Abstract
A characteristic feature of Staphylococcus aureus is its ability to acquire resistance to antimicrobial agents. There is a need, therefore, for new approaches to combat this pathogen; for example, employing a combination of plant-derived products and antibiotics to overcome bacterial resistance. Indigofera suffruticosa is a plant popularly used to treat infections and has verified antimicrobial action. Here, we investigate the antimicrobial activity of different extracts from I. suffruticosa against S. aureus and their synergistic effects with erythromycin. Leaves of I. suffruticosa were extracted sequentially using diethyl ether, chloroform and acetone and the antimicrobial activity of each extract then tested against nine clinical isolates of S. aureus. Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined by microdilution tests, while the fractional inhibitory concentration (FIC) was assessed by checkerboard assay. All organic solvent extracts showed antimicrobial activity against S. aureus strains. The acetone extract was the most potent inhibitor of S. aureus (MIC and MBC of 0.78 and 3.12 mg/mL), followed by the chloroform extract (MIC and MBC of 3.12 and 6.25 mg/mL). Furthermore, acetone or chloroform extracts of I. suffruticosa enhanced the activity of erythromycin against S. aureus (FIC ≤ 0.5). We conclude that organic extracts from leaves of I. suffruticosa, alone or combined with erythromycin, are promising natural products for the development of new anti-S. aureus formulations.
Keywords: plant extracts, antibacterial agents, macrolide antibiotic, S. aureus
Introduction
Patients in hospital intensive care units are at risk of acquiring nosocomial infections due to the use of invasive devices and/ or extended hospital stay (Streit et al., 2004). Long-term hospitalization may further complicate patient health by exposure to various antimicrobial agents. Additionally, the indiscriminate use of antibiotics in treating infections promotes bacterial evolution and emergence of resistance strains (Palmer and Kishony, 2013; Tavares et al., 2013). Staphylococcus aureus is an important pathogen associated with nosocomial human infections, and this microorganism has successfully evolved numerous strategies to resist different antibiotics (Coutinho et al., 2009; Chung et al., 2011). Such increases in antibiotic resistant S. aureus strains drives research discovery of new antimicrobial agents and the development of alternative therapeutic strategies. These include plant extracts, which have considerable antimicrobial potential (Leite et al., 2006; da Silva et al., 2013; Zakavi et al., 2013).
Medicinal plants are important health and economic components used by many cultures for thousands of years (Agra et al., 2008; Silva et al., 2012). According to the World Health Organization approximately 80% of the global population uses medicinal plants or herbal medicine for primary health care (Pereira et al., 2012). Brazil has the highest plant diversity of any country and represents 20% of biodiversity in the world. Indigofera suffruticosa Mill (Fabaceae, Papilionidae) is a plant originally from Antilles and Central America, popularly known as “anileira” or “anil,” and was introduced into Brazil for the extraction of indigo, a blue dye blue (Indigo Blue) widely used by the textile industry. Although some toxic effects are reported for this plant, such as hemolytic anemia and hemoglobinuria in cattle and guinea pig (Salvador et al., 2011), it has been used in traditional medicine both externally and internally (Barros and Teixeira, 2008). Moreover, pharmacological effects of I. suffruticosa have been confirmed scientifically, such as anti-inflammatory (Chen et al., 2013a), anticonvulant (Almeida et al., 2013) and wound healing (Luiz-Ferreira et al., 2011) (Table 1). Previous work by our group has shown that aqueous infusions of I. suffruticosa leaves have inhibitory activity against S. aureus and dermatophyte strains (fungi) (Leite et al., 2006), though their action against clinical isolates and synergic potential have yet to be studied.
Table 1.
Pharmacological potential of Indigofera suffruticosa.
| Scientific account | Related compounds |
|---|---|
| Gastroprotective agent acute ulcer stimulating prostaglandin, mucus and HSP70. (Luiz-Ferreira et al., 2011) | Ethyl acetate fraction from methanolic extract showed the best action and the authors highlighted the role of role of flavonoids and alkaloids presents in AcF as active compounds |
| In vivo action against Pediculosis capitis (García Calixto et al., 2011) | An effective treatment using 5% I. suffruticosa Mill tincture was reported in a patient infested with Pediculosis capitis |
| Immunostimulatory and antitumoral actvities in vitro (Lopes et al., 2011) | This study evaluated the action of both alkaloid fraction and pure indigo. Indigo showed high activity which suggest that it is the major active principle in I. suffruticosa |
| Antimycobacterial (Carli et al., 2010) | These authors did not isolate or detected any compounds. The methanolic extract showed better activity than dichloromethane |
| Anticonvulsant effect (Almeida et al., 2013) | Alkaloids, flavonoids, steroids, proteins, carbohydrates, indigo carmine and essential oils (Linalool and Pinene) were detected in the methanolic extract |
| Anti-inflammatory property in vivo (Chen et al., 2013a) | Eight phenolic compounds were quantified: salicylic acid, syringic acid (major compounds) ρ-coumaric acid, vanillin, syringaldehyde, quercetin, isoliquiritigenin, formononetin. Salicylic acid was found in the plasma of mice fed with I. suffruticosa extracts |
| In vivo increase of Phase II detoxification enzyme and glutathione levels (Chen et al., 2013b) | The authors reported the same compounds quantified by Chen et al. (2013a). Ethanolic extracts showed the best action on the induction of phase II detoxification enzyme, and syringic acid was the most active among phenolic compounds detected, however, it was less potent than ethanolic extracts |
Synergistic assessments have become a key tool in phytomedicine research in recent years, and uses of antibiotics in combination with herbal products have been investigated as antimicrobials for S. aureus resistant strains (Wagner and Ulrich-Merzenich, 2009). Some studies have used erythromycin, a 14-membered ring macrolide antibiotic and therefore part of the Macrolide-Lincosamide-Streptogramin-B (MLSB) family, as a representative drug to evaluate combinatory effects of plant-derived products (Chan et al., 2013, 2015). Antibiotics from the MLSB family serve as an important combatant against S. aureus methicillin resistant (MRSA) strains, which are a major cause of disease in the general population and hospital-acquired infections (Pantosti, 2012). MLSB comprises three unrelated groups (macrolide, lincosamide and streptogramin-B) that share the same binding site in bacterial ribosome. It is possible, therefore, that a synergistic effect for one group might predict a similar action from the other groups.
