Abstract
How the immune system adapts to malnutrition to sustain immunity at barrier surfaces such as the intestine remains unclear. Vitamin A deficiency is one of the most common micronutrient deficiencies, associated with profound defects in adaptive immunity. Here, we found that type 3 innate lymphoid cells (ILC3) are severely diminished under vitamin A deficient settings resulting in compromised immunity to acute bacterial infection. However, deprivation of vitamin A paradoxically resulted in dramatic expansion of IL-13 producing ILC2 and resistance to nematode infection revealing ILC as primary sensors of dietary stress. Further, these data propose that during malnutrition, a switch to innate type 2 immunity may represent a powerful adaptation of the immune system to promote host survival in the face of ongoing barrier challenges.
Maintenance of barrier defense is an absolute requirement for mammalian host health and survival. Intestinal immunity has evolved in the context of constitutive exposure to microbial pressures. In this regard, the gastrointestinal (GI) tract is home to an estimated 100 trillion commensals, and over 2 billion humans remain chronically infected by parasitic worms1, 2. Over the course of evolution, maintenance of barrier immunity had to adapt to unstable nutritional availability in the face of these ongoing challenges. Although, dietary ligands can be sensed by immune cells3–6 how the immune system integrates dietary cues in order to tune immune responses based on the nutritional state of the host remains unclear.
Malnutrition remains the primary cause of immunosuppression worldwide7. Nonetheless, despite profoundly impaired adaptive immunity associated with malnutrition, most humans can survive for extended periods of severe dietary restriction. We postulated that compensatory mechanisms might be in place to sustain defined branches of immunity and in particular responses associated with the protection of barrier tissues. Vitamin A deficiency is one of the most common nutrient deficiencies, affecting an estimated 250 million children in regions of the world where chronic worm infections are prevalent8. This essential micronutrient supports adaptive immunity through its metabolite retinoic acid (RA) that is highly enriched in the gastrointestinal tract9, 10. Herein, we examined the possibility that innate lymphoid cells (ILC) potent mediators of barrier maintenance, tissue repair, and host defense11, 12 may be primary sensors of dietary stress able to sustain barrier defense in the context of vitamin A deficiency.
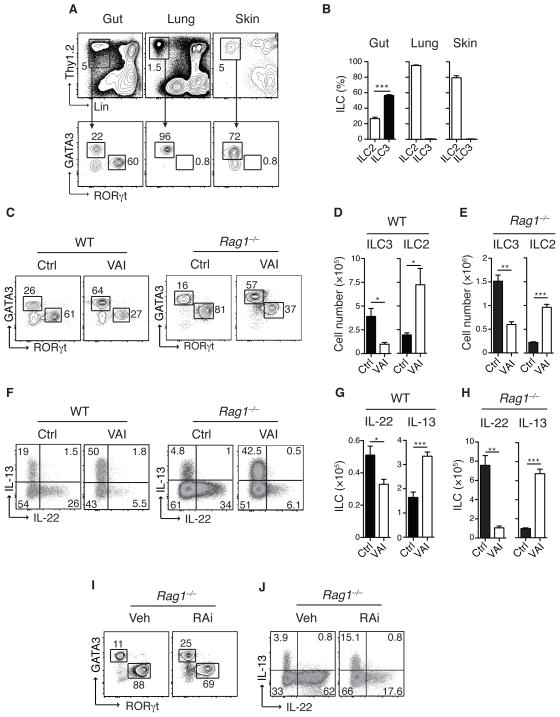
Several subsets of ILC have been described with two dominant tissue resident populations12. At steady state, RORγt+ ILC (ILC3) are dominantly present in the gut while the majority of ILC residing in the lung or skin belonged to the GATA3+ ILC2 subset13–15 (Fig. 1A and B). We explored the possibility that vitamin A metabolites may act as a local cue to control ILC populations. As previously described Th1 and Th17 cells16, 17 but also Th2 cells were significantly reduced in the gut of vitamin A deficient (VAI) mice (Fig. S1A and B). The number of gut resident ILC, ILC1 and LTi remained unchanged in VAI mice (Fig. S2A–D). On the other hand, ILC3 and ILC derived IL-22 and IL-17 were greatly reduced in VAI wild-type (WT) and Rag1−/− mice (Fig. 1C–H, Fig. S3). Impaired cytokine expression was associated with reduced expression of RORγt (Fig. S4A and B). In contrast, we observed a significant increase in ILC2 and ILC derived IL-13, IL-5 and IL-4 in the gut of VAI mice (Fig. 1F, G and H and Fig. S5A and B).
