examples of such fulleroids are shown in Figure 25. In this figure, every patch consisting of a pentagon surrounded by hexagons is replaced by six pentagons surrounded by five heptagons, for three different fullerenes, yielding characteristic ‘spiky’ fulleroids. In Figure 26, heptagons are introduced in a fashion that leads to peanut shaped fulleroids,326 and in fact, many types of interesting shapes can be constructed.
Figure 25.

Spiky fulleroids with negative curvature containing heptagons derived from fullerenes through patch replacement. (a) C260-I[5, 7] fulleroid derived from C140-I. (b) C300-D6h[5, 6, 7] derived from C180-D6h. (c) C310-D5h[5, 6, 7] derived from C190-D5h.
Figure 26.

Peanut shaped fulleroids. (a) C120-D5d[5, 6, 7] with 10 heptagons and 22 pentagons. (b) C168-D3d[5, 6, 7] with 18 heptagons and 30 pentagons.
More interesting fulleroid shapes can be found in a recent paper by Diudea et al.327 Negatively curved fulleroids of higher genus (so-called perforated fullerenes) have been explored by Terrones and Terrones,328 who studied their electronic structures.329 Under specific conditions fullerenes can be combined by coalescence, forming larger carbon structures such as deformed nano-tubes or nano-peapods.330 For an overview of such carbon nano-peapods see Ref 331.
Structures of negatively curved graphitic carbon, which can be periodically extended to a lattice, have been proposed in 1991 by Mckay and Terrones,305 and subsequently explored by experiment.332–335 These so-called ‘spongy’ carbon frameworks or Schwarzites are named in honor of the mathematician Hermann Schwarz (1843–1921), who investigated minimal surfaces with zero mean Gaussian curvature.336 Schwarzites are structures containing (beside hexagons) heptagons and/or octagons and are of genus g ≥ 3. These Schwarzites can be periodically continued through so-called junctions as shown in Figure 27 for the case g = 3 (Figure 27(a)). The two structures in Figure 27 are termed P-type and D-type, and can be approximately represented by the simple formulae derived from the Weierstrass-Enneper parameterization for minimal surfaces,337
| 55 |
 |
56 |
Figure 27.

Periodic P-type (a) and D-type (b) Schwarzite surfaces.
Relevant reviews on such minimal surface carbon networks have been given by Terrones and Mackay311 and by Terrones and Terrones.338
Lenosky et al. have performed local density functional calculations for two different g = 3 periodic Schwarzite structures with 216 atoms and a tessellation of hexagons and 24 heptagons within the unit cell.339 The vertices on such surfaces can be obtained through the Weierstrass representation.340,341 These calculations reveal a higher thermodynamic stability compared to C60, but not surprisingly lower compared to the graphene sheet (or graphite). D-type Schwarzites have the structure of a ‘diamond shaped’ lattice so that the unit cell can be split into two identical elements having 12 heptagons each, whereas P-type Schwarzites have the structure of a simple cubic lattice (see Figure 7). In principle, Schwarzites can be associated to any kind of lattice, either periodic or amorphous, the latter are realized in so-called random Schwarzites observed experimentally.332–335 Such random Schwarzites show rather nice transmission electron microscope (TEM) images (Figure 28) rather different to periodic Schwarzites. A recent comprehensive review over experimental and theoretical studies on Schwarzites has been given by Benedek et al.335
Figure 28.

Comparison between a small portion of a TEM image of a P-type Schwarzite and the surface described by Eq 55 projected onto a plane normal to the z-direction (see Ref 333 for details). (Copyright © 2013 AIP Publishing LLC)
Finally, we consider non-cubic (n-valent) polyhedral structures. In chemistry this is realized by using elements from the periodic table which can share more (or less) than three bonds with its neighbors. Prime examples are all-boron ‘fullerenes’,342 gaudiene C72-Oh (with two- and three-valent carbon as analogs to graphyne),343,344 and golden ‘fullerenes’345–348 (we shall call these structures polyhedral molecules rather than fullerenes). For example, Au32 and Au72 are a triangulated surfaces of icosahedral symmetry.346–348 It is well known that small gold clusters adopt 2D structures with the most coordinated gold atom having a valency of six.349 Extended to infinity, this gives the dual structure of a graphene sheet, that is a triangulation of the plane with vertices of degree six (see Figure 29). In complete analogy to the fullerenes, golden ‘fullerenes’ are the duals of fullerene structures, for example, Au32-Ih is the dual of C60-Ih, Au42-I is the dual of C80-I, and Au72-I is the dual of C140-I (the Goldberg-Coxeter transform of C20 with k = 2 and l = 1). We may ask how many possible isomers are there for Au72? Since the mapping from the fullerene isomer space into the dual space is bijective, there are as many isomers for Au72 as there are for C140, and there are 7,341,204 of these. The stability of these structures have not been explored yet! A smaller dual-type golden fullerene structure, Au , has been observed spectroscopically by Bulusu et al.345 Here the fullerene analog is C28, where we have only two isomers, of Td and D2 symmetry. Only the Au
, has been observed spectroscopically by Bulusu et al.345 Here the fullerene analog is C28, where we have only two isomers, of Td and D2 symmetry. Only the Au -Td structure has been investigated presently.
-Td structure has been investigated presently.
Figure 29.
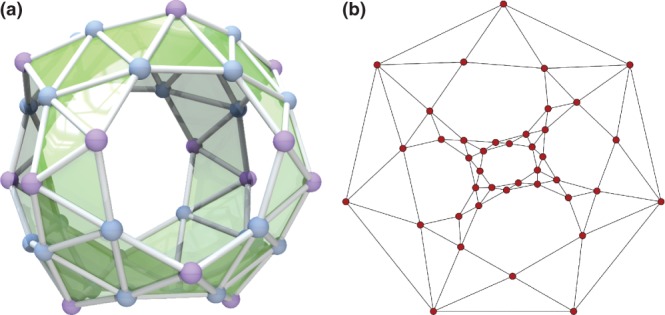
All boron fullerene B40 (adapted from Ref 350). (a) 3D structure; (b) 2D graph.
As a second example, the all-boron B40 fullerene has been identified by photoelectron spectroscopy only very recently by Wang and co-workers (as the anionic species B ),350 consisting of 4 heptagons, 2 hexagons, and 48 triangles (see Figure 29). In this intriguing new polyhedral structure the vertices are of degrees 4 and 5. To be more precise, we have F3 = 48, F6 = 2, F7 = 4, N4 = 16, N5 = 24 for B40, which gives according to Eq 52
E = 92 for the number of edges.
),350 consisting of 4 heptagons, 2 hexagons, and 48 triangles (see Figure 29). In this intriguing new polyhedral structure the vertices are of degrees 4 and 5. To be more precise, we have F3 = 48, F6 = 2, F7 = 4, N4 = 16, N5 = 24 for B40, which gives according to Eq 52
E = 92 for the number of edges.
CONCLUSIONS
To review the many developments in the topology and graph theory of fullerenes or fulleroids would be a monumental task. We have only outlined a few important concepts in order to give the reader a good introduction into this exciting field. There is certainly the need of a more comprehensive review or book. Nevertheless, we hope to have shown that the interplay between mathematics and structural chemistry is both interesting, rich and well alive. As more sophisticated method in the synthesis of carbon and other materials become available, we hope that some of the exotic, but very beautiful, structures become accessible with many useful applications in chemistry and materials science.
Acknowledgments
PS is indebted to the Alexander von Humboldt Foundation (Bonn) for financial support in terms of a Humboldt Research Award, and to both Gernot Frenking and Ralf Tonner (Marburg) for support during his extended stay in Marburg where the topology project began. We also thank Stephan Irle (Nagoya) for valuable information on fullerene formation, Lai-Sheng Wang (Brown University), and Jun Li (Beijing) for information of the non-cubic fulleroid B40, and Ottorino Ori (Parma), Dage Sundholm (Helsinki), Michel Deza (Paris), and Helmut Schwarz (Berlin) for valuable information. JA was supported by VILLUM FONDEN through the network for Experimental Mathematics in Number Theory, Operator Algebras, and Topology.
Footnotes
If we grow Goldberg-Coxeter transforms of C20 to infinity we obtain spherical graphene sheets connected by 12 pentagons. For definitions see below.
An interesting side aspect is that fullerene-like shapes are realized also in viral shapes.20
Most of the useful graph theoretical and topological aspects discussed here are implemented into a Fortran/C++ program called Fullerene, an open-source code freely available at the Massey University website.35
The embedding is unique in the following sense: All the planar embeddings of a three-connected graph are topologically equivalent, which means that they can be continuously deformed into each other without crossing any edges. Consequently, every planar embedding of such a graph defines the same set of faces. The converse it true as well: only three-connected graphs have this property.
Fulleroids are cubic graph fullerene-like structures where we allow also for other polygons than pentagons or hexagons.
In the mathematical sense that they are dense in this set, i.e. any smooth non-negative curvature genus 0 surface is the limit of a sequence of genus 0 triangulations with vertex degree six and less.
The interested reader is directed to the Gauss-Bonnet theorem, which generalizes Euler's theorem to arbitrary Riemann surfaces. In the discrete case they are the same, except that Gauss-Bonnet is stated in terms of the Gaussian curvature. From this identification, we can see that every n-gon, or vertex of degree n in the dual, contributes the angle 2π(6 − n)/6 to the Gaussian curvature, which must sum to 2π(2 − 2g), where g is the surface's genus.
When there are multiple exponentially crowded centers, the Tutte-embedding is still guaranteed planar, but the mapping to the sphere can have crossings. In practice, however, it turns out to not matter.
Counting perfect matchings for general graphs is #P-complete. #P is the complexity class of counting solutions for decision problems in NP. While only exponential algorithms exist both for NP-complete and #P-complete problems, in practice #P-complete problems are dramatically harder than NP-complete.
It is known that not all connected cubic planar graphs are Hamiltonian, and that it is an NP-complete problem to determine whether any one cubic planar graph admits a Hamilton cycle. However, it is a long standing but yet unproven conjecture that fullerene graphs, a small subset of the cubic planar graphs, are all Hamiltonian. It has been verified by Brinkmann, Goedgebeur, and McKay for all 2.4 × 1011 fullerene isomers up to C316.75 In Schwerdtfeger et al. (unpublished manuscript), we investigate in detail the Hamiltonian cycle counts admitted by fullerene graphs.
hentriacontacyclo[29.29.0.02,14.03,12.04,59.05,10.06,58.07,55.08,53.09,21.011,20.013,18.015,30.016,28.017,25.019,24.022,52.023,50.026,49.027,47.029,45.032,44.033,60.034,57.035,43.036,56.037,41.038,54.039,51.040,48.042,46]hexaconta− 1, 3, (10), 6, 8, 11, 13(18), 14, 16, 19, 21, 23, 25, 27, 29(45), 30, 32(44), 33, 35(43), 36, 38(54), 39(51), 40(48), 41, 46, 49, 52, 55, 57, 59 −triacontaene.
Orientability is a property of a surface in Euclidean space describing whether it is possible to make a consistent choice of a surface normal vector at every point.
The genus of a connected and orientable surface is the maximum number of possible cuttings along non-intersecting closed simple curves such that the surface is still connected.
Fulleroids may in general have point group symmetries not admissible to the regular fullerenes.