Given this background, our study aimed to define the antimicrobial activities of different organic extracts from I. suffruticosa leaves against S. aureus strains (MRSA and MSSA), and then to examine synergistic actions with erythromycin.
Materials and methods
Chemicals
Dimethylsulfoxide (DMSO), erythromycin and 7-hydroxy-3H-phenoxazin-3-one-10-oxide sodium salt (Resazurin) was purchased from Sigma-Aldrich Chemical Company, St. Louis, MO, while Mueller-Hinton Agar and Nutrient Agar medium were from HIMEDIA Laboratories®. Diethylether, chloroform and acetone were obtained from Merck, Darmstadt, Germany.
Plant material and preparation of organic extracts
Leaves of I. suffruticosa were collected in São Caetano, Pernambuco, Brazil (latitude: 08° 19′ 33″ S; longitude: 36° 04′ 21″ W) between 10 and 11 a.m. The plant was identified by Dr. Marlene Carvalho Alencar Barbosa (Department of Botany, UFPE) and a voucher specimen deposited at the UFP Geraldo Mariz Herbarium-UFPE (identification number 45.217).
Organic extracts were prepared by successively extracting dried leaves of I. suffruticosa (100 g) with 200 mL of diethyl ether, chloroform or acetone, common solvents arranged in order of increasing polarity. Briefly, the leaf powder was homogenized firstly with 200 mL of diethyl ether for 2 h in a mechanical stirrer, kept refrigerated overnight (4°C) and filtered with Whatman no.1 paper. The solvent was then removed under reduced pressure in a rotary evaporator at 45°C to produce diethyl ether extract. The plant material which was not extracted by diethyl ether was then homogenized with 200 mL chloroform and all extraction process was repeated generating the chloroform extract. Finally, the remaining powder was submitted to acetone extraction to produce acetone extract. All dried organic extracts of I. suffruticosa were stored at −20°C until use and dissolved in dimethyl sulfoxide (DMSO, 1%) before each test.
Phytochemical screening
An approximate amount of diethyl ether, chloroform and acetone extracts from I. suffruticosa leaves were subjected to phytochemical analysis to ascertain the presence of secondary metabolites such as alkaloids, flavonoids, phenylpropanoids, triterpenoids and volatile oil in according to Wagner and Bladt (2009). Briefly, compounds classes were visualized as aid thin layer chromatography (TLC) on silicagel 60 F254 (Merck), mobile phase standard and Dragendorff, NEU-PEG, KOH-Ethanol, Liebermann-Burchard and vanillin-sulfuric acid reagents, respectively. Tests for tannins, saponins and other heterosides were not performed due to the low polarity of the extracts.
Antimicrobial assays
Staphylococcus aureus strains
The antimicrobial activity was tested against the following microorganisms provided by the Departamento de Antibióticos, Universidade Federal de Pernambuco (UFPEDA): Staphylococcus aureus (UFPEDA 02), and some isolated strains of S. aureus originally obtained from: vaginal secretion (UFPEDA 660); catheter tip (UFPEDA 663); urine sample (UFPEDA 670); blood sample (UFPEDA 672); prostate secretion (UFPEDA 676); wound secretion (UFPEDA 677 and 679); ocular secretion (UFPEDA 687). Strains UFPEDA 670 and 672 are classified as MRSA strains (Table 2). All strains were and maintained in Mueller-Hinton Agar (MHA) and stored at 4°C.
Table 2.
Susceptibility to antibiotics of Staphylococcus aureus strainsa.
| UFPEDA | Source | Susceptibility to antibiotics | |||
|---|---|---|---|---|---|
| Oxacillin | Cefoxitin | Erythromycin | Clindamycin | ||
| 02 | ATCC 6538 | S | S | S | S |
| 660 | Vaginal secretion | S | S | S | S |
| 663 | Catheter tip | S | S | S | S |
| 670b | Urine sample | R | R | R | R |
| 672b | Blood sample | R | R | R | R |
| 676 | Prostate secretion | S | S | S | S |
| 677 | Wound secretion | S | R | R | S |
| 679 | Wound secretion | S | S | R | S |
| 687 | Ocular secretion | S | S | S | S |
R, resistant; S, sensitive.