Figure 1. ILC3 are enriched in the GI tract and depend on retinoic acid.
A) Flow cytometric analysis of cells isolated from small intestinal lamina propria (gut), lung and skin of naïve C57Bl/6 mice. Upper panel represents live CD45+ cells stained with Thy1.2 and lineage (lin) markers (NK1.1, TCRβ, TCRγδ, CD11b, CD11c, CD4, CD8a, CD8b, CD19, GR-1, DX5, Ter119). Lower panel displays cells gated on Lin− and Thy1.2 expression (ILC), stained for RORgt (ILC3) and GATA3 (ILC2). B) Frequencies of ILC2 and ILC3 in gut, lung and skin. C) Small intestinal lamina propria (SiLP) cells from control (Ctrl) or vitamin A insufficient (VAI) WT or Rag1−/− mice, gated on Lin−, Thy1.2+ cells and analyzed for GATA3 and RORγt expression. D–E) Total numbers of ILC3 (RORγt+) and ILC2 (GATA3+) cells in the SiLP of WT and Rag1−/− mice. F) Intracellular IL-13 and IL-22 expression in Lin− Thy1.2+ cells following stimulation with PMA and ionomycin and G–H) Total numbers of IL-22 and IL-13 producing ILC in the SiLP. I) SiLP ILC2 and ILC3 from Rag1−/− mice treated with vehicle control (Veh) or retinoic acid receptor inhibitor (RAi) for 8 days and J) Intracellular IL-13 and IL-22 expression in ILC following stimulation with PMA and ionomycin. Results are representative of at least three independent experiments with 3–5 mice in each experimental group. All graphs display means ±SEM.
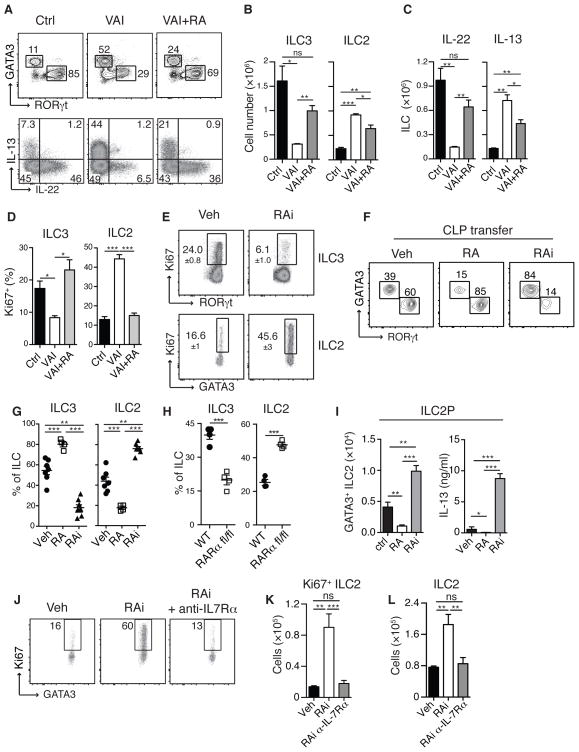
Acute inhibition of RA signaling with the pan-RA receptor inhibitor BMS493 (RAi)18 also resulted in reduced ILC3 and ILC derived IL-22 and inversely increased ILC2 and ILC derived IL-13 in both WT and Rag1−/− mice (Fig. 1I and J and Fig. S6A–D). Alterations in ILC subsets subsequent to RA deprivation occurred independently of commensals (Fig. S7). Short-term treatment of VAI Rag1−/− mice with RA restored ILC3 frequencies and cytokine production to levels found in control mice, while reducing ILC2 numbers (Fig. 2A, B and C). VAI Rag1−/− mice or mice treated with RAi displayed lower frequencies of Ki67 expressing ILC3 than control mice, while the amount of Ki67 expressing ILC2 was greatly increased (Fig. 2D and E). Conversely, addition of RA reversed the proliferative potential of ILC to frequencies observed in control mice (Fig. 2D). Thus, RA acts as a switch to control a proliferative balance between the two intestinal ILC subsets.
Figure 2. Retinoic acid dynamically regulates developmental balance between ILC subsets.