There are many other operations which can be performed on polyhedra resulting in transformed fullerenes or fulleroids, see the review by Vizitiu and Diudea.316
REFERENCES
- Ōsawa E. Superaromaticity. Kagaku (Chem) 1970;25:854–863. [Google Scholar]
- Ōsawa E, Kroto HW, Fowler PW, Wasserman E. The evolution of the football structure for the C60 molecule: a retrospective [and discussion] Phil Trans R Soc Lond A. 1993;343:1–8. . doi: 10.1098/rsta.1993.0035. [Google Scholar]
- Bochvar DA, Gal'pern EG. Electronic structure of the molecules C20 and C60. Proc Acad Sci SSSR. 1973;209:239–241. [Google Scholar]
- Stankevich I, Nikerov M, Bochvar D. Structural chemistry of crystalline carbon: geometry, stability, electronic spectrum. Russ Chem Rev. 1984;53:640–655. . doi: 10.1070/RC1984v053n07ABEH003084. [Google Scholar]
- Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163. . doi: 10.1038/318162a0. [Google Scholar]
- Kroto HW. C60: Buckminsterfullerene, the celestial sphere that fell to earth. Angew Chem Int Ed. 1992;31:111–129. . doi: 10.1002/anie.199201113. [Google Scholar]
- Hare JP, Kroto HW. A postbuckminsterfullerene view of carbon in the galaxy. Acc Chem Res. 1992;25:106–112. . doi: 10.1021/ar00015a002. [Google Scholar]
- Kroto H. Symmetry, space, stars, and C60 (Nobel lecture) Angew Chem Int Ed. 1997;36:1578–1593. . doi: 10.1002/anie.199715781. [Google Scholar]
- Krätschmer W, Lamb LD, Fostiropoulos K, Huffman DR. Solid C60: a new form of carbon. Nature. 1990;347:354–358. . doi: 10.1038/347354a0. [Google Scholar]
- Mattauch J, Ewald H, Hahn O, Strassmann F. Hat ein Caesium-Isotop langer Halbwertszeit existiert? Ein Beitrag zur Deutung ungewöhnlicher Linien in der Massenspektrographie. Z Physik. 1943;120:598–617. [Google Scholar]
- Iijima S. Direct observation of the tetrahedral bonding in graphitized carbon black by high resolution electron microscopy. J Cryst Growth. 1980;50:675–683. . doi: 10.1016/0022-0248(80)90013-5. [Google Scholar]
- Buseck PR, Tsipursky SJ, Hettich R. Fullerenes from the geological environment. Science. 1992;257:215–217. doi: 10.1126/science.257.5067.215. . doi: 10.1126/science.257.5067.215. [DOI] [PubMed] [Google Scholar]
- Cami J, Bernard-Salas J, Peeters E, Malek SE. Detection of C60 and C70 in a young planetary nebula. Science. 2010;329:1180–1182. doi: 10.1126/science.1192035. . doi: 10.1126/science.1192035. [DOI] [PubMed] [Google Scholar]
- Berné O, Tielens AGGM. Formation of buckminsterfullerene (C60) in interstellar space. Proc Natl Acad Sci U S A. 2012;109:401–406. doi: 10.1073/pnas.1114207108. . doi: 10.1073/pnas.1114207108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz HP. Topological organic chemistry. polyhedranes and prismanes. J Org Chem. 1965;30:1361–1364. . doi: 10.1021/jo01016a005. [Google Scholar]
- Prinzbach H, Weiler A, Landenberger P, Wahl F, Worth J, Scott LT, Gelmont M, Olevano D, v Issendorff B. Gas-phase production and photoelectron spectroscopy of the smallest fullerene C20. Nature. 2000;407:60–63. doi: 10.1038/35024037. . doi: 10.1038/35024037. [DOI] [PubMed] [Google Scholar]
- Sutton D. Platonic and Archimedean Solids. New York: Wooden Books; 2002. [Google Scholar]
- Kroto HW. The stability of the fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature. 1987;329:529–531. . doi: 10.1038/329529a0. [Google Scholar]
- Thurston WP. Shapes of polyhedra and triangulations of the sphere. Geo Topol Mono. 1998;1:511–549. [Google Scholar]
- Zhao G, Perilla JR, Yufenyuy EL, Meng X, Chen B, Ning J, Ahn J, Gronenborn AM, Schulten K, Aiken C. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature. 2013;497:643–646. doi: 10.1038/nature12162. doi: 10.1038/nature12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagmatarchis N, Okada K, Tomiyama T, Yoshida T, Kobayashi Y, Shinohara H. A catalytic synthesis and structural characterization of a new [84]fullerene isomer. Chem Commun. 2001;1366–1367 . doi: 10.1039/B103679N. [Google Scholar]
- Popov AA, Yang S, Dunsch L. Endohedral fullerenes. Chem Rev. 2013;113:5989–6113. doi: 10.1021/cr300297r. . doi: 10.1021/cr300297r. [DOI] [PubMed] [Google Scholar]
- Yamada M, Akasaka T, Nagase S. Carbene additions to fullerenes. Chem Rev. 2013;113:7209–7264. doi: 10.1021/cr3004955. . doi: 10.1021/cr3004955. [DOI] [PubMed] [Google Scholar]
- Franco JU, Hammons JC, Rios D, Olmstead MM. New tetraazaannulene hosts for fullerenes. Inorg Chem. 2010;49:5120–5125. doi: 10.1021/ic1002513. . doi: 10.1021/ic1002513. [DOI] [PubMed] [Google Scholar]
- York APE. Inorganic fullerenes, onions, and tubes. J Chem Educ. 2004;81:673. . doi: 10.1021/ed081p673. [Google Scholar]
- Scott LT, Boorum MM, McMahon BJ, Hagen S, Mack J, Blank J, Wegner H, de Meijere A. A rational chemical synthesis of C60. Science. 2002;295:1500–1503. doi: 10.1126/science.1068427. . doi: 10.1126/science.1068427. [DOI] [PubMed] [Google Scholar]
- Kabdulov M, Jansen M, Amsharov KY. Bottom-up C60 fullerene construction from a fluorinated C60H21F9 precursor by laser-induced tandem cyclization. Chem Eur J. 2013;19:17262–17266. doi: 10.1002/chem.201303838. . doi: 10.1002/chem.201303838. [DOI] [PubMed] [Google Scholar]
- Cioslowski J. Electronic Structure Calculations on Fullerenes and Their Derivatives. New York: Wooden Books; 1995. [Google Scholar]
- Balaban AT, Liu X, Klein DJ, Babić D, Schmalz TG, Seitz WA, Randic M. Graph invariants for fullerenes. J Chem Inf Comput Sci. 1995;35:396–404. . doi: 10.1021/ci00025a007. [Google Scholar]
- Zhu HY, Klein DJ. Graph-geometric invariants for molecular structures. J Chem Inf Comput Sci. 1996;36:1067–1075. . doi: 10.1021/ci960025a. [Google Scholar]
- Fowler PW, Manolopoulos DE. An Atlas of Fullerenes. 2nd ed. Mineola, NY: Dover Publications Inc; 2006. [Google Scholar]
- Alcamí M, Sánchez G, Díaz-Tendero S, Wang Y, Martín F. Structural patterns in fullerenes showing adjacent pentagons: C20 to C72. J Nanosci Nanotechnol. 2007;7:1329–1338. doi: 10.1166/jnn.2007.311. . doi: 10.1166/jnn.2007.311. [DOI] [PubMed] [Google Scholar]
- Cioslowski J, Rao N, Moncrieff D. Standard enthalpies of formation of fullerenes and their dependence on structural motifs. J Am Chem Soc. 2000;122:8265–8270. . doi: 10.1021/ja001109+ [Google Scholar]
- Brinkmann G, Goedgebeur J, Mélot H, Coolsaet K. House of graphs: a database of interesting graphs. Discrete Appl Math. 2013;161:311–314. [Google Scholar]
- Schwerdtfeger P, Wirz L, Avery J. Program fullerene: a software package for constructing and analyzing structures of regular fullerenes. J Comput Chem. 2013;34:1508–1526. doi: 10.1002/jcc.23278. . doi: 10.1002/jcc.23278. [DOI] [PubMed] [Google Scholar]
- Fowler PW. Fullerene stability and structure. Contemp Phys. 1996;37:235–247. . doi: 10.1080/00107519608217530. [Google Scholar]
- Baldridge KK, Siegel JS. Of graphs and graphenes: molecular design and chemical studies of aromatic compounds. Angew Chem Int Ed. 2013;52:5436–5438. doi: 10.1002/anie.201300625. . doi: 10.1002/anie.201300625. [DOI] [PubMed] [Google Scholar]
- Trinajstic N. Chemical Graph Theory. Boca Raton, FL: CRC Press; 1983. [Google Scholar]
- Kotschik D. The topology and combinatorics of soccer balls. Am Sci. 2006;94:350–357. . doi: 10.1511/2006.60.1001. [Google Scholar]
- Cataldo F, Graovac A, Ori O. The mathematics and topology of fullerenes. Berlin: Springer; 2011. [Google Scholar]
- Dresselhaus MS, Dresselhaus G, Eklund PC. Science of Fullerenes and Carbon Nanotubes. New York: Academic Press; 1995. [Google Scholar]
- Curl RF. Dawn of the fullerenes: experiment and conjecture. Rev Mod Phys. 1997;69:691–702. . doi: 10.1103/RevModPhys.69.691. [Google Scholar]
- Kadish KM, Ruoff RS, editors. Fullerenes: Chemistry, Physics, and Technology. New York: Wiley-Interscience; 2000. [Google Scholar]
- Hirsch A, Brettreich M, Wudl F. Fullerenes: Chemistry and Reactions. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- Rodríguez-Fortea A, Irle S, Poblet JM. Fullerenes: formation, stability, and reactivity. WIREs Comput Mol Sci. 2011;1:350–367. . doi: 10.1002/wcms.21. [Google Scholar]
- Sheka EF. Fullerenes: Nanochemistry, Nanomagnetism, Nanomedicine, Nanophotonics. Boca Radon, FL: CRC Press, Taylor & Francis; 2011. [Google Scholar]
- Darwish AD. Fullerenes. Annu Rep Prog Chem, Sect A Inorg Chem. 2012;108:464–477. . doi: 10.1039/C2IC90017C. [Google Scholar]
- Whitney H. Non-separable and planar graphs. Trans Am Math Soc. 1932;34:339–362. . doi: 10.1090/S0002-9947-1932-1501641-2. [Google Scholar]
- Steinitz E. Polyeder und Raumeinteilungen. In: Klein F, Meyer W, editors. Encyclopädie der mathematischen Wissenschaften. Vol. 3. Leipzig: B.G. Teubner Verlag; 1922. pp. 1–139. (Geometrie). [Google Scholar]
- Schmalz TG, Seitz WA, Klein DJ, Hite GE. Elemental carbon cages. J Am Chem Soc. 1988;110:1113–1127. . doi: 10.1021/ja00212a020. [Google Scholar]
- Grünbaum G, Motzkin TS. The number of hexagons and the simplicity of geodesics on certain polyhedra. Can J Math. 1963;15:744–751. . doi: 10.4153/CJM-1963-071-3. [Google Scholar]
- Killblane C, Gao Y, Shao N, Zeng XC. Search for lowest-energy nonclassical fullerenes III: C22. J Phys Chem A. 2009;113:8839–8844. doi: 10.1021/jp9016745. . doi: 10.1021/jp9016745. [DOI] [PubMed] [Google Scholar]
- Janežič D, Miličević A, Nikolić S. Trinajstić N. In: Gutman I, editor. Graph Theoretical Matrices in Chemistry. Vol. 3. Kragujevac, Serbia: University of Kragujevac; 2007. (Mathematical Chemistry Monographs). [Google Scholar]
- Tutte WT. How to draw a graph. Proc Lond Math Soc. 1963;13:743–767. [Google Scholar]
- Brandenburg FJ, Himsolt M, Rohrer C. An experimental comparison of force-directed and randomized graph drawing algorithms. In: Brandenburg FJ, editor. Graph Drawing. Vol. 1027. Berlin Heidelberg: Springer; 1996. pp. 76–87. (Lecture Notes in Computer Science). [Google Scholar]
- Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Softw Pract Exp. 1991;21:1129–1164. . doi: 10.1002/spe.4380211102. [Google Scholar]
- Plestenjak B. 1996. An algorithm for drawing Schlegel diagrams. Available at: http://www-lp.fmf.uni-lj.si/plestenjak/Papers/NICEGR.pdf1–10. (Accessed July 1, 2014)
- Manolopoulos DE, May JC, Down SE. Theoretical studies of the fullerenes: C34 to C70. Chem Phys Lett. 1991;181:105–111. . doi: 10.1016/0009-2614(91)90340-F. [Google Scholar]
- Wirz LN, Avery JE, Schwerdtfeger P. Structure and properties of the non-face-spiral fullerenes T-C380D3-C384D3-C440 and D3-C672 and their halma and leapfrog transforms. J Chem Inf Model. 2014;54:121–130. doi: 10.1021/ci4005578. . doi: 10.1021/ci4005578. [DOI] [PubMed] [Google Scholar]
- Manolopoulos DE, Fowler PW. A fullerene without a spiral. Chem Phys Lett. 1993;204:1–7. . doi: 10.1016/0009-2614(93)85597-H. [Google Scholar]
- Brinkmann G, Goedgebeur J, McKay BD. The smallest fullerene without a spiral. Chem Phys Lett. 2012;522:54–55. . doi: 10.1016/j.cplett.2011.11.056. [Google Scholar]
- Fowler P, Cremona J. Fullerenes containing fused triples of pentagonal rings. J Chem Soc Faraday Trans. 1997;93:2255–2262. . doi: 10.1039/A701271C. [Google Scholar]
- Yoshida M, Fowler PW. Dihedral fullerenes of threefold symmetry with and without face spirals. J Chem Soc Faraday Trans. 1997;93:3289–3294. . doi: 10.1039/A702351K. [Google Scholar]
- Brinkmann G, Fowler PW, Yoshida M. New non-spiral fullerenes from old: generalised truncations of isolated-pentagon-triple carbon cages. MATCH Commun Math Comput Chem. 1998;7–17 [Google Scholar]
- Brinkmann G. Problems and scope of spiral algorithms and spiral codes for polyhedral cages. Chem Phys Lett. 1997;272:193–198. . doi: 10.1016/S0009-2614(97)88009-8. [Google Scholar]
- Fowler PW, Graovac A, Žerovnik J, Pisanski T. 1998. A generalized ring spiral algorithm for coding fullerenes and other cubic polyhedra. Preprint series/Institute of Mathematics, Physics and Mechanics, Department of Mathematics, University of Ljubljana.