Data provided by UFPEDA Collection.
MRSA.
Determination of antibacterial activity using the disc diffusion method
The antibacterial activity of the organic extracts of I. suffruticosa leaves was determined by the disc diffusion method (de Oliveira et al., 2012). Briefly, all clinically isolated S. aureus strains were grown on MHA medium at 37°C for 18 h, suspended in distilled water (approximately 1.5 × 108 CFU/mL) and 100 μL aliquots of bacterial suspension were immediately inoculated in Petri dishes containing MHA medium. Sterile paper discs (6 mm diameter) containing 20 μL organic extracts of I. suffruticosa (100 mg/mL) were applied to the agar and the Petri dishes incubated at 37°C for an additional 18 h. Following incubation, the diameter of the inhibition zone (DIZ) of growth was measured, using DMSO as negative control.
Effects of temperature and pH on antimicrobial activity
The antimicrobial activity of each I. suffruticosa extract against S. aureus UFPEDA 02 was determined. Samples were placed in sterile tubes and kept for 30 min at different temperatures (28, 30, 60, and 100°C), or were stored at a different pH for 30 min at 25°C, using 1M NaOH or 1M HCl to adjust the pH range between 3 and 10. The antibacterial activity of treated extracts was tested using the disc diffusion method, as described above.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The minimal inhibitory concentration (MIC) was determined by a microdilution broth susceptibility assay (Clinical and Laboratory Standards Institute, 2011). Two-fold serial dilutions of the organic extracts of I. suffruticosa containing 50–0.20 mg/mL in DMSO were prepared in Mueller-Hinton Broth (MHB; 200 μL) in a 96-well microtiter plate. Bacterial suspensions were prepared from each S. aureus strains freshly grown in Mueller-Hinton broth (Merck) (approximately 1.5 × 108 CFU/mL,) and 10 μL of this suspension was added to each well. After incubation at 37°C for 24 h, bacterial growth was recorded using a Resazurin solution (0.01%). MIC was the lowest concentration at which no color change (from purple to pink) was observed. Afterwards, cultures were seeded in MHA medium and incubated for 24 h at 37°C to determine the minimum bactericidal concentration (MBC), which corresponds to the lowest amount of extract that kills S. aureus. All experiments were performed in triplicate.
Evaluation of combinatory effects of extracts and erythromycin
Combinatory effects between organic extracts of I. suffruticosa and erythromycin were assessed using the checkerboard test against the strain UFPEDA 02. Briefly, samples with different proportions of plant extract:drug (final volume: 20 μL) were prepared from stock solutions of each extract (50 mg/mL) and erythromycin (1 mg/mL) and antibacterial activity was tested as described for MIC determination (da Silva et al., 2013). The Fractional Inhibitory Concentration (Σ FIC) was calculated according to the equation:
MICE+D: minimal inhibitory concentration of extract in combination with erythromycin; MICD+E: minimal inhibitory concentration of erythromycin in combination with extract. Results were considered: synergistic (Σ FIC < 0.5); additive (0.5 < Σ FIC < 1); non-interactive (1 < Σ FIC < 4); or antagonist (Σ FIC > 4) (Vuuren and Viljoen, 2011).
Statistical analysis
Each experiment was performed in triplicate and results are expressed as the mean ± standard deviation (SD). Statistical analyses were performed by ANOVA and unpaired Student's t-test. All analyses were carried out using software StatView, version 4.5, Abacus Concept, Inc, Berkeley, CA. Differences were considered significant at p < 0.05. The correlation indices were calculated using the Pearson coefficient (ρ).
Results
Phytochemical analysis
TLC analysis revealed the presence of flavonoids, phenylpropanoids, triterpenoids and volatile oils in all three extracts. In most of the tests performed, only quantitative differences were found. Thus, flavonoids, phenylpropanoids and volatile oils predominated in acetone, ether and chloroform extracts, respectively. Alkaloids or nitrogen-containing compounds were detected only in the chloroform extract of I. suffruticosa (Table 3).
Table 3.
Phytochemical analysis of organic extract from leaves of Indigofera suffruticosa.
| Compounds class | Indigofera suffruticosa extracts | ||
|---|---|---|---|
| Ether | Chloroform | Acetone | |
| Alkaloids | - | + | - |
| Flavonoids | + | + | ++ |
| Phenylpropanoids | ++ | + | + |
| Triterpenoids | + | + | + |
| Volatile oils | + | ++ | + |
(-) absent, (+) weak, (++) strong.
