Abstract
This study builds on previous work by Kendall, Leonard and McKenzie, which investigated event sequence variability for 12 paired-events during swallowing by healthy volunteers. They identified four event pairs, which always occurred in a stereotyped order as well as a most-common occurring overall order of events during swallowing. In the current study, we investigate overall event sequencing and the same four paired-events in a sample of swallows by healthy, young (under 45 years old) volunteers. Data were collected during a 16-swallow lateral videofluoroscopy protocol, which included manipulations of bolus volume, barium density, bolus viscosity and swallow cueing. Our results agree with previous findings regarding the fact that variable event sequencing is found in healthy swallowing, and regarding the obligatory sequencing of two paired-events: movement of the arytenoids towards the base of the epiglottis begins prior to upper esophageal sphincter (UES) opening; and maximum hyo-laryngeal approximation occurs after UES opening. However, our data failed to replicate the previous finding of obligatory sequencing of maximum pharyngeal constriction after maximal UES distension and UES opening occurring before bolus arrival at the UES. The most-common observed overall event sequence reported by Kendall was observed in only 4/293 swallows in our dataset. Manipulations of bolus volume, bolus viscosity, barium concentration, swallow cueing and swallow repetitions could not completely account for differences observed between the two studies.
Keywords: deglutition, deglutition disorders, swallowing, sequence, variability
Introduction
The pharyngeal phase of swallowing is a highly coordinated neuromuscular process involving a bilateral cascade of inhibition and activation of the muscles of the palate, pharynx, larynx and esophagus [1]. In a healthy individual, this complex sequence of inter-dependent events occurs within approximately one second [2]. Timely and coordinated pharyngeal swallowing ensures safe delivery of the bolus from the mouth to the esophagus. The swallowing literature contains a large number of studies reporting normative data for temporal measures in swallowing (see [3] for a review). Prior data are available both for event durations (such as laryngeal closure duration or hyoid movement duration) and for the latencies between events, which we call swallowing intervals (such as stage transition duration or pharyngeal transit time). However, a relatively smaller base of literature exists describing the sequencing of swallowing events (either bolus or gestural events) in relation to each other (see for example, [4-8]).
In 2007, Mendell and Logemann [7] reported a comprehensive review of studies in which the temporal sequence of events that occur during the healthy pharyngeal swallow hadbeen investigated. Importantly, they pointed out variations in the methodology used in the available literature, particularly with respect to the choice of a reference point or event to which all other events are related. For example, several authors (including Mendell and Logemann themselves) have chosen the onset of UES opening as their reference event [4-7] while others have chosen antecedent reference events such as the onset of oral bolus movement [9,1O] or the onset of hyolaryngeal elevation [11,12].
In a contrasting approach, Kendall and colleagues [13] sequenced both gestural and bolus events from the lateral view videofluoroscopies of 60 healthy volunteers (30 male) between 18 and 62 years of age, who each swallowed one bolus each of 1, 3, and 20ml Liquid Barosperse Barium Sulfate Suspension (60% w/v). The analysis investigated the degree to which the sequence varied for 12 separate event pairs, drawn :from the following events of interest:
onset of arytenoid movement towards the base of the epiglottis (AEstart);
onset of airway closure (AEclose);
opening of the upper esophageal sphincter [UES] (Pop);
bolus head arriving at the UES (BP1);
maximum hyoid displacement (H2);
closest approximation ofhyoid and larynx (HL);
maximum distension of the UES (PESmax); and
maximum constriction of the pharynx (PAmax).
The sequencing approach used by Kendall and colleagues [13] removes the need to define a single reference event and simply asks, ‘How often does event A occur before event B?’. Event pairs were chosen for study because they occurred in proximity to one another [13]. Of the 12 event pairs examined, four sequences were found to occur in a regular pattern in all cases (100% of the time), regardless of bolus volume:
AEstart always began prior to Pop;
The UES always opened (Pop) prior to or simultaneously with BP1;
Maximum larynx-to-hyoid approximation (HL) always occurred after Pop; and
PAmax always occurred after PESmax.
With the exception of these four paired-event sequences, Kendall and colleagues observed a high degree of variation in swallow event sequencing across their healthy participants. They also reported that the greatest variability in sequence was seen with smaller bolus volumes. Finally, they identified a most-common sequence of events during the swallow: AEclose→Pop→BP1→H2→PESmax→HL→PAmax. This sequence was, however, only observed in 25% (45/180) of all swallows in their dataset (7/60 for lml boluses, 25/60 for 3ml, and 23/60 for 20ml).
In the present study, we replicate methods reported by Kendall et al. [13] for investigating sequence variability during pharyngeal swallowing in a new dataset of healthy swallows. We focus on the replication of their primary findings, that is:
to confirm whether or not the four observed regular paired-event sequences (which we refer to as ‘obligatory paired-events’) are seen in a new sample; and
to determine whether the most-common sequence of events described by Kendall et al. [13] is seen during swallowing in a new sample.