- Fowler PW, Horspool D, Myrvold W. Vertex spirals in fullerenes and their implications for nomenclature of fullerene derivatives. Chem Eur J. 2007;13:2208–2217. doi: 10.1002/chem.200601107. . doi: 10.1002/chem.200601107. [DOI] [PubMed] [Google Scholar]
- Cozzi F, Powell WH, Thilgen C. Numbering of fullerenes (IUPAC recommendations 2005) Pure Appl Chem. 2005;77:843–923. . doi: 10.1351/pac200577050843. [Google Scholar]
- Liu X, Klein DJ, Schmalz TG, Seitz WA. Generation of carbon-cage polyhedra. J Comput Chem. 1991;12:1252–1259. . doi: 10.1002/jcc.540121013. [Google Scholar]
- Brinkmann G, Dress AW. A constructive enumeration of fullerenes. J Algor. 1997;23:345–358. . doi: 10.1006/jagm.1996.0806. [Google Scholar]
- Brinkmann G, Franceus D, Fowler PW, Graver JE. Growing fullerenes from seed: growth transformations of fullerene polyhedra. Chem Phys Lett. 2006;428:386–393. . doi: 10.1016/j.cplett.2006.07.040. [Google Scholar]
- Hasheminezhad M, Fleischner H, McKay BD. A universal set of growth operations for fullerenes. Chem Phys Lett. 2008;464:118–121. . doi: 10.1016/j.cplett.2008.09.005. [Google Scholar]
- Balaban AT, Schmalz TG, Zhu H, Klein DJ. Generalizations of the Stone-Wales rearrangement for cage compounds, including fullerenes. J Mol Struct THEOCHEM. 1996;363:291–301. . doi: 10.1016/0166-1280(95)04448-5. [Google Scholar]
- Graver JE, Graves CM, Graves SJ. Fullerene patches II. Ars Math Contemp. 2014;7:405–421. [Google Scholar]
- Brinkmann G, Goedgebeur J, McKay BD. The generation of fullerenes. J Chem Inf Model. 2012;52:2910–2918. doi: 10.1021/ci3003107. . doi: 10.1021/ci3003107. [DOI] [PubMed] [Google Scholar]
- Brinkmann G, Goedgebeur J, Van Cleemput N. The history of the generation of cubic graphs. J Chem Inf Model. 2013;5:67–89. [Google Scholar]
- Goldberg M. A class of multi-symmetric polyhedra. Tohoku Math J. 1937;43:104–108. [Google Scholar]
- Coxeter HSM. Virus macromolecules and geodesic domes. In: Butcher JC, editor. A Spectrum of mathematics—Essays Presented to H. G. Forder. Oxford, UK: Oxford University Press; 1971. pp. 98–107. [Google Scholar]
- Guo X, Hansen P, Zheng M. Boundary uniqueness of fusenes. Discrete Appl Math. 2002;118:209–222. . doi: 10.1016/S0166-218X(01)00180-9. [Google Scholar]
- Graver JE. The (m,k)-patch boundary code problem. MATCH Commun Math Comput Chem. 2003;48:189–196. [Google Scholar]
- Brinkmann G, Graver JE, Justus C. Numbers of faces in disordered patches. J Math Chem. 2009;45:263–278. . doi: 10.1007/s10910-008-9403-6. [Google Scholar]
- Graver JE, Graves CM. Fullerene patches I. Ars Math Contemp. 2010;3:109–120. [Google Scholar]
- Brinkmann G, Fowler PW, Justus C. A catalogue of isomerization transformations of fullerene polyhedra. J Chem Inf Comput Sci. 2003;43:917–927. doi: 10.1021/ci020069l. . doi: 10.1021/ci020069l. [DOI] [PubMed] [Google Scholar]
- Brinkmann G, Fowler PW. A catalogue of growth transformations of fullerene polyhedra. J Chem Inf Comput Sci. 2003;43:1837–1843. doi: 10.1021/ci030017b. . doi: 10.1021/ci030017b. [DOI] [PubMed] [Google Scholar]
- Bornhöft J, Brinkmann G, Greinus J. Pentagon–hexagon-patches with short boundaries. Eur J Comb. 2003;24:517–529. . doi: 10.1016/S0195-6698(03)00034-9. [Google Scholar]
- Stone AJ, Wales DJ. Theoretical studies of icosahedral C60 and some related species. Chem Phys Lett. 1986;128:501–503. . doi: 10.1016/0009-2614(86)80661-3. [Google Scholar]
- Babić D, Bassoli S, Casartelli M, Cataldo F, Graovac A, Ori O, York B. Generalized Stone-Wales transformations. Mol Simulat. 1995;14:395–401. . doi: 10.1080/08927029508022032. [Google Scholar]
- Astakhova TY, Vinogradov GA. New isomerization operations for fullerene graphs. J Mol Struct THEOCHEM. 1998;430:259–268. . doi: 10.1016/S0166-1280(98)90253-6. [Google Scholar]
- Ori O, Putz MV, Gutman I, Schwerdtfeger P. Generalized Stone-Wales transformations for fullerene graphs derived from Berge's switching theorem. In: Gutman I, Pokric B, Vukicevic D, editors. Ante Graovac—Life and Works. Kragujevac, Serbia: University of Kragujevac; 2014. pp. 259–272. (Mathematical Chemistry Monographs). vol. 16. [Google Scholar]
- Berge C. Graphs and Hypergraphs. New York: Elsevier; 1973. [Google Scholar]
- Endo M, Kroto HW. Formation of carbon nanofibers. J Phys Chem. 1992;96:6941–6944. . doi: 10.1021/j100196a017. [Google Scholar]
- Yoshida M, Fowler PW. Systematic relationships between fullerenes without spirals. Chem Phys Lett. 1997;278:256–261. . doi: 10.1016/S0009-2614(97)00980-9. [Google Scholar]
- Podlivaev A, Openov L. Stone-Wales transformation paths in fullerene C60. J Exp Theoret Phys Lett. 2005;81:533–537. . doi: 10.1134/1.1996764. [Google Scholar]
- Ori O, Cataldo F, Putz MV. Topological anisotropy of Stone-Wales waves in graphenic fragments. Int J Mol Sci. 2011;12:7934–7949. doi: 10.3390/ijms12117934. . doi: 10.3390/ijms12117934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Irle S, Morokuma K. Hot giant fullerenes eject and capture C2 molecules: QM/MD simulations with constant density. J Phys Chem C. 2011;115:22707–22716. . doi: 10.1021/jp203614e. [Google Scholar]
- Murry RL, Strout DL, Odom GK, Scuseria GE. Role of sp3 carbon and 7-membered rings in fullerene annealing and fragmentation. Nature. 1993;366:665–667. . doi: 10.1038/366665a0. [Google Scholar]
- Walsh TR, Wales DJ. Relaxation dynamics of C60. J Chem Phys. 1998;109:6691–6700. . doi: 10.1063/1.477319. [Google Scholar]
- Dunk PW, Kaiser NK, Hendrickson CL, Quinn JP, Ewels CP, Nakanishi Y, Sasaki Y, Shinohara H, Marshall AG, Kroto HW. Closed network growth of fullerenes. Nature Commun. 2012;3:855. doi: 10.1038/ncomms1853. . doi: 10.1038/ncomms1853. [DOI] [PubMed] [Google Scholar]
- Irle S, Zheng G, Wang Z, Morokuma K. The C60 formation puzzle “solved”: QM/MD simulations reveal the shrinking hot giant road of the dynamic fullerene self-assembly mechanism. J Phys Chem B. 2006;110:14531–14545. doi: 10.1021/jp061173z. . doi: 10.1021/jp061173z. [DOI] [PubMed] [Google Scholar]
- Tan YZ, Chen RT, Liao ZJ, Li J, Zhu F, Lu X, Xie SY, Li J, Huang RB, Zheng LS. Carbon arc production of heptagon-containing fullerene[68] Nature Commun. 2011;2:420. doi: 10.1038/ncomms1431. . doi: 10.1038/ncomms1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SJ, Fowler PW, Manolopoulos DE, Zerbetto F. The Stone-Wales map for C60. Chem Phys Lett. 1995;235:146–151. . doi: 10.1016/0009-2614(95)00082-F. [Google Scholar]
- Budyka MF, Zyubina TS, Ryabenko AG, Muradyan VE, Esipov SE, Cherepanova NI. Is C2 cluster ingested by fullerene C60. Chem Phys Lett. 2002;354:93–99. . doi: 10.1016/S0009-2614(02)00113-6. [Google Scholar]
- Dutour M, Deza M. Goldberg-Coxeter construction for 3- and 4-valent plane graphs. Electron J Comb. 2004;11:1–49. [Google Scholar]
- Bakowies D, Bühl M, Thiel W. Can large fullerenes be spherical? J Am Chem Soc. 1995;117:10113–10118. . doi: 10.1021/ja00145a025. [Google Scholar]
- Bakowies D, Bühl M, Thiel W. A density functional study on the shape of C180 and C240 fullerenes. Chem Phys Lett. 1995;247:491–493. . doi: 10.1016/S0009-2614(95)01222-2. [Google Scholar]
- Senn P. Computation of the cartesian coordinates of buckminsterfullerene. J Chem Educ. 1995;72:302–303. . doi: 10.1021/ed072p302. [Google Scholar]
- Manolopoulos DE, Fowler PW. Molecular graphs, point groups, and fullerenes. J Chem Phys. 1992;96:7603–7614. . doi: 10.1063/1.462413. [Google Scholar]
- Cvetković D, Fowler P, Rowlinson P, Stevanović D. Constructing fullerene graphs from their eigenvalues and angles. Linear Algebra Appl. 2002;356:37–56. . doi: 10.1016/S0024-3795(02)00280-X. [Google Scholar]
- Pisanski T, Shawe-Taylor J. Characterizing graph drawing with eigenvectors. J Chem Inf Comput Sci. 2000;40:567–571. doi: 10.1021/ci9900938. . doi: 10.1021/ci9900938. [DOI] [PubMed] [Google Scholar]
- Fowler P, Pisanski T, Shawe-Taylor J. Graph drawing. In: Tamassia R, Tollis I, editors. Lecture Notes in Computer Science. Vol. 894. Berlin: Springer; 1995. pp. 282–285. [Google Scholar]
- László I, Graovac A, Pisanski T, Plavšić D. Graph drawing with eigenvectors. In: Putz MV, editor. Carbon Bonding and Structures, Vol. 5: Carbon Materials: Chemistry and Physics. Netherlands: Springer; 2011. pp. 95–115. doi: 10.1007/978-94-007-1733-6_6. [Google Scholar]
- Graver JE. Kekulé structures and the face independence number of a fullerene. Europ J Combin. 2007;28:1115–1130. . doi: 10.1016/j.ejc.2006.03.003. [Google Scholar]
- Kardoš F, Král' D, Miškuf J, Sereni JS. Fullerene graphs have exponentially many perfect matchings. J Math Chem. 2009;46:443–447. . doi: 10.1007/s10910-008-9471-7. [Google Scholar]
- Wu Z, Jelski DA, George TF. Vibrational motions of buckminsterfullerene. Chem Phys Lett. 1987;137:291–294. . doi: 10.1016/0009-2614(87)80221-X. [Google Scholar]
- Weeks DE, Harter WG. Rotation-vibration spectra of icosahedral molecules. II. Icosahedral symmetry, vibrational eigenfrequencles, and normal modes of buckminsterfullerene. J Chem Phys. 1989;90:4744–4771. . doi: 10.1063/1.456571. [Google Scholar]