Antibacterial activity of organic extracts from leaves of I. suffruticosa
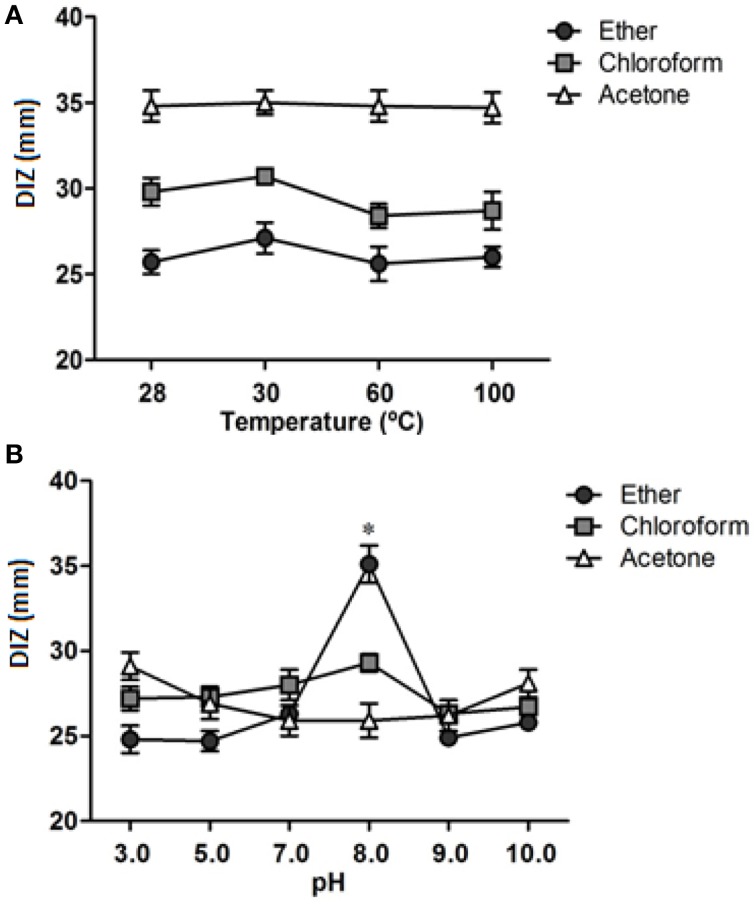
All organic extracts of leaves of I. suffruticosa showed antimicrobial activity against different S. aureus strains. However, the inhibition varied according to the extract and test strain with DIZ values ranging from 25.3 ± 2.1 to 36.0 ± 1.0 mm (Table 4). All extracts were active against both MRSA strains (UFPEDA 670 and UFPEDA 672) with DIZ values >30.0 mm, except for the chloroform extract which gave a DIZ of 27.7 ± 2.5 mm for strain UFPEDA 670. Diethyl ether extracts showed the best inhibition (30.08 ± 2.69 mm), followed by acetone (28.79 ± 3.35 mm) and chloroform (28.7 ± 3.42 mm), however no significant differences were observed between these average DIZ values (p > 0.05). Furthermore, strong correlations were found between the DIZ of all extracts with ρ-values of 0.86, 0.94, and 0.92 for ethyl/chloroform, and chloroform/acetone ethyl/acetone extracts, respectively. The antimicrobial activity of the extracts was not affected (p > 0.05) after high temperature treatment (Figure 1A) or variation of pH (Figure 1B), except for the ether extract which was notably more active at pH 8 (p > 0.05).
Table 4.
Antimicrobial activity of organic extracts from leaves of Indigofera suffruticosa against Staphylococcus aureus strains.
| S. aureus strains | Organic extracts of leaves of Indigofera suffruticosa DIZ | ||
|---|---|---|---|
| Ether | Chloroform | Acetone | |
| 02 | 34.7 ± 0.6a,1 | 36.0 ± 0.0a,1 | 35.7 ± 1.1a,1 |
| 660 | 29.0 ± 1.7b,1 | 28.0 ± 2.0b,1 | 28.0 ± 2.0b,1 |
| 663 | 28.7 ± 0.6b,1 | 27.7 ± 0.6b,1 | 26.7 ± 0.6b,1 |
| 670 | 32.7 ± 1.1a,1 | 27.7 ± 2.5b,2 | 30.7 ± 0.6b,2 |
| 672 | 32.6 ± 1.1a,1 | 32.3 ± 0.6c,1 | 31.0 ± 3.0b,1 |
| 676 | 27.3 ± 0.6b,1 | 25.3 ± 0.6b,1 | 26.3 ± 0.6b,1 |
| 677 | 30.0 ± 1.0b,1 | 29.0 ± 1.7b,1 | 29.7 ± 0.6b,1 |
| 679 | 29.0 ± 1.0b,1 | 26.3 ± 2.3b,1 | 25.7 ± 2.1b,1 |
| 687 | 26.7 ± 2.3b,1 | 26.0 ± 2.6b,1 | 25.3 ± 2.1b,1 |
| Average DIZ | 30.08 ± 2.7 | 28.7 ± 3.4 | 28.78 ± 3.4 |
DIZ values are expressed in mm.
*Same superscript letter (a,b,c) indicates no significant difference (p > 0.05) between DIZ values from different strains for each solvent (same column).
**Same superscript number (1,2) indicates no significant difference (p > 0.05) between DIZ values from different solvents against each strain (same row).
Figure 1.
Stability of organic extracts of leaves of Indigofera suffruticosa. (A) Effect of temperature on the stability of organic extracts of I. suffruticosa. (B) Effect of pH on the stability of organic extracts of I. suffruticosa. DIZ—inhibition zone diameter. *Significant differences in relation to control.