Our experimental design includes a volume manipulation (as per Kendall et al. [13]), as well as additional viscosity (thin versus nectar) and barium concentration (22% w/v versus 40% w/v) manipulations. Further, our method differs from the original study, in that we collected three swallows per bolus condition (rather than one), enabling us to investigate the evolution of sequence across repeated trials within bolus condition. Our null hypothesis was that the four paired-event sequences described by Kendall and colleagues as obligatory would occur in the same order in all tasks, regardless of the influence of viscosity, barium concentration, bolus volume, and task repetition.
Materials and Methods
Participants
A sample of twenty healthy young volunteers balanced for sex (10 male) and stratified by height participated in a 16-swallow videofluoroscopy protocol. All participants were under 45 years old, with a mean age of 31.5 years (standard deviation = 5.7 years). This study was approved by the Research Ethics Board at the local institution and written consent was obtained :from each participant prior to study participation. The investigation of event sequencing is a secondary analysis of this dataset, which has been described elsewhere [14].
VF Procedure
All VF studies were conducted in seated lateral view by a licensed Speech-Language Pathologist and a Radiology Technologist using a Toshiba Ultimax fluoroscope (Toshiba America Medical Systems, Inc., Tustin, CA). Fluoroscopy was pulsed at full resolution (30 pulses per second) with the resulting images captured and recorded on a Digital Swallowing Workstation (KayPentax, Lincoln Park, NJ) at 30 frames per second Each participant swallowed 3×5ml, 3×10ml and 3×20ml boluses of ultra-thin liquid barium suspension at 22% weight/volume (w/v) (Liquid Polibar diluted with water), 3×5ml boluses of thin liquid barium at 40% w/v (Liquid Polibar diluted with water), and 3×5ml boluses of cranberry-flavor nectar thick barium at 40%w/v (Flavor Creations, Bostwick level 12-14 mixed with Bracco E-Z-Paque). All swallows were self-administered by drinking from a 30 ml medicine cup. fu addition, the data set contained a "bolus hold" (or cued swallow) task, for which the participant was instructed to hold a single 1Oml ultra-thin liquid barium bolus (22% w/v) in their mouth for 5 seconds prior to initiating a swallow; all other swallows were initiated using a non-command paradigm. Although the cued swallow task was not included in the primary analysis for this study, the data were subsequently used for a posthoc comparison, investigating the effects of swallow cueing on sequence variation, given reported effects of swallow cueing on swallow timing [15,16].
Boluses were presented in blocks of three with the order of blocks randomized. There were two exceptions to this task randomization: 1) each study routinely began with the bolus-hold task; and 2) the nectar swallows were reserved for the end of the procedure in order to limit potential contamination of residue from the thicker stimuli to thin liquid trials. Strict volumetric control was maintained by weighing each cup before and after swallowing. The average radiation exposure time was 1.75 minutes (SD: +/−0.31 minutes). No clinically significant penetration, aspiration or residue was documented on any of the swallows collected from these healthy volunteer participants.
Analysis
Data processing
Swallows were analyzed in a randomized and blinded fashion by a trained research assistant. Seven swallows were excluded due to the use of multiple swallows to clear a single bolus (I instance at 5ml and 6 instances at 20ml), resulting in a total of 293 swallows in the final dataset. Swallows were advanced frame-by-frame in ImageJ software (National Institutes of Health, Bethesda, MD) to identify the first frame showing each of the following 8 events according to the original methods and the operational definitions inKendall et al. [13]: AEstart, AEclose, Pop, BP1, H2, HL, PESmax and PAmax. In order to limit measurement error, our practice is to use the posterior-superior corner of the laryngeal air column as the location of the larynx in the HL measure. One clarification in our approach compared to that used by Kendall et al. [13] is that we identified the frames of maximum superior (H2Y) and maximum anterior displacement (H2X) separately from frame-by-frame position tracking of the anterior superior corner of the hyoid bone as seen in lateral view VFSS, and chose the later of these two frames to represent H2, the frame of maximum hyoid displacement. The identified timing of all events was then used to document the overall sequence of events for the 8 events studied, and for the four paired-event sequences for which Kendall et al. [13] reported an obligatory order. With the exception of the Pop before or with BP1 event sequence (which allows for both events to occur on the same frame), an obligatory event sequence was considered not to have been followed if the two events occurred on the same frame. This is a direct replication of the original analysis rules used by Kendall et al. [13].