- Cyvin SJ, Brendsdal E, Cyvin BN, Brunvoll J. Molecular vibrations of footballene. Chem Phys Lett. 1988;143:377–380. . doi: 10.1016/0009-2614(88)87050-7. [Google Scholar]
- Jishi RA, Mirie RM, Dresselhaus MS. Force-constant model for the vibrational modes in C60. Phys Rev B. 1992;45:13685–13689. doi: 10.1103/physrevb.45.13685. . doi: 10.1103/PhysRevB.45.13685. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Broughton JQ, Boyer LL, Reich DE, Kluge MD. Intramolecular-force-constant model for C60. Phys Rev B. 1992;46:12731–12736. doi: 10.1103/physrevb.46.12731. . doi: 10.1103/PhysRevB.46.12731. [DOI] [PubMed] [Google Scholar]
- Ceulemans A, Fowler PW, Vos I. C60 vibrates as a hollow sphere. J Chem Phys. 1994;100:5491–5500. . doi: 10.1063/1.467167. [Google Scholar]
- Hands ID, Dunn JL, Bates CA. A complete nearest-neighbor force field model for C60. J Chem Phys. 2004;120:6912–6923. doi: 10.1063/1.1683105. . doi: 10.1063/1.1683105. [DOI] [PubMed] [Google Scholar]
- Ceulemans A, Titeca BC, Chibotaru LF, Vos I, Fowler PW. Complete bond force field for trivalent and deltahedral cages: group theory and applications to cubane, closo-dodecahedrane, and buckminsterfullerene. J Phys Chem A. 2001;105:8284–8295. . doi: 10.1021/jp0036792. [Google Scholar]
- Zheng G, Irle S, Morokuma K. Performance of the DFTB method in comparison to DFT and semiempirical methods for geometries and energies of C20–C86 fullerene isomers. Chem Phys Lett. 2005;412:210–216. . doi: 10.1016/j.cplett.2005.06.105. [Google Scholar]
- Mani P. Automorphismen von polyedrischen Graphen. Math Ann. 1971;192:279–303. . doi: 10.1007/BF02075357. [Google Scholar]
- Deza M, Dutour-Sikirić M, Fowler PW. The symmetries of cubic polyhedral graphs with face size no larger than 6. MATCH Commun Math Comput Chem. 2009;61:589–602. [Google Scholar]
- Balasubramanian K. Applications of combinatorics and graph theory to spectroscopy and quantum chemistry. Chem Rev. 1985;85:599–618. . doi: 10.1021/cr00070a005. [Google Scholar]
- Faghani M. Symmetry of a toroidal fullerene. Optoelectron Adv Mater Rapid Commun. 2010;4:1844–1846. [Google Scholar]
- Fujita S. Application of coset representations to the construction of symmetry adapted functions. Theoret Chim Acta. 1990;78:45–63. . doi: 10.1007/BF01112352. [Google Scholar]
- King RB, Diudea MV. The chirality of icosahedral fullerenes: a comparison of the tripling (leapfrog), quadrupling (chamfering), and septupling (capra) transformations. J Math Chem. 2006;39:597–604. . doi: 10.1007/s10910-005-9048-7. [Google Scholar]
- David WIF, Ibberson RM, Matthewman JC, Prassides K, Dennis TJS, Hare JP, Kroto HW, Taylor R, Walton DRM. Crystal structure and bonding of ordered C60. Nature. 1991;353:147–149. . doi: 10.1038/353147a0. [Google Scholar]
- Devadoss SL, O'Rourke J. Discrete and Computational Geometry. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- Pisanski T, Kaufman M, Bokal D, Kirby EC, Graovac A. Isoperimetric quotient for fullerenes and other polyhedral cages. J Chem Inf Comput Sci. 1997;37:1028–1032. . doi: 10.1021/ci970228e. [Google Scholar]
- Sylvester JJ. A question in the geometry of situation. Q J Pure Appl Math. 1857;1:79. [Google Scholar]
- Elzinga DJ, Hearn DW. The minimum covering sphere problem. Manag Sci. 1972;19:96–104. [Google Scholar]
- Hopp TH, Reeve CP. 1996. . An algorithm for computing the minimum covering sphere in any dimension. In: COMMERCE UDO ed. NIST IR5381, Gaithersburg, MD: NIST;1–8.
- Nielsen F, Nock R. Approximating smallest enclosing balls. In: Laganá A, Gavrilova M, Kumar V, Mun Y, Tan C, Gervasi O, editors. Computational Science and Its Applications – ICCSA 2004. Vol. 3045. Berlin Heidelberg: Springer; 2004. pp. 147–157. (Lecture Notes in Computer Science). [Google Scholar]
- Yıldırım E. Two algorithms for the minimum enclosing ball problem. SIAM J Optimiz. 2008;19:1368–1391. . doi: 10.1137/070690419. [Google Scholar]
- Fowler P. Cylindrical fullerenes: the smallest nanotubes? J Phys Chem Solids. 1993;54:1825–1833. . doi: 10.1016/0022-3697(93)90295-3. [Google Scholar]
- Kumar P, Yıldırım E. Minimum-volume enclosing ellipsoids and core sets. J Optim Theory Appl. 2005;126:1–21. . doi: 10.1007/s10957-005-2653-6. [Google Scholar]
- Kumar P, Yıldırım E. Computing minimum-volume enclosing axis-aligned ellipsoids. J Optim Theory Appl. 2008;136:211–228. . doi: 10.1007/s10957-007-9295-9. [Google Scholar]
- Sun P, Freund RM. Computation of minimum-volume covering ellipsoids. Oper Res. 2004;52:690–706. . doi: 10.1287/opre.1040.0115. [Google Scholar]
- Todd MJ, Yıldırım E. On Khachiyan's algorithm for the computation of minimum-volume enclosing ellipsoids. Discrete Appl Math. 2007;155:1731–1744. . doi: 10.1016/j.dam.2007.02.013. [Google Scholar]
- Nasu K, Taketsugu T, Nakano T, Nagashima U, Hosoya H. Stability of small fullerenes Cnn = 36, 40 and 60): a topological and molecular orbital approach. Theoret Chim Acta. 1995;90:75–86. . doi: 10.1007/BF01113841. [Google Scholar]
- Díaz-Tendero S, Martín F, Alcamí M. Structure and electronic properties of fullerenes C52q+: is C522+ an exception to the pentagon adjacency penalty rule? ChemPhysChem. 2005;6:92–100. doi: 10.1002/cphc.200400273. . doi: 10.1002/cphc.200400273. [DOI] [PubMed] [Google Scholar]
- Díaz-Tendero S, Alcamí M, Martín F. Fullerene C50: sphericity takes over, not strain. Chem Phys Lett. 2005;407:153–158. . doi: 10.1016/j.cplett.2005.03.065. [Google Scholar]
- Fowler PW, Heine T, Zerbetto F. Competition between even and odd fullerenes: C118, C119, and C120. J Phys Chem A. 2000;104:9625–9629. . doi: 10.1021/jp0019815. [Google Scholar]
- Fischer JE, Heiney PA, Smith AB. Solid-state chemistry of fullerene-based materials. Acc Chem Res. 1992;25:112–118. . doi: 10.1021/ar00015a003. [Google Scholar]
- Heiney PA, Fischer JE, McGhie AR, Romanow WJ, Denenstein AM, McCauley JP, Jr, Smith AB, Cox DE. Orientational ordering transition in solid C60. Phys Rev Lett. 1991;66:2911–2914. doi: 10.1103/PhysRevLett.66.2911. . doi: 10.1103/PhysRevLett.66.2911. [DOI] [PubMed] [Google Scholar]
- Liu S, Lu YJ, Kappes MM, Ibers JA. The structure of the C60 molecule: X-ray crystal structure determination of a twin at 110 K. Science. 1991;254:408–410. doi: 10.1126/science.254.5030.408. . doi: 10.1126/science.254.5030.408. [DOI] [PubMed] [Google Scholar]
- Vaughan GBM, Heiney PA, Fischer JE, Luzzi DE, Ricketts-Foot DA, McGhie AR, Hui YW, Smith AL, Cox DE, Romanow WJ. Orientational disorder in solvent-free solid C70. Science. 1991;254:1350–1353. doi: 10.1126/science.254.5036.1350. doi: 10.1126/science.254.5036.1350. [DOI] [PubMed] [Google Scholar]
- Kauczor J, Norman P, Saidi WA. Non-additivity of polarizabilities and van der Waals C6 coefficients of fullerenes. J Chem Phys. 2013;138:114107. doi: 10.1063/1.4795158. . doi: 10.1063/1.4795158. [DOI] [PubMed] [Google Scholar]
- Lundin A, Sundqvist B, Skoglund P, Fransson A, Pettersson S. Compressibility, specific heat capacity, and Grüneisen parameter for C60/C70. Solid State Commun. 1992;84:879–883. . doi: 10.1016/0038-1098(92)90451-E. [Google Scholar]
- Kawamura H, Akahama Y, Kobayashi M, Shinohara H, Sato H, Saito Y, Kikegawa T, Shimomura O, Aoki K. Solid C70 high pressure and high temperature. J Phys Chem Solids. 1993;54:1675–1678. . doi: 10.1016/0022-3697(93)90281-U. [Google Scholar]
- Zettl A, Cumings J. Elastic properties of fullerenes. In: Levy M, Bass H, Stern R, editors. Handbook of Elastic Properties of Solids, Liquids, and Gases. Vol. 2. San Diego, CA: Academic Press; 2001. pp. 163–170. (Lecture Notes in Computer Science). [Google Scholar]
- Schwerdtfeger P, Gaston N, Krawczyk RP, Tonner R, Moyano GE. Extension of the Lennard-Jones potential: theoretical investigations into rare-gas clusters and crystal lattices of He, Ne, Ar, and Kr using many-body interaction expansions. Phys Rev B. 2006;73:064112. . doi: 10.1103/PhysRevB.73.064112. [Google Scholar]
- Cohen ML. Calculation of bulk moduli of diamond and zinc-blende solids. Phys Rev B. 1985;32:7988–7991. doi: 10.1103/physrevb.32.7988. . doi: 10.1103/PhysRevB.32.7988. [DOI] [PubMed] [Google Scholar]
- Faccio R, Denis PA, Pardo H, Goyenola C, Mombrú AW. Mechanical properties of graphene nanoribbons. J Phys Condens Mat. 2009;21:285304. doi: 10.1088/0953-8984/21/28/285304. . doi: 10.1088/0953-8984/21/28/285304. [DOI] [PubMed] [Google Scholar]
- Milyavskiy VV, Borodina TI, Sokolov SN. Phase transitions of C70 fullerite with hexagonal closed-packed structure under shock-wave loading. Fuller Nanotub Carbon Nanostruct. 2008;16:494–498. . doi: 10.1080/15363830802282433. [Google Scholar]
- Hales TC. Proceedings of the International Congress of Mathematicians. III. Beijing: Higher Ed. Press; 2002. A computer verification of the Kepler conjecture; pp. 795–804. [Google Scholar]
- Hales TC. A proof of the Kepler conjecture. Ann Math. 2005:1065–1185. . doi: 10.4007/annals.2005.162.1065. [Google Scholar]
- Stoyan Y, Gil N, Scheithauer G, Pankratov A, Magdalina I. 2003. Packing of convex polytopes into a parallelepiped. Tech. Rep. MATH-NM-04-2004, Technische Universität Dresden.