The MIC and MBC values ranged from 0.78 to 6.25 mg/mL and 3.12 to 25.0 mg/mL, respectively, with the acetone extract having the lowest values (Table 5). The MIC50 (minimum concentration able to inhibit 50% of strains) was 1.56 mg/mL for the acetone extract, and 6.25 mg/ml for both ether and chloroform extracts. Similarly, the MBC50 (minimum concentration able to kill 50% of strains), for the acetone extract was 6.25 mg/mL, but 12.5 mg/mL for ether and chloroform extracts. Additionally, the average MIC and MBC of acetone extract (2.16 ± 0.9 and 7.63 ± 3.8, respectively) were lower (p > 0.05) than other extracts (4.85 ± 1.6 and 15.27 ± 7.7 for ether extract; and 5.9 ± 1.0 mg/mL and 16.67 ± 6.2 mg/mL for chloroform). The three extracts also differed in their MBC/MIC ratio (Pankey and Sabath, 2004); although ether and chloroform extracts showed exclusively bactericidal effects (MBC/MIC ratios ranged from 2 to 4), the acetone extract had both bactericidal and bacteriostatic actions, however this extract was a bactericidal agent for almost all S. aureus strains tested (77.78%).
Table 5.
Minimum inhibitory concentration and minimum bactericidal concentration of organic extracts from leaves of Indigofera suffruticosa against Staphylococcus aureus strains.
| S. aureus strains | Organic extracts from leaves of Indigofera suffruticosa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ether | Chloroform | Acetone | |||||||
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| 02 | 3.12 | 12.5 | 4 | 3.12 | 12.5 | 4 | 1.56 | 3.12 | 2 |
| 660 | 6.25 | 12.5 | 2 | 6.25 | 25.0 | 4 | 1.56 | 6.25 | 4 |
| 663 | 6.25 | 25.0 | 4 | 6.25 | 25.0 | 4 | 3.12 | 12.5 | 4 |
| 670 | 6.25 | 25.0 | 4 | 6.25 | 25.0 | 4 | 1.56 | 12.5 | 8 |
| 672 | 6.25 | 12.5 | 2 | 6.25 | 12.5 | 2 | 3.12 | 6.25 | 2 |
| 676 | 6.25 | 12.5 | 2 | 3.12 | 12.5 | 4 | 3.12 | 3.12 | 1 |
| 677 | 6.25 | 25.0 | 4 | 3.12 | 6.25 | 2 | 3.12 | 6.25 | 2 |
| 679 | 6.25 | 12.5 | 2 | 3.12 | 6.25 | 2 | 1.56 | 6.25 | 4 |
| 687 | 6.25 | 12.5 | 2 | 6.25 | 12.5 | 2 | 0.78 | 12.5 | 16 |
| MIC50 | 6.25 | 6.25 | 1.56 | ||||||
| MBC50 | 12.5 | 12.5 | 6.25 | ||||||
| Average MIC | 5.9 ± 1.0 | 4.85 ± 1.6 | 2.16 ± 0.9 | ||||||
| Average MBC | 16.67 ± 6.2 | 15.27 ± 7.7 | 7.63 ± 3.8 | ||||||
MIC, minimal inhibitory concentration; MBC, minimal bactericidal concentration.
MIC50, concentration able to inhibit 50% of strains; MBC50, concentration able to kill 50% of strains.
MIC, MIC50, MBC and MBC50 are expressed in mg/mL.
Combinatory effects of organic extracts of I. suffruticosa and erythromycin
When the antimicrobial actions of erythromycin and I. suffruticosa organic extracts were tested in combination, additive, synergistic and non-interactive actions were observed (Table 6); importantly, no antagonistic effects were noted. Acetone extract and erythromycin showed synergistic effects (in five ratios (55.56%; Σ FIC values ranged from 0.3 to 0.5), additive effects (0.6 ≤ Σ FIC ≤ 0.8) in three and a non-interactive effect in only one (ratio of 1:9, drug:extract; Σ FIC = 1.7). For the chloroform extract and erythromycin combinations both synergistic (0.2 ≤ Σ FIC ≤ 0.4) and additive (0.7 ≤ Σ FIC ≤ 0.9) effects were equally found in four ratios and only one ratio gave a non-interaction (1:9, drug:extract; Σ FIC = 1.7). No synergistic effect was seen with ether extracts, but 8 ratios resulted in additive effects (0.6 ≤ Σ FIC ≤ 0.9) and 1 ratio a non-interactive effect (3:7, drug:extract; Σ FIC = 1.2). Strong correlations were observed between Σ FIC values from erythromycin/acetone and erythromycin/chloroform combinations (ρ = 0.82), although no significant difference was found between the mean of their Σ FIC values (0.68 ± 0.46 and 0.644 ± 0.44; p < 0.05). The best Σ FIC values were 0.2 for erythromycin/chloroform at 5:5, followed by 0.3 for all these combinations: erythromycin/acetone (at 7:3 and 3:7) and for erythromycin/chloroform (at 3:7 and 6:4).
Table 6.