SiLP cells from WT Ctrl and VAI or VAI Rag1−/− mice treated with all-trans retinoic acid every three days for 12 days (VAI + RA). A) GATA3 and RORγt expression in Lin−, Thy1.2+ cells (upper panel). Intracellular IL-13 and IL-22 expression in Lin− Thy1.2+ cells following stimulation with PMA and ionomycin (lower panel). B) Total numbers of ILC3 and ILC2 cells in SiLP and C) total numbers of IL-22 and IL-13 producing ILC in the SiLP. D) Frequencies of Ki67 expression in ILC3 and ILC2 isolated from SiLp of Ctrl, VAI or VAI +RA Rag1−/− mice. E) SiLP cells from Rag1−/− mice treated with RAi or Veh for 8 days stained for intracellular Ki67 in ILC3 (upper panel) cells and ILC2 (lower panel). F) 100,000 CD45.1+ common lymphoid progenitors (CLP) were transferred into congenic CD45.2+ Rag2−/−/gc−/− mice and treated either with vehicle (Veh), RA or RAi. 14 Days after transfer, SiLP cells were isolated, stained for GATA3 and RORγt and gated on CD45.1+, Lin− and Thy1.2+ cells. G) Quantification of relative proportion of ILC3 and ILC2 in recipient mice and H) frequencies of ILC2 and ILC3 from RARafl/fl mice. Transferred cells were gated on GFP+ cells I) ILC2 progenitors (ILC2P) were sort purified from bone marrow and cultured in vitro with IL-7 and SCF in the presence of Veh, RA and RAi.for 7 days. Total numbers of Thy1.2+ GATA3+ cells (left panel) and IL-13 production in the culture supernatant (right panel). J) Ki67 expression in small intestinal ILC2 from mice treated with RAi or Veh and anti-IL7Ra. K) Total number of Ki67+ ILC2 and L) total number of ILC2. Data are representative of three (A–E, G–H) or two (I–L) independent experiments with 3–4 mice in each experimental group or at least two independent experiments with 3 experimental groups of cells isolated from 2 mice each (F). (G) displays pooled data from three independent experiments. All graphs display means ±SEM.
To address if RA signaling influenced the fate of ILC, we transferred common lymphoid progenitors (CLP) to mice devoid of ILC (Rag2−/−γc−/− mice)12. Transferred CLP gave rise to both ILC2 and ILC3 that accumulated in the GI tract19, 20 (Fig. 2F and G). Exogenous delivery of RA led to a dominant accumulation of ILC3 in the intestine while inhibition of RA signaling favored ILC2 accumulation (Fig. 2F and G). Both ILC2 and ILC3 selectively express the nuclear receptor RARα21 (Fig. S8). We retrovirally transfected lymphoid progenitors obtained from mice carrying a WT or floxed alleles of the RARα gene (RARαfl/fl) with retroviral Cre recombinase (Fig. S9). Transfer of control progenitors resulted in the preferential accumulation of ILC3, while RARα-deleted progenitors predominately gave rise to ILC2 (Fig. 2H). Further, addition of RA impaired ILC2 development from ILC2 progenitors (ILC2P) while inhibition of RA signaling resulted in increase ILC2 accumulation and IL-13 production (Fig. 2I). These results reveal a cell intrinsic suppressive role for RA on ILC2 maturation.
We next postulated that some effects of RA may result from a direct action on mature ILC. Highly purified ILC2 exposed to RA or ILC3 treated with RAi for several days maintained their phenotype arguing against an interconversion of these two populations (Fig. S10A–D). As previously reported RA directly increased IL-2221 and RORγt expression by mature ILC3 and promote ILC3 accumulation (Fig. S11A–D). Conversely, RA had a negative impact on ILC2 fitness while treatment with RAi increased their proliferation, numbers and IL-13 production in both mouse (Fig. S12A–C) and human cells (Fig. S13).
We next explored the possibility that vitamin A deficiency may tune the responsiveness of ILC2 to factors contributing to their survival or proliferation11, 12. Using mice deficient in IL-25 or TSLP and IL-33 receptor allowed us to exclude a dominant role for these factors (Fig. S14A–D and Fig. S15A and B). Of note, expression of the IL-7Rα, required for ILC development and survival11, 12 was reduced on ILC2 and ILC2p in the presence of RA while the absence of RA signaling increased IL-7Rα expression by murine ILC2 and ILC2p as well as human ILC2 (Fig. S16A–E). Increased IL7Rα expression conferred enhanced signaling capacity in response to IL-7 as measured by increased STAT5 phosphorylation (Fig. S16F). Blockade of IL-7 signaling in vivo abrogated the increase in proliferation and accumulation of ILC2 following RA inhibition but not in control mice (Fig. 2J, K and L and Fig. S17). Together these results demonstrate that vitamin A deficiency is associated with altered ILC homeostasis and in particular, increased IL-13 producing ILC2 mediated by increased IL-7 responsiveness.