- Stoyan Y, Gil N, Pankratov A. 2004. Packing of non-convex polytopes into a parallelepiped. Tech. Rep. MATH-NM-06-2004, Technische Universität Dresden.
- Yıldırım E. On the minimum volume covering ellipsoid of ellipsoids. SIAM J Optimiz. 2006;17:621–641. . doi: 10.1137/050622560. [Google Scholar]
- Donev A, Stillinger FH, Chaikin PM, Torquato S. Unusually dense crystal packings of ellipsoids. Phys Rev Lett. 2004;92:255506. doi: 10.1103/PhysRevLett.92.255506. . doi: 10.1103/PhysRevLett.92.255506. [DOI] [PubMed] [Google Scholar]
- Wiener H. Structural determination of paraffin boiling points. J Am Chem Soc. 1947;69:17–20. doi: 10.1021/ja01193a005. . doi: 10.1021/ja01193a005. [DOI] [PubMed] [Google Scholar]
- Ori O, D'Mello M. A topological study of the structure of the C76 fullerene. Chem Phys Lett. 1992;197:49–54. . doi: 10.1016/0009-2614(92)86020-I. [Google Scholar]
- Ori O, Cataldo F, Vukičević D, Graovac A. Wiener way to dimensionality. Iranian J Math Chem. 2010;1:5–15. [Google Scholar]
- Graovac A, Ori O, Faghani M, Ashrafi A. Distance property of fullerenes. Iranian J Math Chem. 2011;2:99–107. [Google Scholar]
- Koorepazan-Moftakhar F, Ashrafi AR, Ori O, Putz MV. Fullerenes: Chemistry, Natural Sources and Technological Applications. New York: NIST. Nova Publications; 2014. Sphericality of some classes of fullerenes measured by topology. [Google Scholar]
- Vukičević D, Cataldo F, Ori O, Graovac A. Topological efficiency of C66 fullerene. Chem Phys Lett. 2011;501:442–445. . doi: 10.1016/j.cplett.2010.11.055. [Google Scholar]
- Ori O, Cataldo F, Graovac A. Topological ranking of C28 fullerenes reactivity. Fuller Nanotub Carbon Nanostruct. 2009;17:308–323. . doi: 10.1080/15363830902782332. [Google Scholar]
- Wang CR, Kai T, Tomiyama T, Yoshida T, Kobayashi Y, Nishibori E, Takata M, Sakata M, Shinohara H. Materials science: C66 fullerene encaging a scandium dimer. Nature. 2000;408:426–427. doi: 10.1038/35044195. . doi: 10.1038/35044195. [DOI] [PubMed] [Google Scholar]
- Katritzky AR, Karelson M, Petrukhin R. 2005. The CODESSA PRO project.
- Fowler PW, Caporossi G, Hansen P. Distance matrices, Wiener indices, and related invariants of fullerenes. J Phys Chem A. 2001;105:6232–6242. . doi: 10.1021/jp0104379. [Google Scholar]
- Hosoya H. On some counting polynomials in chemistry. Discrete Appl Math. 1988;19:239–257. . doi: 10.1016/0166-218X(88)90017-0. [Google Scholar]
- Gutman I. A formula for the Wiener number of trees and its extension to graphs containing cycles. Graph Theory Notes NY. 1994;27:9–15. [Google Scholar]
- Balaban AT. Topological indices based on topological distances in molecular graphs. Pure Appl Chem. 1983;55:199–206. . doi: 10.1351/pac198855020199. [Google Scholar]
- Balaban AT. Chemical graphs: looking back and glimpsing ahead. J Chem Inf Comput Sci. 1995;35:339–350. . doi: 10.1021/ci00025a001. [Google Scholar]
- Zhang H, Balasubramanian K. Spectral moments of fullerene cages. Mol Phys. 1993;79:727–745. . doi: 10.1080/00268979300101581. [Google Scholar]
- Estrada E. Characterization of 3D molecular structure. Chem Phys Lett. 2000;319:713–718. . doi: 10.1016/S0009-2614(00)00158-5. [Google Scholar]
- de la Peña JA, Gutman I, Rada J. Estimating the Estrada index. Linear Algebra Appl. 2007;427:70–76. . doi: 10.1016/j.laa.2007.06.020. [Google Scholar]
- Diudea MV, Gutman I, Jantschi L. Molecular Topolgy. New York: Nova Science; 2001. [Google Scholar]
- Ju Y, Liang H, Zhang J, Bai F. A note on Fowler-Manolopoulos predictor of fullerene stability. MATCH Commun Math Comput Chem. 2010;64:419–424. [Google Scholar]
- Austin SJ, Fowler PW, Manolopoulos DE, Orlandi G, Zerbetto F. Structural motifs and the stability of fullerenes. J Phys Chem. 1995;99:8076–8081. . doi: 10.1021/j100020a035. [Google Scholar]
- Stevanović D. Remarks on Fowler-Manolopoulos predictor of fullerene stability. MATCH Commun Math Comput Chem. 2011;66:285–292. [Google Scholar]
- Karton A, Chan B, Raghavachari K, Radom L. Evaluation of the heats of formation of corannulene and C60 by means of high-level theoretical procedures. J Phys Chem A. 2013;117:1834–1842. doi: 10.1021/jp312585r. . doi: 10.1021/jp312585r. [DOI] [PubMed] [Google Scholar]
- Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. . doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys. 2005;7:3297–3305. doi: 10.1039/b508541a. . doi: 10.1039/B508541A. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Alizadeh Y, Mirzaie S. Computing Wiener polynomial, Wiener index and Hyper Wiener index of C80 fullerene by GAP program. Fuller Nanotub Carbon Nanostruct. 2009;17:560–566. . doi: 10.1080/15363830903133204. [Google Scholar]
- Charlier JC, Eklund P, Zhu J, Ferrari A. Electron and phonon properties of graphene: their relationship with carbon nanotubes. In: Jorio A, Dresselhaus G, Dresselhaus M, editors. Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications. Berlin: Springer; 2008. [Google Scholar]
- Khamatgalimov AR, Kovalenko VI. Electronic structure and stability of C80 fullerene IPR isomers. Fuller Nanotub Carbon Nanostruct. 2011;19:599–604. . doi: 10.1080/1536383X.2010.504951. [Google Scholar]
- Xu L, Cai W, Shao X. Prediction of low-energy isomers of large fullerenes from C132 to C160. J Phys Chem A. 2006;110:9247–9253. doi: 10.1021/jp057181h. . doi: 10.1021/jp057181h. [DOI] [PubMed] [Google Scholar]
- Shao N, Gao Y, Yoo S, An W, Zeng XC. Search for lowest-energy fullerenes: C98 to C110. J Phys Chem A. 2006;110:7672–7676. doi: 10.1021/jp0624092. . doi: 10.1021/jp0624092. [DOI] [PubMed] [Google Scholar]
- Shao N, Gao Y, Zeng XC. Search for lowest-energy fullerenes 2: C38 to C80 and C112 to C120. J Phys Chem C. 2007;111:17671–17677. . doi: 10.1021/jp0701082. [Google Scholar]
- Klein DJ, Balaban AT. Clarology for conjugated carbon nano-structures: molecules, polymers, graphene, defected graphene, fractal benzenoids, fullerenes, nano-tubes, nano-cones, nano-tori, etc. Open Org Chem J. 2011;5:27–61. . doi: 10.2174/1874364101105010027. [Google Scholar]
- Klein DJ, Schmalz TG, Hite GE, Seitz WA. Resonance in C60 buckminsterfullerene. J Am Chem Soc. 1986;108:1301–1302. . doi: 10.1021/ja00266a032. [Google Scholar]
- Vukičević D, Kroto HW, Randić M. Atlas of Kekulé valence structures of buckminsterfullerene. Croat Chem Acta. 2005;78:223–234. [Google Scholar]
- Randić M, Kroto HW, Vukičević D. Numerical Kekulé structures of fullerenes and partitioning of π-electrons to pentagonal and hexagonal rings. J Chem Inf Model. 2007;47:897–904. doi: 10.1021/ci600484u. . doi: 10.1021/ci600484u. [DOI] [PubMed] [Google Scholar]
- Austin S, Fowler P, Hansen P, Monolopoulos D, Zheng M. Fullerene isomers of C60. Kekulé counts versus stability. Chem Phys Lett. 1994;228:478–484. . doi: 10.1016/0009-2614(94)00965-1. [Google Scholar]
- Esperet L, Kardoš F, King AD, Král' D, Norine S. Exponentially many perfect matchings in cubic graphs. Adv Math. 2011;227:1646–1664. . doi: 10.1016/j.aim.2011.03.015. [Google Scholar]
- Lovász L, Plummer MD. Matching Theory. Amsterdam: Elsevier; 1986. [Google Scholar]
- Pauling L, Brockway LO, Beach J. The dependence of interatomic distance on single bond-double bond resonance. J Am Chem Soc. 1935;57:2705–2709. . doi: 10.1021/ja01315a105. [Google Scholar]
- Narita S, Morikawa T, Shibuya T. Linear relationship between the bond lengths and the Pauling bond orders in fullerene molecules. J Mol Struct THEOCHEM. 2000;532:37–40. . doi: 10.1016/S0166-1280(00)00563-7. [Google Scholar]
- Rogers KM, Fowler PW. Leapfrog fullerenes, Hückel bond order and Kekulé structures. J Chem Soc, Perkin Trans 2. 2001:18–22. . doi: 10.1039/B007520P. [Google Scholar]
- Gross L, Mohn F, Moll N, Schuler B, Criado A, Guitián E, Peña D, Gourdon A, Meyer G. Bond-order discrimination by atomic force microscopy. Science. 2012;337:1326–1329. doi: 10.1126/science.1225621. . doi: 10.1126/science.1225621. [DOI] [PubMed] [Google Scholar]
- Graver JE, Hartung EJ, Souid AY. Clar and Fries numbers for benzenoids. J Math Chem. 2013;51:1981–1989. . doi: 10.1007/s10910-013-0193-0. [Google Scholar]
- Bernáth A, Kovács ER. 2011. pp. 1–3. . NP-hardness of the Clar number in general plane graphs. Available at http://www.cseltehu/egres/qp/egresqp-11-07ps.