Combinatory effects of organic extracts from leaves of Indigofera suffruticosa and erythromycin against S. aureus UFPEDA 02.
| Erythromycin/Extracts proportion | Organic extracts from Indigofera suffruticosa leaves (Σ FIC) | ||
|---|---|---|---|
| Ether | Chloroform | Acetone | |
| 9:1 | 0.9 | 0.9 | 0.4 |
| 8:2 | 0.9 | 0.4 | 0.4 |
| 7:3 | 0.7 | 0.7 | 0.3 |
| 6:4 | 0.6 | 0.3 | 0.6 |
| 5:5 | 0.6 | 0.2 | 0.5 |
| 4:6 | 0.8 | 0.8 | 0.8 |
| 3:7 | 1.2 | 0.3 | 0.3 |
| 2:8 | 0.8 | 0.8 | 0.8 |
| 1:9 | 0.8 | 1.7 | 1.7 |
| Average Σ FIC | 0.81 ± 0.18 | 0.68 ± 0.46 | 0.644 ± 0.44 |
Discussion
S. aureus is a pathogen long-recognized to be capable of developing drug resistance which increases patient treatment time, rate of morbidity and mortality, and associated financial costs (Pantosti, 2012). These factors make the search for new active agents against S. aureus highly relevant. In contrast to the well-known antimicrobial effects of I. suffruticosa (Leite et al., 2006; Carli et al., 2010), our present work is the first to evaluate organic solvent extracts for activity against clinical isolates of S. aureus strains (including two MRSA strains), as well their combinatory effects with a macrolide drug (erythromycin).
The organic extracts from I. suffruticosa leaves showed antimicrobial activity against all tested strains of S. aureus and, importantly, high inhibition zones were found against MRSA strains (UFPEDA 670 and UFPEDA 672). These two strains were isolated from different sources and exhibited multidrug-resistant profile (oxacillin-cefoxitin-erythromycin-clindamycin). The best anti-S. aureus activity was shown by the acetone extract, since its MIC50 was 4-fold lower than the MIC50 values of the two other extracts. From chemical point of view, the acetone extract contains more flavonoids than ether and chloroform extracts. It is known that different species of genus Indigofera including I. suffruticosa are rich source of bioactive flavonoids (Hasan et al., 1993; Narender et al., 2006; Varanda et al., 2011; Perez et al., 2013). Previous chemical analysis from I. suffruticosa resulted in the identification of four quercetin derivatives. Although our result revealed that the antimicrobial properties might be associated with the presence of flavonoids, a characterization of acetone extract is necessary, even though this has not been our major focus.
We also showed that high temperature (up to 100°C) had negligible effect on the anti-S. aureus activity of each extract, which may explain the effective traditional usage of I. suffruticosa in infusions prepared by prolonged boiling of its leaves (Corrêa, 1984). Similarly, the antimicrobial activities of our three organic extracts showed little change when submitted to pH values ranging from pH 3 to pH 10. Thermal and pH stabilities are noteworthy factors for development of new antimicrobial formulations by the cosmetic, food and pharmaceutical industries, and our findings encourage further research into use of our organic extracts.
Exploring combinatory effects of antimicrobial agents and natural products is an attractive strategy to overcome bacterial resistance (Betoni et al., 2006; Wink et al., 2012). Diverse targets are involved in the synergistic effects of drugs and plant-derived products such as enzymes and substrates, metabolites, receptors, ion channels, transport proteins, DNA and RNA (Wagner, 2011; Yang et al., 2014). Our study establishes that all organic extracts from I. suffruticosa induce at least additive effects with erythromycin. In addition to its more potent antimicrobial activity, the synergestic effect of the acetone extract was higher than that of the chloroform extract, although this did not reach statistical significance and the Σ FIC values of the two were strongly correlated. In contrast, the I. suffruticosa ether extract only showed additive effects or, in one tested ratio, a non-interactive effect. These results suggest these as a promising source of potential compounds to be used in combination of erythromycin (and other members of MLSB family).
I. suffruticosa extracts have been target of a various studies in order to prove their medicinal potential. Most of these works have shown that polar solvent extracts are more active (Table 1) as they are rich in phenolic compounds, flavonoids, carbohydrates, glycoproteins, indigo, alkaloids, and triterpenes (Leite et al., 2006; Carli et al., 2010; Lopes et al., 2011; Luiz-Ferreira et al., 2011; Almeida et al., 2013; Chen et al., 2013a,b). Furthermore, extracts from I. suffruticosa have been also shown key features to be used as a medicine such as lethal dose 50% (1600 mg/kg (ip) in mice (Almeida et al., 2013) and induction of phase II detoxification enzyme and increase of glutathione levels in rat Clone 9 liver cells (Chen et al., 2013b).
In summary this paper showed that organic extracts of I. suffruticosa are promising natural products for the development of new anti-S. aureus formulation given their antimicrobial inhibiting MRSA strains and their combination with erythromycin seems to be very perspective, thus deserving further studies in order to understand their mechanism of action.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Ciência do Estado de Pernambuco (FACEPE) for the financial support to this study. We express our gratitude to Prof. James Stuart Owen, from University College of London, for correcting the English language.