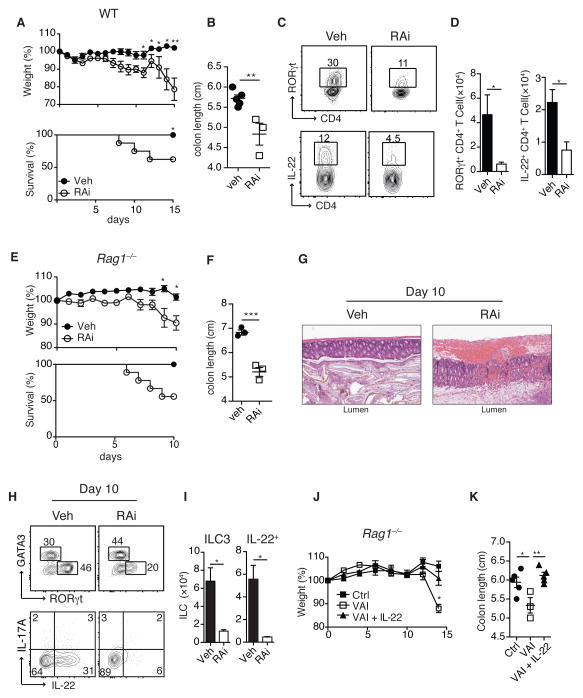
A corollary of our findings is that differential levels of vitamin A could promote different classes of barrier immunity with physiological levels of vitamin A associated with ILC3 responses, while reduced levels associated with enhanced innate type 2 immunity. Indeed, both WT VAI mice and mice treated with RAi displayed enhanced susceptibility and pathology to C. rodentium compared to control mice, a phenotype associated with impaired Th1, Th17 and ILC3 responses (Fig. 3A–D and Fig. S18A–E). Treatment with RAi of Rag1−/− mice in which ILC are the dominant source of IL-2222 dramatically increased pathology and mortality following infection, an effect reversed by exogenous delivery of IL-22 (Fig. 3E–K). This observation provides a potential explanation for the profound susceptibility to gastrointestinal bacterial infections observed in children suffering from vitamin A deficiency23,24.
Figure 3. Vitamin A deficiency results in impaired immunity to bacterial infections.
WT (A–D) and Rag1−/− (E–K) mice treated with Veh or RAi were infected with C. rodentium. A) Percentile change of original body weight and frequency of surviving animals and B) colon length of WT mice treated with RAi or Veh. C) Large intestine lamina propria (LiLP) cells isolated from Veh or RAI treated WT mice 10 days after infection with C. rodentium, gated on CD4+ and TCRb+ cells and analyzed for RORγt (upper panel) and IL-22 expression (lower panel). D) Total numbers of RORγt+ and IL-22+ CD4+ T cells. E) Percentile change of original body weight and frequency of surviving Rag1−/− mice treated with Veh or RAi and infected with C. rodentium. F) Colon length and G) representative H&E histological sections of colonic tissue analyzed 10 days post-infection. H) LILP ILC2 and ILC3 from Veh or RAI treated Rag1−/− mice 10 days after infection with C. rodentium (upper panel) and intracellular IL-17A and IL-22 expression in ILC following stimulation with PMA and ionomycin (lower panel). I) Total numbers of RORγt expressing ILC and total numbers of IL-22 producing ILC per colon. J) Weight loss of Ctrl, VAI or VAI Rag1−/− mice treated with IL-22 (VAI+IL-22) and infected with C. rodentium. K) Colon length analyzed 13 days post-infection. Data represent at least two independent experiments with 3–5 mice in each experimental group. All graphs display means ±SEM.