- Ye D, Zhang H. Extremal fullerene graphs with the maximum Clar number. Discrete Appl Math. 2009;157:3152–3173. . doi: 10.1016/j.dam.2009.06.007. [Google Scholar]
- Zhang H, Ye D, Liu Y. A combination of Clar number and Kekulé count as an indicator of relative stability of fullerene isomers of C60. J Math Chem. 2010;48:733–740. . doi: 10.1007/s10910-010-9706-2. [Google Scholar]
- Fowler PW. Localised models and leapfrog structures of fullerenes. J Chem Soc, Perkin Trans 2. 1992:145–146. . doi: 10.1039/P29920000145. [Google Scholar]
- Došlić T. Leapfrog fullerenes have many perfect matchings. J Math Chem. 2008;44:1–4. . doi: 10.1007/s10910-007-9287-x. [Google Scholar]
- Hartung E. Fullerenes with complete Clar structure. Discrete Appl Math. 2013;161:2952–2957. . doi: 10.1016/j.dam.2013.06.009. [Google Scholar]
- Fowler PW, Austin SJ, Dunning OJ, Dias JR. Symmetry properties of the leapfrog transformation for fullerenes and benzenoids. Chem Phys Lett. 1994;224:123–130. . doi: 10.1016/0009-2614(94)00525-7. [Google Scholar]
- Zhang H, Ye D. An upper bound for the Clar number of fullerene graphs. J Math Chem. 2007;41:123–133. . doi: 10.1007/s10910-006-9061-5. [Google Scholar]
- Herndon WC. Resonance energies of aromatic hydrocarbons. Quantitative test of resonance theory. J Am Chem Soc. 1973;95:2404–2406. . doi: 10.1021/ja00788a073. [Google Scholar]
- Herndon WC, Ellzey JM. Lawrence. Resonance theory. V. Resonance energies of benzenoid and nonbenzenoid pi systems. J Am Chem Soc. 1974;96:6631–6642. . doi: 10.1021/ja00828a015. [Google Scholar]
- Babić D, Balaban AT, Klein DJ. Nomenclature and coding of fullerenes. J Chem Inf Comput Sci. 1995;35:515–526. . doi: 10.1021/ci00025a020. [Google Scholar]
- Liu Y, O'Brien S, Zhang Q, Heath J, Tittel F, Curl R, Kroto H, Smalley R. Negative carbon cluster ion beams: new evidence for the special nature of C60. Chem Phys Lett. 1986;126:215–217. . doi: 10.1016/S0009-2614(86)80042-2. [Google Scholar]
- Slanina Z, Uhlík F, Zhao X, Adamowicz L, Nagase S. Relative stabilities of C74 isomers. Fuller Nanotub Carbon Nanostruct. 2007;15:195–205. . doi: 10.1080/15363830701236423. [Google Scholar]
- Beckhaus HD, Verevkin S, Rüchardt C, Diederich F, Thilgen C, Meer HUT, Mohn H, Müller W. C70 ist stabiler als C60: experimentelle Bestimmung der Bildungswärme von C70. Angew Chem. 1994;33:996–998. . doi: 10.1002/ange.19941060916. [Google Scholar]
- Wang Z, Ke X, Zhu Z, Zhu F, Ruan M, Chen H, Huang R, Zheng L. A new carbon solid made of the world's smallest caged fullerene C20. Phys Lett A. 2001;280:351–356. . doi: 10.1016/S0375-9601(00)00847-1. [Google Scholar]
- Lin F, Sorensen ES, Kallin C, Berlinsky AJ. C20, the smallest fullerene. In: Sattler KD, editor. Handbook of Nanophysics: Clusters and Fullerenes, Handbook of Nanophysics, chap. 29. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- Babić D, Brinkmann G, Dress A. Topological resonance energy of fullerenes. J Chem Inf Comput Sci. 1997;37:920–923. . doi: 10.1021/ci9700283. [Google Scholar]
- Bühl M, Hirsch A. Spherical aromaticity of fullerenes. Chem Rev. 2001;101:1153–1184. doi: 10.1021/cr990332q. . doi: 10.1021/cr990332q. [DOI] [PubMed] [Google Scholar]
- Haddon R, Brus L, Raghavachari K. Electronic structure and bonding in icosahedral C60. Chem Phys Lett. 1986;125:459–464. . doi: 10.1016/0009-2614(86)87079-8. [Google Scholar]
- Haddon R, Brus L, Raghavachari K. Rehybridization and π-orbital alignment: the key to the existence of spheroidal carbon clusters. Chem Phys Lett. 1986;131:165–169. . doi: 10.1016/0009-2614(86)80538-3. [Google Scholar]
- Bakowies D, Thiel W. MNDO study of large carbon clusters. J Am Chem Soc. 1991;113:3704–3714. . doi: 10.1021/ja00010a012. [Google Scholar]
- Fowler PW, Hansen P, Stevanović D. A note on the smallest eigenvalue of fullerenes. MATCH Commun Math Comput Chem. 2003;48:37–48. [Google Scholar]
- Došlić T. The smallest eigenvalue of fullerene graphs – closing the gap. MATCH Commun Math Comput Chem. 2013;70:73–78. [Google Scholar]
- Randić M, Trinajstić N, Živković T. Molecular graphs having identical spectra. J Chem Soc, Faraday Trans 2. 1976;72:244–256. . doi: 10.1039/F29767200244. [Google Scholar]
- Aihara J. A new definition of Dewar-type resonance energies. J Am Chem Soc. 1976;98:2750–2758. . doi: 10.1021/ja00426a013. [Google Scholar]
- Gutman I, Milun M, Trinajstic N. Graph theory and molecular orbitals. 19. Nonparametric resonance energies of arbitrary conjugated systems. J Am Chem Soc. 1977;99:1692–1704. . doi: 10.1021/ja00448a002. [Google Scholar]
- Balasubramanian K. Exhaustive generation and analytical expressions of matching polynomials of fullerenes C20-C50. J Chem Inf Comput Sci. 1994;34:421–427. . doi: 10.1021/ci00018a032. [Google Scholar]
- Salvador JM, Hernandez A, Beltran A, Duran R, Mactutis A. Fast partial-differential synthesis of the matching polynomial of C72-100. J Chem Inf Comput Sci. 1998;38:1105–1110. doi: 10.1021/ci9800155. . doi: 10.1021/ci9800155. [DOI] [PubMed] [Google Scholar]
- Randić M. Novel insight into Clar's aromatic π-sextets. Chem Phys Lett. 2014;601:1–5. . doi: 10.1016/j.cplett.2014.03.073. [Google Scholar]
- Lu X, Chen Z. Curved π-conjugation, aromaticity, and the related chemistry of small fullerenes (<C60) and single-walled carbon nanotubes. Chem Rev. 2005;105:3643–3696. doi: 10.1021/cr030093d. . doi: 10.1021/cr030093d. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wu JI, Corminboeuf C, Bohmann J, Lu X, Hirsch A, Schleyer PR. Is C60 buckminsterfullerene aromatic? Phys Chem Chem Phys. 2012;14:14886–14891. doi: 10.1039/c2cp42146a. . doi: 10.1039/C2CP42146A. [DOI] [PubMed] [Google Scholar]
- Hirsch A, Ruoff Z, Jiao H. Spherical aromaticity in Ih symmetrical fullerenes: the 2(n+1)2 rule. Angew Chem Int Ed. 2000;39:3915–3917. doi: 10.1002/1521-3773(20001103)39:21<3915::AID-ANIE3915>3.0.CO;2-O. . doi: 10.1002/1521-3773(20001103)39:21<3915::AID-ANIE3915>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Fedorov AS, Fedorov DA, Kuzubov AA, Avramov PV, Nishimura Y, Irle S, Witek HA. Relative isomer abundance of fullerenes and carbon nanotubes correlates with kinetic stability. Phys Rev Lett. 2011;107:175506. doi: 10.1103/PhysRevLett.107.175506. . doi: 10.1103/PhysRevLett.107.175506. [DOI] [PubMed] [Google Scholar]
- Fowler PW. Fullerene graphs with more negative than positive eigenvalues: the exceptions that prove the rule of electron deficiency? J Chem Soc Faraday Trans. 1997;93:1–3. . doi: 10.1039/A605413G. [Google Scholar]
- Manolopoulos DE, Woodall DR, Fowler PW. Electronic stability of fullerenes: eigenvalue theorems for leapfrog carbon clusters. J Chem Soc Faraday Trans. 1992;88:2427–2435. . doi: 10.1039/FT9928802427. [Google Scholar]
- Fowler PW, Austin SJ. Closed-shell carbon frameworks: leapfrog fullerenes and decorated spheriphane hydrocarbons. J Chem Inf Comput Sci. 1994;34:264–269. . doi: 10.1021/ci00018a006. [Google Scholar]
- Deza M, Fowler PW, Rassat A, Rogers KM. Fullerenes as tilings of surfaces. J Chem Inf Comput Sci. 2000;40:550–558. doi: 10.1021/ci990066h. . doi: 10.1021/ci990066h. [DOI] [PubMed] [Google Scholar]
- Fowler P, Rogers K, Fajtlowicz S, Hansen P, Caporossi G. Facts and conjectures about fullerene graphs: leapfrog, cylinder and Ramanujan fullerenes. In: Betten A, Kohnert A, Laue R, Wassermann A, editors. Algebraic Combinatorics and Applications. Berlin Heidelberg: Springer; 2001. pp. 134–146. [Google Scholar]
- Bakowies D, Gelessus A, Thiel W. Quantum-chemical study of C78 fullerene isomers. Chem Phys Lett. 1992;197:324–329. . doi: 10.1016/0009-2614(92)85777-8. [Google Scholar]
- Bakowies D, Kolb M, Thiel W, Richard S, Ahlrichs R, Kappes MM. Quantum-chemical study of C84 fullerene isomers. Chem Phys Lett. 1992;200:411–417. . doi: 10.1016/0009-2614(92)87013-F. [Google Scholar]
- Chen Z, Thiel W. Performance of semiempirical methods in fullerene chemistry: relative energies and nucleus-independent chemical shifts. Chem Phys Lett. 2003;367:15–25. . doi: 10.1016/S0009-2614(02)01660-3. [Google Scholar]
- Noël Y, De La Pierre M, Zicovich-Wilson CM, Orlando R, Dovesi R. Structural, electronic and energetic properties of giant icosahedral fullerenes up to C6000: insights from an ab initio hybrid DFT study. Phys Chem Chem Phys. 2014;16:13390–13401. doi: 10.1039/c4cp01442a. . doi: 10.1039/C4CP01442A. [DOI] [PubMed] [Google Scholar]
- Lu X, Chen Z, Thiel W, Schleyer PR, Huang R, Zheng L. Properties of fullerene[50] and D5h decachlorofullerene[50]: a computational study. J Am Chem Soc. 2004;126:14871–14878. doi: 10.1021/ja046725a. . doi: 10.1021/ja046725a. [DOI] [PubMed] [Google Scholar]
- Fowler PW, Sandall JPB. Predictions of special signatures of fullerenes. Second-order Jahn-Teller effects on the structures of C44, C56, C68 and C92. J Chem Soc, Perkin Trans 2. 1994:1917–1921. . doi: 10.1039/P29940001917. [Google Scholar]
- Paulus B. Electronic and structural properties of the cage-like molecules C20 to C36. Phys Chem Chem Phys. 2003;5:3364–3367. . doi: 10.1039/B304539K. [Google Scholar]
- Grossman JC, Mitas L, Raghavachari K. Structure and stability of molecular carbon: importance of electron correlation. Phys Rev Lett. 1995;75:3870–3873. doi: 10.1103/PhysRevLett.75.3870. . doi: 10.1103/PhysRevLett.75.3870. [DOI] [PubMed] [Google Scholar]
- Zhang BL, Wang CZ, Ho KM, Xu CH, Chan CT. The geometry of small fullerene cages: C20 to C70. J Chem Phys. 1992;97:5007–5011. . doi: 10.1063/1.463854. [Google Scholar]
- Chen Z, Jiao H, Bühl M, Hirsch A, Thiel W. Theoretical investigation into structures and magnetic properties of smaller fullerenes and their heteroanalogues. Theoret Chem Acc. 2001;106:352–363. . doi: 10.1007/s002140100284. [Google Scholar]
- Chen Z, Heine T, Jiao H, Hirsch A, Thiel W, Schleyer PR. Theoretical studies on the smallest fullerene: from monomer to oligomers and solid states. Chem Eur J. 2004;10:963–970. doi: 10.1002/chem.200305538. . doi: 10.1002/chem.200305538. [DOI] [PubMed] [Google Scholar]