References
- Agra M. F., Silva K. N., Basílio D. I. J. L., Freitas P. F., Barbosa-Filho J. M. (2008). Survey of medicinal plants used in the region Northeast of Brazil. Rev. Bras. Farmacogn. 10, 472–508 10.1590/S0102-695X2008000300023 [DOI] [Google Scholar]
- Almeida E. R., Chaves T. M., Luna R. L. A., Silva A. R., Aragão-Neto A. C., Silva L. L. S., et al. (2013). Anticonvulsant effect of Indigofera suffruticosa Mill: indication of involvement of the GABAergic system. Afr. J. Pharm. Pharmacol. 7, 622–628 10.5897/AJPP12.1262 [DOI] [Google Scholar]
- Barros G. M. C. C., Teixeira S. D. P. (2008). Pharmacobotanical studies of wild indigo species (Indigofera suffruticosa and Indigofera truxillensis, Leguminosae,) with pharmacological properties. Rev. Bras. Farmacogn. 18, 287–294 10.1590/S0102-695X2008000200024 [DOI] [Google Scholar]
- Betoni J. E. C., Mantovani R. P., Barbosa L. N., Di Stasi L. C., Fernandes A., Jr. (2006). Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz 101, 387–390. 10.1590/S0074-02762006000400007 [DOI] [PubMed] [Google Scholar]
- Carli C. B. A., Quilles M. B., Maia D. C., Lopes F. C., Santos R., Jr., Fujimura-Leite C. Q., et al. (2010). Antimycobacterial activity of Indigofera suffruticosa with activation potential of the innate immune system. Pharm. Biol. 48, 878–882. 10.3109/13880200903303471 [DOI] [PubMed] [Google Scholar]
- Chan B. C., Han X. Q., Lui S. L., Wong C. W., Wang T. B., Cheung D. W., et al. (2015). Combating against methicillin−resistant Staphylococcus aureus–two fatty acids from Purslane (Portulaca oleracea L.) exhibit synergistic effects with erythromycin. J. Pharm. Pharmacol. 67, 107–116. 10.1111/jphp.12315 [DOI] [PubMed] [Google Scholar]
- Chan B. C., Ip M., Gong H., Lui S. L., See R. H., Jolivalt C., et al. (2013). Synergistic effects of diosmetin with erythromycin against ABC transporter over-expressed methicillin-resistant Staphylococcus aureus (MRSA) RN4220/pUL5054 and inhibition of MRSA pyruvate kinase. Phytomedicine 20, 611–614. 10.1016/j.phymed.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Chen C. C., Liu C. S., Li C. C., Tsai C. W., Yao H. T., Liu T. C., et al. (2013b). Indigofera suffruticosa Mill extracts up-regulate the expression of the π class of glutathione S-transferase and NAD (P) H: quinone oxidoreductase 1 in rat Clone 9 liver cells. Food Chem. Toxicol. 59, 610–617. 10.1016/j.fct.2013.06.042 [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Sun H. L., Yao H. T., Lii C. K., Chen H. W., Chen P. Y., et al. (2013a). Suppressive effects of Indigofera suffruticosa Mill extracts on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. Food Chem. Toxicol. 55, 257–264. 10.1016/j.fct.2012.12.056 [DOI] [PubMed] [Google Scholar]
- Chung P. Y., Navaratnam P., Chung L. Y. (2011). Synergistic antimicrobial activity between pentacyclic triterpernoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 10, 1–6. 10.1186/1476-0711-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (2011). Performance Standards for Antimicrobial Susceptibility Testing; Twenty First Informational Supplement, M100S21. Wayne, PA.
- Corrêa M. P. (ed.). (1984). Dicionário de plantas úteis do Brasil e das exóticas cultivadas, in Dicionário de Plantas úteis do Brasil e das Exóticas Cultivadas (Brasília: Imprensa Nacional; ), 1–172. [Google Scholar]
- Coutinho H. D. M., Costa J. G. M., Lima E. O., Falcão-Silva V. S., Junior J. P. S. (2009). Herbal therapy associated with antibiotic therapy: potentiation of the antibiotic activity against methicillin- resistant Staphylococcus aureus by Turnera ulmifolia L. BMC Complem. Alter. Med. 9:13. 10.1186/1472-6882-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva L. C. N., Sandes J. M., de Paiva M. M., de Araújo J. M., Figueiredo R. C. B. Q. D., da Silva M. V., et al. (2013). Anti-Staphylococcus aureus action of three Caatinga fruits evaluated by electron microscopy. Nat. Prod. Res. 27, 1492–1496. 10.1080/14786419.2012.722090 [DOI] [PubMed] [Google Scholar]
- de Oliveira Y. L., Nascimento da Silva L. C., da Silva A. G., Macedo A. J., de Araújo J. M., Correia M. T. S., et al. (2012). Antimicrobial activity and phytochemical screening of Buchenavia tetraphylla (Aubl.) R. A. Howard (Combretaceae: Combretoideae). Sci. World. J. 2012, 1–6, 10.1100/2012/849302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Calixto T., Rodríguez Gonzalez M. E., Pinera Wiltshire M. C., Martínez Monier M. A., Santana Suárez Y., Hernández Contreras N. (2011). Effective treatment of a patient infested with pediculus capitis by using 5% Indigofera suffruticosa Mill tincture. Rev. Cubana Med. Trop. 63, 275–277. [PubMed] [Google Scholar]
- Hasan A., Farman M., Ahmed I. (1993). Flavonoid glycosides from Indigofera hebepetala. Phytochemistry 35, 275–276. 10.1016/S0031-9422(00)90552-18987878 [DOI] [Google Scholar]