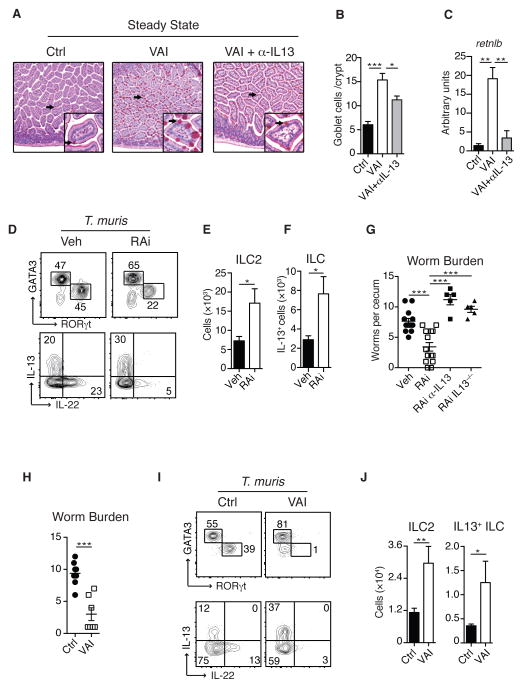
Our results thus far, predict that although Th1, Th17, and ILC3 collapse under vitamin A deficiency, type 2 immunity might be paradoxically augmented via an enhanced ILC2 response. In support of this, withdrawal of vitamin A heightened mucus production16. Goblet cells play a central role in barrier protection by the secretion of mucus and antimicrobial peptides25. Consistent with increased ILC2 observed in the absence of vitamin A, VAI Rag1−/− mice displayed significant goblet cell hyperplasia and increased goblet cell associated expression of the RELM-β gene, retnlb, an effect largely dependent upon IL-13 (Fig. 4A, B and C). Under these settings, IL-13 production was predominantly ILC2 derived (Fig. S19). We next addressed if enhanced ILC2 responses could compensate for the defect in Th2 immunity during vitamin A deficiency26–30. To this end we utilized a high dose of Trichuris muris eggs associated with Th2 induction31. Although blocking of RA impaired Th2 induction, ILC2 numbers were significantly increased compared to infected control mice (Fig. S20A and B). Remarkably, RAi treated mice controlled parasite burden comparably to control mice supporting the idea that ILC2 sustained worm control (Fig. S20C). Further, both VAI and RAi treated mice showed enhanced protection to physiological low dose T.muris a response associated with significant increase in the numbers of ILC2 and IL-13 producing ILC (Fig. 4D–G and Fig. S21A). In agreement with the role of IL-13 in T.muris control32, accelerated worm clearance was critically dependent on this cytokine (Fig. 4G). We next assessed the role of ILC2 in mediating worm expulsion independently of adaptive immunity. Remarkably, VAI Rag1−/− mice displayed enhanced protection and reduced worm burden associated with substantial increase in ILC2 numbers and IL-13 production (Fig. 4H, I and J). Thus, vitamin A insufficiency leads to sustained and in some cases augmented control of nematode infection via the promotion of ILC2 dependent type 2 immunity.
Figure 4. Vitamin A deficiency increases ILC2 mediated immunity to helminth infections.
A). Small intestine histologic sections of Ctrl, VAI or VAI Rag1−/− mice treated with anti-IL13 antibody (VAI+αIL13) stained with PAS to visualize goblet cells. B) Total numbers of PAS-positive goblet cells per crypt and C) Retnlb (Relm-β) gene expression in the small intestine of Ctrl, VAI or VAI+αIL13 Rag1−/− mice. D) Lamina propria ILC2 and ILC3 isolated from the cecum of Ctrl or VAI WT mice 13 days after oral infection with T. muris (upper panel) and intracellular IL-13 and IL-22 expression in ILC after stimulation with PMA and ionomycin (lower panel). E) Total numbers of ILC2 and F) total numbers of IL-13 producing ILC in the cecum. G) Worm burden in the cecum of Veh, RAi, RAi treated IL-13−/− (RAi IL-13−/−) and RAi mice treated with neutralizing anti-IL13 antibody (RAi a-IL13) 12 days after infection. H) Number of worms in Ctrl and VAi Rag1−/− mice 12 days after infection and I) intracellular GATA3 and RORγt (upper panel) and IL-13 and IL-22 expression (lower panel) in cecal ILC. J) Total numbers of ILC2 and IL-13+ ILC in the cecum of T. muris infected mice. Data represents at least two (A–B, H–J) or three (D–G) independent experiments with 3–5 mice in each experimental group. Data in G) Veh and RAi is pooled from three experiments, RAi a-IL13 and RAi IL-13−/− represent one experiment each. All graphs display means ±SEM.