- Gerhardt P, Löffler S, Homann KH. Polyhedral carbon ions in hydrocarbon flames. Chem Phys Lett. 1987;137:306–310. . doi: 10.1016/0009-2614(87)80889-8. [Google Scholar]
- Zhang QL, O'Brien SC, Heath JR, Liu Y, Curl RF, Kroto HW, Smalley RE. Reactivity of large carbon clusters: spheroidal carbon shells and their possible relevance to the formation and morphology of soot. J Phys Chem. 1986;90:525–528. . doi: 10.1021/j100276a001. [Google Scholar]
- Smalley RE. Self-assembly of the fullerenes. Acc Chem Res. 1992;25:98–105. . doi: 10.1021/ar00015a001. [Google Scholar]
- Wakabayashi T, Achiba Y. A model for the C60 and C70 growth mechanism. Chem Phys Lett. 1992;190:465–468. . doi: 10.1016/0009-2614(92)85174-9. [Google Scholar]
- Wakabayashi T, Kikuchi K, Shiromaru H, Suzuki S, Achiba Y. Ring-stacking considerations on higher fullerene growth. Z Phys D. 1993;26:258–260. . doi: 10.1007/BF01425683. [Google Scholar]
- von Helden G, Gotts NG, Bowers MT. Experimental evidence for the formation of fullerenes by collisional heating of carbon rings in the gas phase. Nature. 1993;363:60–63. . doi: 10.1038/363060a0. [Google Scholar]
- George S, Hammond VJK, editors. Fullerenes: Synthesis, Properties, and Chemistry of Large Carbon Clusters. Washington DC: ACS; 1992. (ACS Symposium Series). [Google Scholar]
- Ōsawa E. Formation mechanism of C60 under nonequilibrium and irreversible conditions – an annotation. Fuller Nanotub Carbon Nanostruct. 2012;20:299–309. . doi: 10.1080/1536383X.2012.655104. [Google Scholar]
- Sasaki K, Wakasaki T, Matsui S, Kadota K. Distributions of C2 and C3 radical densities in laser-ablation carbon plumes measured by laser-induced fluorescence imaging spectroscopy. J Appl Phys. 2002;91:4033–4039. . doi: 10.1063/1.1455151. [Google Scholar]
- Curl RF, Lee MK, Scuseria GE. C60 buckminsterfullerene high yields unraveled. J Phys Chem A. 2008;112:11951–11955. doi: 10.1021/jp806951v. . doi: 10.1021/jp806951v. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bowles FL, Bearden DW, Ray WK, Fuhrer T, Ye Y, Dixon C, Harich K, Helm RF, Olmstead MM. A missing link in the transformation from asymmetric to symmetric metallofullerene cages implies a top-down fullerene formation mechanism. Nature Chem. 2013;5:880–885. doi: 10.1038/nchem.1748. doi: 10.1038/NCHEM.1748. [DOI] [PubMed] [Google Scholar]
- Shinohara H. Endohedral metallofullerenes. Rep Prog Phys. 2000;63:843–892. . doi: 10.1088/0034-4885/63/6/201. [Google Scholar]
- Chaur MN, Melin F, Ortiz AL, Echegoyen L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew Chem Int Ed. 2009;48:7514–7538. doi: 10.1002/anie.200901746. . doi: 10.1002/anie.200901746. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Nagase S, Yoshida M, Ōsawa E. Endohedral metallofullerenes. Are the isolated pentagon rule and fullerene structures always satisfied? J Am Chem Soc. 1997;119:12693–12694. . doi: 10.1021/ja9733088. [Google Scholar]
- Beavers CM, Zuo T, Duchamp JC, Harich K, Dorn HC, Olmstead MM, Balch AL. Tb3N@C84: an improbable, egg-shaped endohedral fullerene that violates the isolated pentagon rule. J Am Chem Soc. 2006;128:11352–11353. doi: 10.1021/ja063636k. . doi: 10.1021/ja063636k. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fortea A, Alegret N, Balch AL, Poblet JM. The maximum pentagon separation rule provides a guideline for the structures of endohedral metallofullerenes. Nature Chem. 2010;2:955–961. doi: 10.1038/nchem.837. . doi: 10.1038/nchem.837. [DOI] [PubMed] [Google Scholar]
- Ugarte D. Curling and closure of graphitic networks under electron-beam irradiation. Nature. 1992;359:707–709. doi: 10.1038/359707a0. . doi: 10.1038/359707a0. [DOI] [PubMed] [Google Scholar]
- Ugarte D. Canonical structure of large carbon clusters: Cnn > 100. Europhys Lett. 1993;22:45–50. . doi: 10.1209/0295-5075/22/1/009. [Google Scholar]
- Bühl M, Thiel W. Ab initio helium NMR chemical shifts of endohedral fullerene compounds He@Cnn = 32–180) Chem Phys Lett. 1995;233:585–589. . doi: 10.1016/0009-2614(94)01459-9. [Google Scholar]
- Ugarte D. Onion-like graphitic particles. Carbon. 1995;33:989–993. . doi: 10.1016/0008-6223(95)00027-B. [Google Scholar]
- Bühl M, Patchkovskii S, Thiel W. Interaction energies and NMR chemical shifts of noble gases in C60. Chem Phys Lett. 1997;275:14–18. . doi: 10.1016/S0009-2614(97)00733-1. [Google Scholar]
- Heggie MI, Terrones M, Eggen BR, Jungnickel G, Jones R, Latham CD, Briddon PR, Terrones H. Quantitative density-functional study of nested fullerenes. Phys Rev B. 1998;57:13339–13342. . doi: 10.1103/PhysRevB.57.13339. [Google Scholar]
- Zhang M, He D, Ji L, Wei B, Wu D, Zhang X, Xu Y, Wang W. Macroscopic synthesis of onion-like graphitic particles. Nanostruc Mat. 1998;10:291–297. . doi: 10.1016/S0965-9773(98)00069-5. [Google Scholar]
- Tomita S, Fujii M, Hayashi S, Yamamoto K. Electron energy-loss spectroscopy of carbon onions. Chem Phys Lett. 1999;305:225–229. . doi: 10.1016/S0009-2614(99)00374-7. [Google Scholar]
- Iglesias-Groth S. Fullerenes and buckyonions in the interstellar medium. Astrophys J Lett. 2004;608:L37. . doi: 10.1086/422216. [Google Scholar]
- Grimme S, Mück-Lichtenfeld C, Antony J. Noncovalent interactions between graphene sheets and in multishell (hyper)fullerenes. J Phys Chem C. 2007;111:11199–11207. . doi: 10.1021/jp0720791. [Google Scholar]
- Yamada M, Akasaka T, Nagase S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc Chem Res. 2010;43:92–102. doi: 10.1021/ar900140n. . doi: 10.1021/ar900140n. [DOI] [PubMed] [Google Scholar]
- Korona T, Dodziuk H. Small molecules in C60 and C70: which complexes could be stabilized? J Chem Theory Comput. 2011;7:1476–1483. doi: 10.1021/ct200111a. . doi: 10.1021/ct200111a. [DOI] [PubMed] [Google Scholar]
- Botkin N, Turova-Botkina V. An algorithm for finding the Chebyshev center of a convex polyhedron. Appl Math Optim. 1994;29:211–222. . doi: 10.1007/BF01204183. [Google Scholar]
- Mantina M, Chamberlin AC, Valero R, Cramer CJ, Truhlar DG. Consistent van der Waals radii for the whole main group. J Phys Chem A. 2009;113:5806–5812. doi: 10.1021/jp8111556. . doi: 10.1021/jp8111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiske T, Böhme DK, Hrušák J, Krätschmer W, Schwarz H. Endohedral cluster compounds: inclusion of helium within C60+ and C70+ through collision experiments. Angew Chem Int Ed. 1991;30:884–886. . doi: 10.1002/anie.199108841. [Google Scholar]
- Weiske T, Hrusak J, Böhme DK, Schwarz H. Formation of endohedral carbon-cluster noble-gas compounds with high-energy bimolecular reactions: C60Hen+n = 1,2) Chem Phys Lett. 1991;186:459–462. . doi: 10.1016/0009-2614(91)90209-R. [Google Scholar]
- Weiske T, Schwarz H. Sequential insertion of 3He and 4He in C60+ Angew Chem Int Ed. 1992;31:605–606. . doi: 10.1002/anie.199206051. [Google Scholar]
- Weiske T, Wong T, Krätschmer W, Terlouw JK, Schwarz H. The neutralization of He@C60+ in the gas phase: compelling evidence for the existence of an endohedral structure for He@C60. Angew Chem Int Ed. 1992;31:183–185. . doi: 10.1002/anie.199201831. [Google Scholar]
- Weiske T, Hrušák J, Böhme DK, Schwarz H. Endohedral fullerene-noble gas clusters formed with high-energy bimolecular reactions of Cxn+x = 60, 70; n = 1, 2, 3) Helvet Chim Acta. 1992;75:79–89. . doi: 10.1002/hlca.19920750106. [Google Scholar]
- Weiske T, Schwarz H, Giblin DE, Gross ML. High-energy collisions of Kr@C60+. with helium. Evidence for the formation of HeKr@C60+ Chem Phys Lett. 1994;227:87–90. . doi: 10.1016/0009-2614(94)00786-1. [Google Scholar]
- Saunders M, Jimenez-Vazquez HA, Cross RJ, Mroczkowski S, Gross ML, Giblin DE, Poreda RJ. Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high pressure. J Am Chem Soc. 1994;116:2193–2194. . doi: 10.1021/ja00084a089. [Google Scholar]
- Son MS, Sung YK. The atom-atom potential. exohedral and endohedral complexation energies of complexes of X@C60 between fullerene and rare-gas atoms (X = He, Ne, Ar, Kr, and Xe) Chem Phys Lett. 1995;245:113–118. . doi: 10.1016/0009-2614(95)00952-Z. [Google Scholar]
- Straka M, Lantto P, Vaara J. Toward calculations of the 129Xe chemical shift in Xe@C60 at experimental conditions: relativity, correlation, and dynamics. J Phys Chem A. 2008;112:2658–2668. doi: 10.1021/jp711674y. . doi: 10.1021/jp711674y. [DOI] [PubMed] [Google Scholar]
- Autschbach J, Zurek E. Relativistic density-functional computations of the chemical shift of 129Xe in Xe@C60. J Phys Chem A. 2003;107:4967–4972. . doi: 10.1021/jp0346559. [Google Scholar]
- Kurotobi K, Murata Y. A single molecule of water encapsulated in fullerene C60. Science. 2011;333:613–616. doi: 10.1126/science.1206376. . doi: 10.1126/science.1206376. [DOI] [PubMed] [Google Scholar]
- Thilgen C. A single water molecule trapped inside hydrophobic C60. Angew Chem Int Ed. 2012;51:587–589. doi: 10.1002/anie.201107379. . doi: 10.1002/anie.201107379. [DOI] [PubMed] [Google Scholar]
- Zhang RQ, Ma WY, Han KL, Lee CS. A thermodynamic and kinetic study of the formation of C20 compounds encapsulating H, He and Ne atoms. Theoret Chem Acc. 2003;109:278–283. . doi: 10.1007/s00214-003-0438-2. [Google Scholar]
- Breslavskaya N, Levin A, Buchachenko A. Endofullerenes: size effects on structure and energy. Russian Chem Bull. 2004;53:18–23. . doi: 10.1023/B:RUCB.0000024824.35542.0e. [Google Scholar]
- Pyykkö P, Wang C, Straka M, Vaara J. A London-type formula for the dispersion interactions of endohedral A@B systems. Phys Chem Chem Phys. 2007;9:2954–2958. doi: 10.1039/b704695b. . doi: 10.1039/B704695B. [DOI] [PubMed] [Google Scholar]
- Ruoff RS, Ruoff AL. The bulk modulus of C60 molecules and crystals: a molecular mechanics approach. Appl Phys Lett. 1991;59:1553–1555. . doi: 10.1063/1.106280. [Google Scholar]