Reliability
Twenty percent of swallows were re-rated by the original rater as well as by an experienced Speech-Language Pathologist (first author). The reliability analysis was conducted in two steps. First, we explored agreement with respect to sequencing of the four event pairs described by Kendall and colleagues to be obligatory [13]. As will be described below, the BP1 and POP events were found to be synchronous (i.e., occurring on the same frame) in 10% of the swallows in the current data set; thus, for reliability we explored the extent to which the AEStart event was located prior to these events across and within raters, and the extent to which the HL even occurred after these events across and within raters. Similarly, the PESmax and PAmax frames were found to be synchronous in 10% of the swallows in the dataset; therefore, for reliability purposes, we explored the extent to which HL occurred prior to these events across and within raters. Table 1 shows agreement with respect to event sequencing using Cohen’s Kappa scores [17]. Given the modest agreement obtained for some of these comparisons (particularly for later event pairs in the swallow), we also explored agreement for event latencies, which were calculated by expressing each event relative to a fixed reference point, operationally defined as 10 frames prior to the AEstart frame. We compared event latencies from this reference point within and across raters using averaged two-way mixed intraclass coefficients (ICC) for consistency. All scores for the derived latency comparisons achieved excellent reliability (Table 2) with inter-rater scores ranging from 0.89 to 0.99 and intra-rater scores ranging from 0.97 to 0.99 [18]. Given that AEstart had a fixed latency from the selected reference frame, ICCs were not calculated for this variable. Descriptive statistics for AEstart revealed that the repeated ratings by the original rater were, on average, within a single frame of her original ratings (mean 0.23 frames, i.e., 0.007 seconds) and that the secondary (inter) rater was, on average, within 2 frames (mean= 1.58 frames, i.e. 0.053 seconds) of the original rater’s frame of choice.
Table 1.
Intra- and inter-rater agreement statistics for event sequence. Interpretation of Cohen's Kappa scores for agreement is cited as suggested by Landis & Koch [17].
| Event Order | Intra-rater | Inter-rater | ||||
|---|---|---|---|---|---|---|
|
%
Agreement |
Kappa |
Kappa
Interpretation* |
%
Agreement |
Kappa |
Kappa
Interpretation* |
|
| AEStart before POP and BP1 | 98% | 0.65 | Substantial | 98% | 0.66 | Substantial |
| BP1 and POP before HL | 62% | 0.55 | Moderate | 98% | 0.49 | Moderate |
| HL before PESmax and Pamax | 45% | 0.14 | Slight | 65% | 0.34 | Fair |
Table 2.
Inter-rater and Intra-rater reliability scores and corresponding 95% Confidence Intervals for event latencies from AEstart, derived from event frame selection using intra-class correlation coefficients. [AEstart = onset of arytenoid elevation, Pop = UES opening, BP1 = bolus arriving at the UES, H2 = maximum hyoid elevation, HL = maximum approximation of hyoid and larynx, PESmax = maximum distension of the UES, PAmax = maximum constriction of the pharynx].
| Event | Interclass Correlation Coefficient (ICC) |
95% Confidence Interval |
|
|---|---|---|---|
| Pop | Inter-rater | 0.98 | (0.97-0.99) |
| Intra-rater | 0.97 | (0.95-0.98) | |
| BP1 | Inter-rater | 0.98 | (0.97-0.99) |
| Intra-rater | 0.98 | (0.96-0.98) | |
| H2 | Inter-rater | 0.99 | (0.99-1.00) |
| Intra-rater | 0.99 | (0.99-1.00) | |
| PESmax | Inter-rater | 0.96 | (0.94-0.98) |
| Intra-rater | 0.98 | (0.97-0.99) | |
| HL | Inter-rater | 0.89 | (0.81-0.93) |
| Intra-rater | 0.97 | (0.95-0.98) | |
| PAmax | Inter-rater | 0.93 | (0.88-0.96) |
| Intra-rater | 0.98 | (0.96-0.99) | |
Statistics
We calculated the frequencies of the different swallow sequences seen in our dataset, including the number of swallows that adhered to Kendall’s most-common event sequence (AEclose < Pop ≤ BP1 < H2 <PESmax <HL <PAmax). The frequency distributions of the four paired-event sequences described as obligatory by Kendall et al. [13] were explored by bolus volume, viscosity and barium concentration and by swallow-number-within-bolus-condition using frequency tables and bar charts. Descriptive statistics (mean and 95% confidence intervals) were calculated for the latencies between the two events in each paired-event sequence. A post-hoc analysis compared the frequency distribution of cued event-pairs with non-cued event-pairs.
Results
Our analysis identified remarkable variability in swallow event sequencing. A total of 214 different 8-event sequences in the current dataset, of which only 3 sequences were found to occur 4 or more times, each accounting for 1.4-2.0% of the dataset. Within this presentation of remarkable variability, several consistent patterns were noted:
• AEstart was the first event in the overall sequence 92% of the time, while an initial BP1 event accounted for a further 5% of cases;
• a pattern beginning with AEstart < AEclose ≤ BP1 ≤ POP accounted for 37% of the recorded swallows;
• a pattern beginning with AEstart < BP1 <AEclose ≤ POP accounted for a further 23% of the recorded swallows;
• AEclose either preceded or occurred simultaneously with BP1 in 29% of the swallows in the dataset, with an average anticipation of 0.77 frames, i.e., 25 msec (95% confidence interval: −16 to 67 msec).