- Leite S. P., Vieira J. R. C., Medeiros P. L., Leite R. M. P., Lima V. L. M., Xavier H. S., et al. (2006). Antimicrobial activity of Indigofera suffruticosa. Evid. Based Complement. Alternat. Med. 3, 261–265. 10.1093/ecam/nel010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes F. C., Calvo T. R., Colombo L. L., Vilegas W., Carlos I. Z. (2011). Immunostimulatory and cytotoxic activities of Indigofera suffruticosa (Fabaceae). Nat. Prod. Res. 25, 1796–1806. 10.1080/14786419.2010.488624 [DOI] [PubMed] [Google Scholar]
- Luiz-Ferreira A., Cola M., Barbastefano V., Farias-Silva E., Calvo T. R., de Almeida A. B., et al. (2011). Indigofera suffruticosa Mill as new source of healing agent: involvement of prostaglandin and mucus and heat shock proteins. J. Ethnopharmacol. 137, 192–198. 10.1016/j.jep.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Narender T., Khaliq T., Puri A., Chander R. (2006). Antidyslipidemic activity of furano-flavonoids isolated from Indigofera tinctoria. Bioorg. Med. Chem. Lett. 16, 3411–3414. 10.1016/j.bmcl.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Palmer A. C., Kishony R. (2013). Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nat. Rev. Genet. 14, 243–248. 10.1038/nrg3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankey G. A., Sabath L. D. (2004). Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 38, 864–870. 10.1086/381972 [DOI] [PubMed] [Google Scholar]
- Pantosti A. (2012). Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front. Microbiol. 3:127. 10.3389/fmicb.2012.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F. L., Fernandes J. M., Leite J. P. V. (2012). Ethnopharmacological survey: a selection strategy to identify medicinal plants for a local phytotherapy program. Braz. J. Pharm. Sci. 48, 299–313 10.1590/S1984-82502012000200014 [DOI] [Google Scholar]
- Perez L. B., Li J., Lantvit D. D., Pan L., Ninh T. N., Chai H. B., et al. (2013). Bioactive Constituents of Indigofera spicata. J. Nat. Prod. 76, 1498–1504. 10.1021/np400567c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador I. S., Medeiros R. M. T., Pessoa C. R. M., Oliveira D. M., Duarte A. L. A., Fighera R. A., et al. (2011). Experimental poisoning of guinea pig (Cavia porcellus) with Indigofera suffruticosa. Toxicon 57, 927–931. 10.1016/j.toxicon.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Silva M. I. G., Melo C. T. V., Vasconcelos L. F., Carvalho A. M. R., Souza F. C. F. (2012). Bioactivity and potential therapeutic benefits of some medicinal plants from the Caatinga (semi-arid) vegetation of Northeast Brazil: a review of the literature. Rev. Bras. Farmacogn. 22, 193–207 10.1590/S0102-695X2011005000171 [DOI] [Google Scholar]
- Streit J. M., Jones R. N., Sader H. S. (2004). Daptomycin activity and spectrum: a worldwide sample of 6,737 Gram-positive organisms. J. Antimicrob. Chemother. 53, 669–674. 10.1093/jac/dkh143 [DOI] [PubMed] [Google Scholar]
- Tavares L. S., Silva C. S. F., Souza V. C., Silva V., Diniz C. G., Santos M. O. (2013). Strategies and molecular tools to fight antimicrobial resistance: resistome, transcriptome, and antimicrobial peptides. Front. Microbiol. 4:412. 10.3389/fmicb.2013.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanda E. A., Calvo T. R., Cardoso C. R. P., Da Silva Moura A. C., Dos Santos L. C., Colus I. M. S., et al. (2011). Mutagenic activity of Indigofera truxillensis and I. suffruticosa aerial parts. Evid. Based Complement. Alternat. Med. 2011, 1–9. 10.1093/ecam/nep123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuuren S., Viljoen A. (2011). Plant-based antimicrobial studies—methods and approaches to study the interaction between natural products. Planta Med. 77, 1168–1182. 10.1055/s-0030-1250736 [DOI] [PubMed] [Google Scholar]
- Wagner H. (2011). Synergy research: approaching a new generation of phytopharmaceuticals. Fitoterapia 82, 34–37. 10.1016/j.fitote.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Wagner H., Bladt S. (2009). Plant drug analysis—A thin layer chromatography atlas. Berlin: Springer Verlag. [Google Scholar]
- Wagner H., Ulrich-Merzenich G. (2009). Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 16, 97–110. 10.1016/j.phymed.2008.12.018 [DOI] [PubMed] [Google Scholar]
- Wink M., Ashour M. L., El-Readi M. Z. (2012). Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 3:130. 10.3389/fmicb.2012.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang Z., Li S., Ye X., Li X., He K. (2014). Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia 92, 133–147. 10.1016/j.fitote.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Zakavi F., Golpasand Hagh L., Daraeighadikolaei A., Farajzadeh Sheikh A., Daraeighadikolaei A., Leilavi Shooshtari Z. (2013). Antibacterial effect of juglans regia bark against oral pathologic bacteria. Int. J. Dent. 2013, 1–5. 10.1155/2013/854765 [DOI] [PMC free article] [PubMed] [Google Scholar]