- Tonner R, Frenking G, Lein M, Schwerdtfeger P. Packed to the rafters: filling up C60 with rare gas atoms. ChemPhysChem. 2011;12:2081–2084. doi: 10.1002/cphc.201100360. . doi: 10.1002/cphc.201100360. [DOI] [PubMed] [Google Scholar]
- Tomańek D, Zhong W, Krastev E. Stability of multishell fullerenes. Phys Rev B. 1993;48:15461–15464. doi: 10.1103/physrevb.48.15461. . doi: 10.1103/PhysRevB.48.15461. [DOI] [PubMed] [Google Scholar]
- Bates KR, Scuseria GE. Why are buckyonions round? Theoret Chem Acc. 1998;99:29–33. . doi: 10.1007/s002140050299. [Google Scholar]
- Casella G, Bagno A, Saielli G. Spectroscopic signatures of the carbon buckyonions C60@C180 and C60@C240: a dispersion-corrected DFT study. Phys Chem Chem Phys. 2013;15:18030–18038. doi: 10.1039/c3cp53273a. . doi: 10.1039/C3CP53273A. [DOI] [PubMed] [Google Scholar]
- Mackay AL, Terrones H. Diamond from graphite. Nature. 1991;352:762. . doi: 10.1038/352762a0. [Google Scholar]
- Vanderbilt D, Tersoff J. Negative-curvature fullerene analog of C60. Phys Rev Lett. 1992;68:511–513. doi: 10.1103/PhysRevLett.68.511. . doi: 10.1103/PhysRevLett.68.511. [DOI] [PubMed] [Google Scholar]
- Ching WY, Huang MZ, Yn X. Electronic and optical properties of the Vanderbilt-Tersoff model of negative-curvature fullerene. Phys Rev B. 1992;46:9910–9912. doi: 10.1103/physrevb.46.9910. . doi: 10.1103/PhysRevB.46.9910. [DOI] [PubMed] [Google Scholar]
- Ceulemans A, Fowler PW. Symmetry extensions of Euler's theorem for polyhedral, toroidal and benzenoid molecules. J Chem Soc Faraday Trans. 1995;91:3089–3093. . doi: 10.1039/FT9959103089. [Google Scholar]
- Chuang C, Fan Y, Jin B. Systematics of toroidal, helically-coiled carbon nanotubes, high-genus fullerenes, and other exotic graphitic materials. Procedia Eng. 2011;14:2373–2385. . doi: 10.1016/j.proeng.2011.07.299. [Google Scholar]
- Schein S, Gayed JM. Fourth class of convex equilateral polyhedron with polyhedral symmetry related to fullerenes and viruses. Proc Natl Acad Sci U S A. 2014;111:2920–2925. doi: 10.1073/pnas.1310939111. . doi: 10.1073/pnas.1310939111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrones H, Mackay A. The geometry of hypothetical curved graphite structures. Carbon. 1992;30:1251–1260. . doi: 10.1016/0008-6223(92)90066-6. [Google Scholar]
- Ayuela A, Fowler PW, Mitchell D, Schmidt R, Seifert G, Zerbetto F. C62: theoretical evidence for a nonclassical fullerene with a heptagonal ring. J Phys Chem. 1996;100:15634–15636. . doi: 10.1021/jp961306o. [Google Scholar]
- Charlier JC, Ebbesen TW, Lambin P. Structural and electronic properties of pentagon-heptagon pair defects in carbon nanotubes. Phys Rev B. 1996;53:11108–11113. doi: 10.1103/physrevb.53.11108. . doi: 10.1103/PhysRevB.53.11108. [DOI] [PubMed] [Google Scholar]
- Kirby E, Pisanski T. Aspects of topology, genus and isomerism in closed 3-valent networks. J Math Chem. 1998;23:151–167. . doi: 10.1023/A%1019104804788. [Google Scholar]
- An J, Gan LH, Zhao JQ, Li R. A global search for the lowest energy isomer of C26. J Chem Phys. 2010;132:154304. doi: 10.1063/1.3364801. . doi: 10.1063/1.3364801. [DOI] [PubMed] [Google Scholar]
- Vizitiu AE, Diudea MV. C60 structural relatives – an omega-aided topological study. In: Cataldo F, Graovac A, Ori O, editors. The Mathematics and Topology of Fullerenes. Vol. 16. Berlin: Springer; 2011. pp. 39–60. [Google Scholar]
- Terrones H, Terrones M. The transformation of polyhedral particles into graphitic onions. J Phys Chem Solids. 1997;58:1789–1796. . doi: 10.1016/S0022-3697(97)00067-X. [Google Scholar]
- Avron JE, Berger J. Tiling rules for toroidal molecules. Phys Rev A. 1995;51:1146–1149. doi: 10.1103/physreva.51.1146. . doi: 10.1103/PhysRevA.51.1146. [DOI] [PubMed] [Google Scholar]
- Berger J, Avron JE. Classification scheme for toroidal molecules. J Chem Soc Faraday Trans. 1995;91:4037–4045. . doi: 10.1039/FT9959104037. [Google Scholar]
- Tamura R, Ikuta M, Hirahara T, Tsukada M. Positive magnetic susceptibility in polygonal nanotube tori. Phys Rev B. 2005;71:045418. . doi: 10.1103/PhysRevB.71.045418. [Google Scholar]
- Borštnik B, Lukman D. Molecular mechanics of toroidal carbon molecules. Chem Phys Lett. 1994;228:312–316. . doi: 10.1016/0009-2614(94)00944-9. [Google Scholar]
- Diudea MV, Kirby EC. The energetic stability of tori and single-wall tubes. Fuller Sci Technol. 2001;9:445–465. . doi: 10.1081/FST-100107148. [Google Scholar]
- Kang MH. Toroidal fullerenes with the Cayley graph structures. Discrete Math. 2011;311:2384–2395. . doi: 10.1016/j.disc.2011.06.018. [Google Scholar]
- Yoshida M, Fujita M, Fowler PW, Kirby EC. Non-bonding orbitals in graphite, carbon tubules, toroids and fullerenes. J Chem Soc Faraday Trans. 1997;93:1037–1043. . doi: 10.1039/A607401D. [Google Scholar]
- Iijima S, Ichihashi T, Ando Y. Pentagons, heptagons and negative curvature in graphite microtubule growth. Nature. 1992;356:776–778. . doi: 10.1038/356776a0. [Google Scholar]
- Diudea MV, Nagy CL, Silaghi-Dumitrescu I, Graovac A, Janežić D, Vikic-Topiić D. Periodic cages. J Chem Inf Model. 2005;45:293–299. doi: 10.1021/ci049738g. . doi: 10.1021/ci049738g. [DOI] [PubMed] [Google Scholar]
- Diudea MV, Bende A, Nagy CL. Carbon multi-shell cages. Phys Chem Chem Phys. 2014;16:5260–5269. doi: 10.1039/c3cp55309d. . doi: 10.1039/C3CP55309D. [DOI] [PubMed] [Google Scholar]
- Terrones H, Terrones M. Quasiperiodic icosahedral graphite sheets and high-genus fullerenes with nonpositive Gaussian curvature. Phys Rev B. 1997;55:9969–9974. . doi: 10.1103/PhysRevB.55.9969. [Google Scholar]
- Ricardo-Chávez JL, Dorantes-Dávila J, Terrones M, Terrones H. Electronic properties of fullerenes with nonpositive Gaussian curvature: finite zeolites. Phys Rev B. 1997;56:12143–12146. . doi: 10.1103/PhysRevB.56.12143. [Google Scholar]
- Bandow S, Takizawa M, Hirahara K, Yudasaka M, Iijima S. Raman scattering study of double-wall carbon nanotubes derived from the chains of fullerenes in single-wall carbon nanotubes. Chem Phys Lett. 2001;337:48–54. . doi: 10.1016/S0009-2614(01)00192-0. [Google Scholar]
- Terrones H. Beyond carbon nanopeapods. ChemPhysChem. 2012;13:2273–2276. doi: 10.1002/cphc.201200321. . doi: 10.1002/cphc.201200321. [DOI] [PubMed] [Google Scholar]
- Donadio D, Colombo L, Milani P, Benedek G. Growth of nanostructured carbon films by cluster assembly. Phys Rev Lett. 1999;83:776–779. . doi: 10.1103/PhysRevLett.83.776. [Google Scholar]
- Barborini E, Piseri P, Milani P, Benedek G, Ducati C, Robertson J. Negatively curved spongy carbon. Appl Phys Lett. 2002;81:3359–3361. . doi: 10.1063/1.1516635. [Google Scholar]
- Benedek G, Vahedi-Tafreshi H, Barborini E, Piseri P, Milani P, Ducati C, Robertson J. The structure of negatively curved spongy carbon. Diamond Relat Mater. 2003;12:768–773. . doi: 10.1016/S0925-9635(03)00082-7. [Google Scholar]
- Benedek G. The topological background of schwarzite physics. In: Cataldo F, Graovac A, Ori O, editors. The Mathematics and Topology of Fullerenes. Vol. 16. Berlin: Springer; 2011. pp. 214–247. [Google Scholar]
- Schwarz HA. Gesammelte Mathematische Abhandlungen. Berlin: Springer; 1933. Sur une définition erronée de l'aire d'une surface courbe. [Google Scholar]
- Dierkes U, Hildebrandt S. Global Analysis of Minimal Surfaces. Vol. 341. Berlin: Springer; 2010. [Google Scholar]
- Terrones H, Terrones M. Curved nanostructured materials. New J Phys. 2003;5:126.1–126.37. . doi: 10.1088/1367-2630/5/1/126. [Google Scholar]
- Lenosky T, Gonze X, Teter M, Elser V. Energetics of negatively curved graphitic carbon. Nature. 1992;355:333–335. . doi: 10.1038/355333a0. [Google Scholar]
- Weierstrass K. Untersuchungen über die Flächen, deren mittlere Krümmung überall gleich Null ist. Monatsber Akad Wiss Berlin. 1866;612–625 [Google Scholar]
- Terrones H, Mackay A. Negatively curved graphite and triply periodic minimal surfaces. J Math Chem. 1994;15:183–195. . doi: 10.1007/BF01277558. [Google Scholar]
- Gonzalez Szwacki N, Sadrzadeh A, Yakobson BI. B80 fullerene: an ab initio prediction of geometry, stability, and electronic structure. Phys Rev Lett. 2007;98:166804. doi: 10.1103/PhysRevLett.98.166804. . doi: 10.1103/PhysRevLett.98.166804. [DOI] [PubMed] [Google Scholar]
- Enyashin AN, Ivanovskii AL. Graphene allotropes. Phys Status Sol (b) 2011;248:1879–1883. . doi: 10.1002/pssb.201046583. [Google Scholar]
- Sundholm D. C72: gaudiene, a hollow and aromatic all-carbon molecule. Phys Chem Chem Phys. 2013;15:9025–9028. doi: 10.1039/c3cp51042e. . doi: 10.1039/C3CP51042E. [DOI] [PubMed] [Google Scholar]
- Bulusu S, Li X, Wang LS, Zeng XC. Evidence of hollow golden cages. Proc Natl Acad Sci U S A. 2006;103:8326–8330. doi: 10.1073/pnas.0600637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MP, Sundholm D, Vaara J. Au32: a 24-carat golden fullerene. Angew Chem Int Ed. 2004;43:2678–2681. doi: 10.1002/anie.200453986. . doi: 10.1002/anie.200453986. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zeng XC. Au42: an alternative icosahedral golden fullerene cage. J Am Chem Soc. 2005;127:3698–3699. doi: 10.1021/ja050435s. . doi: 10.1021/ja050435s. [DOI] [PubMed] [Google Scholar]
- Karttunen AJ, Linnolahti M, Pakkanen TA, Pyykkö P. Icosahedral Au72: a predicted chiral and spherically aromatic golden fullerene. Chem Commun. 2008;465–467 doi: 10.1039/b715478j. . doi: 10.1039/B715478J. [DOI] [PubMed] [Google Scholar]
- Assadollahzadeh B, Schwerdtfeger P. A systematic search for minimum structures of small gold clusters Aunn = 2-20) and their electronic properties. J Chem Phys. 2009;131:064306. doi: 10.1063/1.3204488. . doi: 10.1063/1.3204488. [DOI] [PubMed] [Google Scholar]
- Zhai HJ, Zhao YF, Li WL, Chen Q, Bai H, Hu HS, Piazza ZA, Tian WJ, Lu HG, Wu YB. Observation of an all-boron fullerene. Nature Chem. 2014;6:727–731. doi: 10.1038/nchem.1999. doi: 10.1038/nchem.1999. [DOI] [PubMed] [Google Scholar]