Strict adherence to the most-common event sequence as reported by Kendall et al. [13] (AEclose→ Pop→ BP1→ H2→PESmax→ HL→ PAmax) was observed in only 4 (1.3%) swallows in our dataset. In the 3% of cases where neither AEstart or BP1 initiated the sequence, the initial even was either H2 or Pop, suggesting anticipatory hyoid movement or UES opening.
Frequency distributions for the four paired-event sequences of interest are displayed in Table 3 by volume, viscosity and barium concentration. When examining the distribution of these paired-event sequences, both the AEstart before Pop and HL after Pop sequences were found to occur with similar dominance to that observed by Kendall et al. [13], with less than 3% of the swallows in the overall dataset presenting with the reversed pattern. Interestingly, however, the Pop before/with BP1 sequence occurred predominantly in reverse order in our dataset, i.e., UES opening occurred after bolus arrival at the sphincter in 259/293 cases, with only 11.6% of our data following the pattern described by Kendall et al. [13]. Interestingly, discussion with colleagues regarding this observation raised the possibility that the definition of BP1 (i.e., arrival of the bolus head at the UES) is somewhat open to interpretation, and that some individuals may operationally require entry of the bolus into the open sphincter for the BP1 event (which would automatically fix the order of these two events as POP ≤ BP1). This was not required in this study, as per our interpretation of Kendall et al.’s definitions, rather we required the bolus head to arrive at the base of the pharynx or pyriform sinuses in order to score the BP1 event as having occurred. Finally, the PAmax after PESmax sequence demonstrated a mixed result compared to Kendall et al.’s observations [13] with 83% of cases following Kendall et al.’s observed sequence, and 17% occurring either in the reverse order or synchronously. When we examined the average latency (Table 4) between the first and second event in each of the paired-event sequences we see that the Pop before/with BP1 sequence and the PAmax after PESmax sequence had shorter latencies than the remaining two paired-event sequences.
Table 3.
Frequency distribution of paired-event sequences across bolus volumes, viscosities, and barium concentration. Percentages are based on a ‘YES’ response. [AEstart = onset of arytenoid elevation, Pop = UES opening, BP1 = bolus arriving at the UES, HL = maximum approximation of hyoid and larynx, PESmax = maximum distension of the UES, PAmax = maximum constriction of the pharynx, w/v = weight/volume, ml = milliliters].
|
Obligatory Event
Sequence |
Adherence? | Ultra-thin 22% 5ml |
Ultra-thin 22% 10ml |
Ultra-thin 22% 20ml |
Thin 40% 5ml |
Nectar 40% 5ml |
Total |
|---|---|---|---|---|---|---|---|
| AEstart before Pop? | YES | 100% | 100% | 94% | 97% | 100% | 98% |
| NO | 0% | 0% | 6% | 3% | 0% | 2% | |
|
| |||||||
| Pop before/with BP1? |
YES | 10% | 17% | 22% | 2% | 8% | 12% |
| NO | 90% | 83% | 78% | 98% | 92% | 88% | |
|
| |||||||
| HL after Pop? | YES | 95% | 100% | 100% | 95% | 98% | 98% |
| NO | 5% | 0% | 0% | 5% | 2% | 2% | |
|
| |||||||
| PAmax after PESmax? |
YES | 71% | 73% | 93% | 90% | 87% | 83% |
| NO | 29% | 27% | 7% | 10% | 13% | 17% | |
Table 4.
Descriptive statistics for event latencies for the four event sequences of interest. Latencies are calculated as: Event 2 minus Event 1. Negative latencies represent a pattern in which event 2 occurred prior to event 1. [AEstart = onset of arytenoid elevation, Pop = UES opening, BP1 = bolus arriving at the UES, HL = maximum approximation of hyoid and larynx, PESmax = maximum distension of the UES, PAmax = maximum constriction of the pharynx].