Our work suggests that vitamin A and its metabolite RA, functions as a dietary alarm signal allowing the host to immunologically respond to its nutritional state. Contrary to the current paradigm, we show that nutrient deficiency is not associated with global immunosuppression but rather can selectively activate a distinct arm of barrier immunity. Notably we found that ILC2 act as primary sensors of dietary stress able to compensate for the collapse of adaptive immunity in settings of nutrient deprivation. Type 2 immunity and in particular IL-13 is associated with tissue repair, increased mucus production, and physiological responses all aimed at reinforcing barrier integrity and defense33, 34. Further enhanced type 2 responses are clearly beneficial in the context of exposure to worms that have been partners throughout human evolution and still represent the major form of parasitic infection worldwide. Since nematodes compete with the host for nutritional resources reinforcement of anti-helminth immunity can provide a substantial advantage to the host. Thus, in settings of malnutrition, a rapid switch to type 2 barrier immunity imposed by vitamin A deficiency may represent a powerful adaptation of the immune system to transiently promote host survival in the face of dominant barrier exposures. Such a strategy leaves the host vulnerable to potential encounters with acute diarrheal pathogens but could provide a survival strategy to temporarily reduce the pressure from its constitutive evolutionary partners, worms and commensals, in settings of nutritional deprivation.
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases (NIAID), Office of dietary supplements grant (NAI12023), NIH grant F30 DK094708 (S.P.S.), Human Frontier Science Program (C.W.), the Damon Runyon Cancer Research Foundation (Dale F. and Betty Ann Frey Fellow, J.A.H.) and by funds from the USDA/ARS Project Plan #1254-32000-094-00D (J.F.Jr.U.). We thank the NIAID animal facility staff; K. Holmes and the NIAID sorting facility, in particular C. Eigsti and E. Stregevsky; K. Beacht and the NIAID gnotobiotic facility and in particular C. Avecedo and D. Trageser-cesler for technical assistance. We thank Dr. Artis for providing C. rodentium, Dr. W. J. Leonard for providing TSLPR−/− mice and Dr. W. Paul for providing IL33R−/− mice. We thank the Belkaid lab for critical discussions regarding the manuscript. The data presented in this manuscript are tabulated in the main paper and the supplementary materials.
Footnotes
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Soil-transmitted helminth infections (factsheet) World Health Organization; 2013. [Google Scholar]
- 3.Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nature immunology. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS medicine. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency. World Health Organization; 2009. [Google Scholar]
- 9.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldhoen M, Brucklacher-Waldert V. Dietary influences on intestinal immunity. Nature reviews. Immunology. 2012;12:696–708. doi: 10.1038/nri3299. [DOI] [PubMed] [Google Scholar]
- 11.Spits H, et al. Innate lymphoid cells - a proposal for uniform nomenclature. Nature reviews. Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 12.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature immunology. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 13.Roediger B, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature immunology. 2013 doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BS, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Science translational medicine. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews. Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 16.Cha HR, et al. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 2010;184:6799–6806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- 17.Hall JA, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastner P, et al. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001;97:1314–1320. doi: 10.1182/blood.v97.5.1314. [DOI] [PubMed] [Google Scholar]
- 19.Possot C, et al. Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nature immunology. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mielke LA, et al. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima AA, Guerrant RL. Persistent diarrhea in children: epidemiology, risk factors, pathophysiology, nutritional impact, and management. Epidemiologic reviews. 1992;14:222–242. doi: 10.1093/oxfordjournals.epirev.a036088. [DOI] [PubMed] [Google Scholar]
- 24.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094. doi: 10.1136/bmj.d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nature reviews. Microbiology. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 26.Carman JA, Hayes CE. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J Immunol. 1991;147:1247–1252. [PubMed] [Google Scholar]
- 27.Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology. 1993;80:581–586. [PMC free article] [PubMed] [Google Scholar]
- 28.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. International immunology. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 29.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. The Journal of nutrition. 2002;132:3736–3739. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- 30.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 2008;180:1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- 31.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 33.Wynn TA. IL-13 effector functions. Annual review of immunology. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 34.Chen F, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nature medicine. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapellier B, et al. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- 36.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoyler T, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage(−)Sca1+c-Kit(−)CD25+ cells are IL-33-responsive type 2 innate cells in the mouse bone marrow. J Immunol. 2011;187:5795–5804. doi: 10.4049/jimmunol.1102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halim TY, et al. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Yang G, et al. Therapeutic dosing with anti-interleukin-13 monoclonal antibody inhibits asthma progression in mice. The Journal of pharmacology and experimental therapeutics. 2005;313:8–15. doi: 10.1124/jpet.104.076133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.