| Event 1 | Event 2 | Mean latency (frames) |
95% Confidence Interval | Mean Latency (ms) |
95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Lower boundary (frames) |
Upper boundary (frames) |
Lower boundary (ms) |
Upper boundary (ms) |
||||
| AEstart | Pop | 6.68 | 6.24 | 7.11 | 223 | 208 | 237 |
| Pop | BP1 | −2.26 | −2.53 | −1.98 | −75 | −84 | −66 |
| Pop | HL | 5.12 | 4.13 | 6.10 | 171 | 138 | 204 |
| PESmax | PAmax | 2.09 | 1.84 | 2.23 | 70 | 61 | 74 |
We found no clear influence of bolus volume when comparing paired-event sequence distributions for the 5, 10 and 20 ml boluses within a single barium concentration (22% w/v). Similarly, with respect to the contribution of bolus viscosity, no obvious trends emerge when comparing the 5ml 40% w/v thin liquid boluses to the 5ml 40% w/v nectar thick boluses, with the exception of a slight increase in the number of swallows displaying the Pop before/with BP1 sequence in the nectar condition (5/60) compared to the thin condition (1/60). Finally, no clear patterns emerge for barium concentration when comparing the 5ml 22% w/v ultra-thin sequences to the 5ml 40% w/v thin sequences, with the exception of a slightly lower frequency of the reversed order of the PAmax after PESmax sequence in the 22% w/v condition (17/59) compared with the 40% w/v condition (6/60). It should be noted that the nectar-thick stimulus in this study was cranberry flavored, while all other stimuli were composed of water and barium without any additional flavor, thus the consistency comparison may reflect some influence of stimulus flavor.
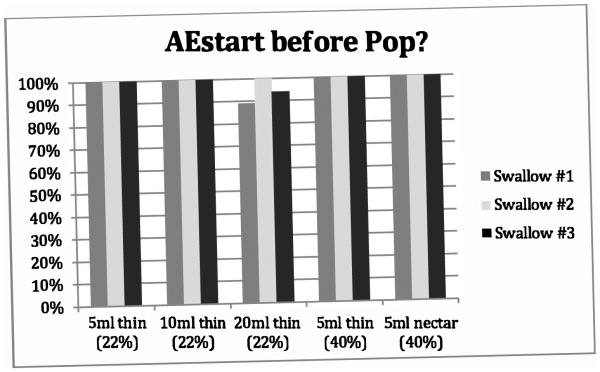
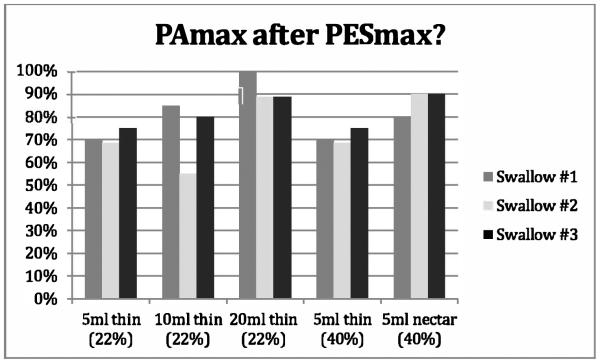
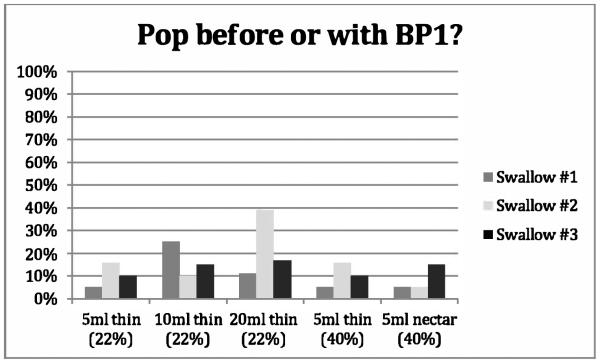
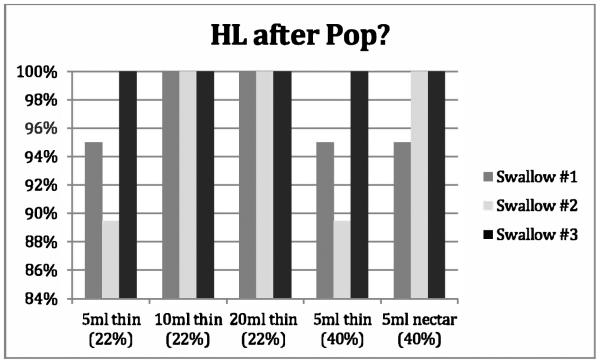
Figures 1-4 provide details regarding sequence variability observed across repeated swallows within bolus condition. There were no order effects noted in our analyses. Although these group data do not display a completely consistent pattern across sequences, it appears that the swallow sequences trend toward stable sequencing across successive swallows for the later HL after Pop and the PAmax after PESmax event pairs in the sequence. Consistent with Kendall et al.’s observation [13], there appears to be greater variation with the smaller bolus volumes for the HL after Pop sequence but not for the other event pairs. Further, additional post-hoc visual inspection of individual participant data within bolus condition blocks revealed variation both within and across individuals. Sequence variation across trials of a bolus condition within individual participants was observed over half of the time (56/100 blocks). Importantly, none of the twenty participants showed a consistent pattern for all four paired-event sequences of interest across all three iterations of all five bolus conditions.
Figure 1.
Proportion of swallows adhering to the obligatory sequence described by Kendall et al. [12] AEstart before Pop across repeated swallows for five bolus conditions. Percentages are based on a ‘YES’ response. [AEstart = onset of arytenoid elevation, Pop = UES opening].
Figure 4.
Proportion of swallows adhering to the obligatory sequence described by Kendall’s et al. [12] PAmax after PESmax across the five bolus conditions. Percentages are based on a ‘YES’ response. [PAmax. =maximum constriction of the pharynx, PESmax = maximum distension of the UES].
Cued vs. non-cued swallows
Swallow cueing is known to influence swallow timing [15, 16], however very little is known about its influence on swallow sequencing. In a post-hoc analysis of our data, we decided to examine the influence of swallow cueing on sequence variability given that the initial Kendall et al. 2003 experiment used a cued swallow paradigm [Leonard, R, 2013, email communication, 21st May]. We compared the frequency distributions of the four paired-event sequences of interest in cued and non-cued 10ml ultra-thin liquid barium swallows. Results appear in Table 5. Cueing did not influence the findings for the AEstart before Pop and HL after Pop sequences, both of which continued to display complete agreement with Kendall et al.’s [13] findings. By contrast, cueing appeared to further reduce the number of Pop before or with BP1 swallows within the 10 ml volume condition (20% to 5%) and slightly increase the frequency of PAmax after PESmax swallows (36% to 50%). Thus, differences inmethodology with respect to cueing cannot be ruled out as, nor considered a satisfactory explanation for the differences between our findings and those reported in the original Kendall et al. study [13].
Table 5.
Frequency distribution of paired-event sequences for non-cued vs. cued 10ml thin liquid barium boluses at 22 % w/v. Percentages are based on a ‘YES’ response. [AEstart = onset of arytenoid elevation, Pop = UES opening, BP1 = bolus arriving at the UES, HL = maximum approximation of hyoid and larynx, PESmax = maximum distension of the UES, PAmax = maximum constriction of the pharynx, w/v = weight/volume, ml = milliliters]
| Sequence | Adherence? |
Non-Cued
10ml thin (22% w/v) |
Cued (Bolus Hold)
10ml thin (22% w/v) |
||
|---|---|---|---|---|---|
| AEstart before Pop? | YES | 60 | 100% | 20 | 100% |
| NO | 0 | 0 | |||
| Pop before or with BP1? | YES | 10 | 20% | 1 | 5% |
| NO | 50 | 19 | |||
| HL after Pop? | YES | 60 | 100% | 20 | 100% |
| NO | 0 | 0 | |||
| PAmax after PESmax? | YES | 44 | 36% | 10 | 50% |
| NO | 16 | 10 | |||
| TOTAL | 60 | 20 | |||
Discussion
This study builds on existing research that explores sequence variability. Our results concur with previous observations by Kendall et al. [13] in identifying remarkable variability in the overall sequence of events within the pharyngeal phase of liquid swallowing in healthy adults. Within the remarkable variation seen in overall event sequencing, certain patterns emerge, with a clear trend towards AEstart <BP1 ≤ AEclose being the usual events at the beginning of the sequence. We examined the sequencing of four paired-events, which were previously reported to occur in an obligatory order [13] .Two of these sequences (AEstart before Pop and HL after Pop), were observed to occur in a similar pattern in our dataset, with < 3% occurring in reverse order. However, the remaining two sequences demonstrated different patterns of occurrence to those previously described. While the majority of swallows were observed to follow the PAmax after PESmax sequence order (83% overall), this proportion did not achieve complete agreement with the data from Kendall et al.’s study [13]. It is worthwhile noting that PAmax as a phenomenon may span several frames during the swallow. Our operational definitions captured the first of these frames, however disparity may exist between our definition and that used by Kendall et al. [13] and may account for the differences found in results. Finally, the order of the Pop before/with BP1 event-pair was largely reversed in our dataset. Kendall describes the UES opening in anticipation of the bolus arriving at the UES 100% of the time, while we fmmd this pattern in only 12% of swallows. Variations in swallow cueing methods between the two studies are suspected to have contributed to this difference in results, although a post-hoc analysis suggests this explanation does not completely account for the reversal in sequence. Similarly, manipulations inbolus volwne, bolus viscosity, and bariwn concentration do not clearly account for reversals in the Pop before/with BP1 and PAmax after PESmax sequences, compared to the patterns observed as obligatory by Kendall et al. [13]. The average latency between the paired-events that did not demonstrate the hypothesized obligatory patterning was shorter than those that did (see Table 3). This may suggest greater complexity in determining the order between two events that are more closely aligned temporally, or that have a greater tendency to occur simultaneously.
One source of sequence variability in our dataset appears to be the fact that we sampled three repeated swallows per condition, however we did not observe systematic patterns of sequence variation across repeated swallows for any of the event-pairs of interest. Variable sequencing across repeated trials of any given bolus consistency was seen in 100% of our healthy participants. Thus, we feel it is important to point out that in this sample of young healthy adults, individual variation in swallow sequence is normal within a bolus condition and should be expected across differences in bolus volwne, viscosity and barium concentration. In their study of event sequencing in 80 healthy individuals balanced for age and gender, Mendell and Logemann [7] reported that despite not seeing an effect of trial (no significant difference between the sequence of swallow #1 and #2), no single pattern was observed for all participants. Kendall and colleagues [13] also recognized a wide range of variability in swallow sequence in their original study, which examined 12 event pairs. They acknowledge that if variability for some events is the norm, then use of sequence evaluation inpatients is not likely to yield clinically useful information. Thus, they suggested that only four obligatory paired-events in their dataset may have utility for identifying disordered swallow coordination in patients (AEstart before Pop, Pop before/with BP1, HL after Pop and PAmax after PESmax). Based on our analysis of sequence variation in 20 healthy young volunteers, we concur that the onset of arytenoid movement towards the base of the epiglottis should occur before the onset of UES opening and that maximum approximation of the hyoid and larynx should occur after UES opening. There are strong physiological and anatomical relationships that exist for these paired-events. Both AEstart and HL are components of the anterior-superior trajectory of the hyolaryngeal complex. It is well established that this upward and forward movement contributes to the opening of the UES via traction forces (see for example [19]). Thus, it is not surprising that these events unfold in an obligatory order in a sample of healthy adults. However, our data failed to find support for the assertion that Pop before/with BP1 and PAmax after PESmax are obligatory sequences. Further research is required before obligatory ordering of these events can be considered characteristic of healthy versus disordered swallowing.
It also remains to been seen, whether other event pairs are candidates for obligatory ordering. For example, in a later study, Kendall et al., [20] found that AEclose occurred prior to BP1 in 93% of normal subjects while the remaining 7% of subjects AEclose occurred within 0.1 seconds of BP1. This particular event pairing has obvious salience for swallowing safety. It is arguably more important to know whether and when complete closure of the laryngeal vestibule is achieved, relative to the arrival of the bolus at or near the laryngeal additus than to know when the arytenoids commence movement towards the base of the epiglottis. In our data, AEclose anticipated BP1 with a lower frequency (29% of the time), but the observed latency agreed with the observations of Kendall et al., with a 95% confidence interval spanning −0.016 to 0.07 seconds. Similarly, it is of interest to confinn whether PES opening is always an antecedent of maximwn pharyngeal constriction (even if maximwn PES opening may not yet have been achieved). Post-hoc exploration of this sequence for the current dataset confirmed that the PA Max frame occurred after the POP frame 100% of the time in our data set, with an average latency of 5.91 frames (197 ms).
There are some important methodological differences to acknowledge between our study and Kendall et al.’s [13], which may partially explain or contribute to the differences in study findings. First, Kendall et al.’s [13] study protocol involved single boluses for three bolus volwnes (1, 3, and 20ml) whereas our study involved 3 task repetitions at different bolus volwnes (5, 10, and 20ml). This difference in methods is important given that our analysis showed that sequence was not always stable across the evolution of repeated swallows within a condition. Second, Kendall et al.’s [13] bariwn concentration was 60% w/v while we used lower concentrations of 22% and 40% w/v. Finally, our dataset only included participants under the age of 45 while Kendall’s study sampled from a larger range of ages.
Conclusions
Our results concur with Kendall and colleagues [13] in displaying extraordinary variability in event sequencing during healthy swallowing, and in showing that the onset of artyenoid movement towards the base of the epiglottis almost always occurs prior to the onset of UES opening, while maximwn approximation of the hyoid and larynx occurs after the onset of UES opening. However, discrepancies exist between our study and Kendall et al.’s [13] with respect to the event sequence observed for UES opening and bolus arrival at the UES and for maximal constriction of the pharynx and maximal distension of the UES. Manipulations of bolus volume, bolus viscosity, barium concentration, swallow cueing and swallow repetitions did not reliably elicit directional differences within our dataset to account for differences observed between the two studies. We conclude that healthy swallowing is characterized by flexibility in event sequencing rather than a fixed order of events, even in the context of a rigorously controlled experimental protocol, designed to limit variation in swallowing tasks. This situation appears optimal for accommodating a variety of task demands and unexpected perturbations in swallowing. Whether patients with dysphagia display reduced variability in sequencing, which might impact their ability to successfully handle variations in the manner in which the bolus is travelling through the pharynx remains an important question for future research.
Figure 2.
Proportion of swallows adhering to the obligatory sequence described by Kendall et al. [12] Pop before or with BP1 across repeated swallows for five bolus conditions. Percentages are based on a ‘YES’ response. [Pop = UES opening, BPI = bolus arriving at the UES].
Figure 3.
Proportion of swallows adhering to the obligatory sequence described by Kendall et al. [12] HL after Pop across repeated swallows for five bolus conditions. Percentages are based on a ‘YES’ response. [HL = maximum approximation of hyoid and larynx, Pop = UES opening].
Acknowledgments
These data have been submitted for poster presentation at the 2013 ASHA Convention. The first author received funding for her doctoral studies from the Natural Sciences and Engineering Research Council (Canada) Create CARE program, the Ontario Student Opportunity Trust Fund and the Ontario Graduate Studies scholarship program. The authors would like to thank Ms. Helen Wang for assistance with reliability analysis and acknowledge the support of Toronto Rehabilitation Institute – University Health Network who receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-term Care in Ontario. The views expressed do not necessarily reflect those of the Ministry. Finally, the authors would like to thank Dr. Rebecca Leonard for correspondence regarding the methodology of her original study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 2.Logemann JA. Evaluation and Treatment of Swallowing Disorders. 1998 [Google Scholar]
- 3.Molfenter SM, Steele CM. Temporal variability inthe deglutition literature. Dysphagia. 2012;27(2):162–177. doi: 10.1007/s00455-012-9397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohmae Y, Logemann JA, Kaiser P, Hanson DG, Kahrilas PJ. Timing of glottic closure during normal swallow. Head and Neck. 1995;17(5):394–402. doi: 10.1002/hed.2880170506. [DOI] [PubMed] [Google Scholar]
- 5.Logemann JA, Pauloski B, Rademaker AW, Kahrilas PJ. Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. J Speech Lang Hear Res. 2002;45(3):434–445. doi: 10.1044/1092-4388(2002/034). [DOI] [PubMed] [Google Scholar]
- 6.Logemann JA, Pauloski B, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and Biomechanical Characteristics of Oropharyngeal Swallow inYounger and Older Men. J Speech Lang Hear Res. 2000;43(5):1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 7.Mendell DA, Logemann JA. Temporal sequence of swallow events during the oropharyngeal swallow. J Speech Lang Hear Res. 2007;50(5):1256–1271. doi: 10.1044/1092-4388(2007/088). [DOI] [PubMed] [Google Scholar]
- 8.Kendall KA. Oropharyngeal swallowing variability. Laryngoscope. 2002;112(3):547–551. doi: 10.1097/00005537-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Cook IJ, Dodds WJ, Dantas RO, Kern MK, Massey BT, Shaker, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4(1):8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 10.Kendall KA, McKenzie S, Leonard RJ, Gon alves MI, Walker A. Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia. 2000;15(2):74–83. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- 11.Gay T, Rendell JK, Spiro J, Mosier K, Lurie AG. Coordination of oral cavity and laryngeal movements during swallowing. J Appl Physiol. 1994;77(1):357–365. doi: 10.1152/jappl.1994.77.1.357. [DOI] [PubMed] [Google Scholar]
- 12.McConnel FMS, Cerenko D, Jackson RT, Guffm TN., Jr. Timing of major events of pharyngeal swallowing. Archives of Otolaryngology - Head and Neck Surgery. 1988;114(12):1413–1418. doi: 10.1001/archotol.1988.01860240063025. [DOI] [PubMed] [Google Scholar]
- 13.Kendall KA, Leonard RJ, McKenzie SW. Sequence variability during hypopharyngeal bolus transit. Dysphagia. 2003;18(2):85–91. doi: 10.1007/s00455-002-0086-z. [DOI] [PubMed] [Google Scholar]
- 14. Reference withheld for blinding purposes.
- 15.Daniels SK, Schroeder MF, DeGeorge PC, Corey DM, Rosenbek JC. Effects of verbal cue on bolus flow during swallowing. Am J Speech Lang Pathol. 2007;16(2):140–147. doi: 10.1044/1058-0360(2007/018). [DOI] [PubMed] [Google Scholar]
- 16.Nagy A, Leigh C, Hori SF, Molfenter SM, Shariff T, Steele CM. Timing Differences Between Cued and Noncued Swallows inHealthy Young Adults. Dysphagia. 2013:1–7. doi: 10.1007/s00455-013-9456-y. (Online first) [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of obseiver agreement for categorical data. Biometrics. 1977;33(1):159–174. (1977) [PubMed] [Google Scholar]
- 18.Fleiss JL. The design and analysis of clinical experiments. Wiley; New York: 1986. [Google Scholar]
- 19.Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97(6):1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- 20.Kendall KA, Leonard RJ, McKenzie S. Airway protection: Evaluation with videofluoroscopy. Dysphagia. 2004;19:65–70. doi: 10.1007/s00455-003-0500-1. [DOI] [PubMed] [Google Scholar]